Abstract
OBJECTIVE
Islet autoimmunity has long been recognized in the pathogenesis of type 1 diabetes and is becoming increasingly acknowledged as a component in the pathogenesis of type 2 diabetes. Islet reactive T cells and autoantibodies have been demonstrated in type 1 diabetes, whereas islet autoimmunity in type 2 diabetes has been limited to islet autoantibodies. In this study, we investigated whether islet reactive T cells might also be present in type 2 diabetic patients and how islet reactive T cells correlate with β-cell function.
RESEARCH DESIGN AND METHODS
Adult phenotypic type 2 diabetic patients (n = 36) were screened for islet reactive T-cell responses using cellular immunoblotting and five islet autoantibodies (islet cell antibody, GADA, insulin autoantibody, insulinoma-associated protein-2 autoantibody, and zinc transporter autoantibody).
RESULTS
We identified four subgroups of adult phenotypic type 2 diabetic patients based on their immunological status (Ab−T−, Ab+T−, Ab−T+, and Ab+T+). The Ab−T+ type 2 diabetic patients demonstrated T-cell responses similar to those of the Ab+T+ type 2 diabetic patients. Data were adjusted for BMI, insulin resistance, and duration of diabetes. Significant differences (P < 0.02) were observed among groups for fasting and glucagon-stimulated C-peptide responses. T-cell responses to islet proteins were also demonstrated to fluctuate less than autoantibody responses.
CONCLUSIONS
We have identified a group of adult autoimmune phenotypic type 2 diabetic patients who are Ab−T+ and thus would not be detected using autoantibody testing alone. We conclude that islet autoimmunity may be more prevalent in adult phenotypic type 2 diabetic patients than previously estimated.
Type 1 diabetes results from cell-mediated autoimmune β-cell dysfunction and destruction, whereas type 2 diabetes has been historically considered a metabolic disease (1). However, increasing evidence is pointing toward a relationship among inflammation, insulin resistance, and the subsequent development of type 2 diabetes. In fact, inflammation in the pancreatic islets of type 2 diabetes includes the presence of cytokines (2,3) and the infiltration of immune cells (3,4).
Despite the fact that the pathological process in autoimmune diabetes involves T cells, immune markers of autoimmune diabetes have primarily centered on the presence of circulating serum autoantibodies to various islet antigens (5,6). However, ∼20% of patients with newly diagnosed autoimmune type 1 diabetes are autoantibody negative (7). It was also found that 9% of autoantibody-negative type 1 diabetic patients carry the highest risk HLA genotype (DR3-DQ2/DR4-DQ8), strongly suggesting that these patients had autoimmune diabetes that was undetected with autoantibody testing alone (7). Furthermore, patients with fulminant type 1 diabetes have been reported to be autoantibody negative but demonstrate islet-specific T-cell responses (8). Therefore, we hypothesized that there may exist a group of autoimmune phenotypic type 2 diabetic patients who are autoantibody negative, similar to the Ab− type 1 diabetic patients, but who demonstrate autoimmunity with islet reactive T cells.
Over the years, we have been investigating islet-specific T-cell responses using cellular immunoblotting in diabetic patients (9–13). Our assay has been validated to have excellent specificity and sensitivity for the detection of islet reactive T cells in type 1 diabetic patients (9,10). Moreover, we have previously observed that T-cell reactivity to islet proteins correlates more strongly with impaired β-cell function than with autoantibody positivity (12). In this study, we used our validated T-cell assay for the detection of islet reactive T cells to investigate whether autoantibody-negative T-cell reactive adult phenotypic type 2 diabetic patients could be identified. We provide evidence for the existence of this group of autoimmune phenotypic type 2 diabetic patients. We conclude that islet autoimmunity may be more prevalent in adult phenotypic type 2 diabetic patients than previously estimated and assessing autoimmunity through T cells is of importance.
RESEARCH DESIGN AND METHODS
In this study, we wanted to determine the autoimmune status of patients aged 35–70 years with recently diagnosed type 2 diabetes. We screened patients within 5 years of diagnosis. However, because diagnosis of type 2 diabetes is often not as definitive a diagnosis as type 1 diabetes, our inclusion criteria included A1C <8.0%, no insulin treatment, and use of only one oral diabetes medication to control glucose levels. The inclusion criterion of A1C <8.0% was used to ensure that all patients were type 2 diabetic patients without “severe” disease and thus not requiring insulin treatment. These patients were either obese or had an increased waist-to-hip ratio, had no history of ketonuria or ketoacidosis, and were consecutively chosen from patients meeting the criteria. Thirty-six phenotypic type 2 diabetic patients participated in this study. Patients were evaluated for T-cell responses, using cellular immunoblotting, and autoantibody responses to islet proteins (islet cell antibody [ICA], GADA, insulin autoantibody [IAA], insulinoma-associated protein-2 autoantibody [IA-2A], and zinc transporter autoantibody [ZnT8A]). Written informed consent was obtained from each patient before sample collection. Patients were subjected to blood draws on two or three occasions 3 months apart. Patients were classified as having autoimmune diabetes if two of two or two of three test results were positive for any antibody or T-cell assay or were classified as having nonautoimmune diabetes if two of two or two of three test results were negative.
Autoantibody assays
Assays for autoantibodies (ICA, GADA, IAA, and IA-2A) were first performed (screening assays) in Seattle, Washington, and then were confirmed in other laboratories (confirmatory assays). All serum assays, both screening and confirmatory, were blinded when performed in the laboratories.
Screening autoantibody assays
Screening autoantibody assays for IAA, IA-2A, and GADA were performed at the Northwest Lipid, Metabolism, and Diabetes Research Laboratories at the University of Washington (Seattle, WA). ICA assays were performed in our laboratory. ZnT8A assays were performed by Dr. Liping Yu at the Barbara Davis Center (Denver, CO).
GAD-65 autoantibody assay
GADAs were measured in a radiobinding immunoassay on coded serum samples as described previously (14). The levels of GADA were expressed as a relative index (GAD index) using one positive serum (Juvenile Diabetes Foundation [JDF] World Standard for ICA) and three negative standard sera from healthy subjects. The GAD index was calculated, and a positive result was set at >0.085, which is the 99th percentile based on 200 normal control subjects. Positive and negative controls were run in duplicate in each assay. In the Immunology of Diabetes Society (IDS)–sponsored 2007 Diabetes Antibody Standardization Program (DASP) workshop, the sensitivity of the GAD assay was 78% and specificity was 98% (15).
IA-2A assay
Autoantibodies to IA-2 were measured under conditions identical to those described for GADAs (14) using the plasmid containing the cDNA coding for the cytoplasmic portion of IA-2. The IA-2A index for each sample was calculated using the same JDF standard serum and control sera that were used in the GADA assay. An IA-2 index >0.017, the 99th percentile based on 200 normal control subjects, was the cutoff for positivity. In the IDS-sponsored 2007 DASP workshop, the sensitivity of the IA-2A assay was 64% and specificity was 99% (15).
IAA assay
125I-Insulin was incubated with serum and separation was achieved using a 50% protein A/8% protein G-Sepharose mixture. As with the other assays, positivity was set at the 99th percentile of normal control subjects. In the IDS-sponsored 2007 DASP workshop, the sensitivity of the IAA assay was 16% and specificity was 99% (15).
ZnT8A assay
The presence of the zinc transporter autoantibody (16) was tested in serum from the patients using a radiobinding assay by Dr. Liping Yu at the Barbara Davis Center. Cutoff values were set as the mean + 4 SD of the within-assay controls.
ICA assay
This assay was performed as described previously (17). All sera with detectable ICA were end point titered. The lower detection limit of our assay was 1 JDF unit and the 95th percentile positivity threshold was established at 6 JDF units based on ∼4,000 normal school children (17). Our laboratory had participated in a total of eight Immunology of Diabetes Society Workshops and IDS-sponsored proficiency programs for ICA with an average sensitivity of 80% and specificity of 100%. In the IDS-sponsored Combined Antibody Workshop, our ICA assay had a specificity of 98% and a sensitivity of 76%. Our ICA assay had been validated in a serum exchange with the Diabetes Prevention Trial–type 1 Diabetes (DPT-1) ICA core laboratory. In this exchange, the sensitivity of our assay was 85% with a specificity of 100%.
Confirmatory autoantibody testing
For confirmation of autoantibody results, autoantibody assays were performed for ICA in the laboratory of Dr. William Winter at the University of Florida (Gainesville, FL) (18). Assays for GADA, IAA, and IA-2A were performed in the laboratory of Drs. Liping Yu and George Eisenbarth at the Barbara Davis Center (19).
ICA measurement
Confirmatory ICA assays were performed by the indirect immunofluorescence method using cryostat-cut frozen sections of human blood type O pancreas. The results were expressed in JDF units, and a value ≥10 JDF units was set as positive (18).
GADA and IA-2A assays
Confirmatory GADA and IA-2A assays were performed by a combined radiobinding assay as described previously (19). The interassay coefficients of variation (CVs) were 10 and 5% for GADA and IA-2A, respectively. The upper limits of normal, nondiabetic sera were established as the 99th percentile of 198 healthy control subjects. In the 2005 DASP workshop, the sensitivity and specificity were 76 and 99% for GADA and 64 and 100% for IA-2A, respectively.
IAAs
IAAs were measured by a microradiobinding assay as described previously (20). The interassay CV was 20% at low positive levels. In the 2005 DASP workshop, the sensitivity and specificity for micro-IAAs were 58 and 99%, respectively.
HLA haplotyping
HLA haplotyping was performed at the University of Pittsburgh (Pittsburgh, PA) by Dr. Massimo Trucco's laboratory (21).
T-cell assay: cellular immunoblotting
Cellular immunoblotting was performed on freshly isolated peripheral blood mononuclear cells to test for the presence of islet reactive T cells (9–13). In brief, normal human islet cell preparations were subjected to preparative one-dimensional 10% SDS-PAGE and electroblotted onto nitrocellulose. The nitrocellulose particles containing islet proteins were used to stimulate peripheral blood mononuclear cells from patients. Positive responses were determined to be T-cell proliferative responses to ≥4 blot sections. Human pancreatic islets were obtained from the National Institutes of Health (NIH)–supported Islet Cell Resource Centers. The specificity of the T-cell responses from diabetic patients to islet proteins has been demonstrated previously (11). We have participated in two distinct NIH-supported T-cell validation workshops designed to test the ability of several different assays, including cellular immunoblotting, to distinguish T-cell responses to islet proteins of type 1 diabetic patients from those of control subjects (9,10). In both workshops, with the use of masked specimens, cellular immunoblotting distinguished type 1 diabetic patients from control subjects with high sensitivity and specificity: 94 and 83% in the Immune Tolerance Network (ITN) workshop and 74 and 88% in the Trial Net workshop, respectively (9,10). To control for interassay variation of the islet antigen preparations, the quantity and quality of islets are held constant among preparations, and new antigen preparations are compared with and run alongside older preparations.
Confirmatory T-cell responses
Peripheral blood mononuclear cells were analyzed for reactivity to 15 test antigens as described previously (10). A response was considered positive if reactivity was positive to ≥4 antigens. Split samples were available from 11 patients (3 in each patient group except the Ab−T+ group for which only 2 patient samples were available) to be sent to Dr. Michael Dosch's laboratory (Hospital for Sick Children, Toronto, ON, Canada). The T-cell proliferation assay performed by Dr. Dosch's laboratory was validated in the ITN-supported study (9) with a sensitivity of 58% and a specificity of 94%. Responses obtained from Dr. Dosch's laboratory confirmed our data (results not shown).
C-peptide assays
Fasting and glucagon-stimulated C-peptide responses were used as a measure of endogenous β-cell function in all patients. Stimulated C-peptide was measured 6 min after the intravenous injection of 1 mg glucagon. The C-peptide assay is a two-site immunoenzymometric assay, performed using a Tosoh 600 II autoanalyzer (Tosoh Bioscience, South San Francisco, CA) at the Northwest Lipid, Metabolism, and Diabetes Research Laboratories (12). The interassay and intra-assay precision analysis showed a CV <10%. The assay has a sensitivity level of 0.04 ng/ml.
Homeostasis model assessment of insulin resistance
To estimate insulin resistance, the homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from fasting insulin (microunits per milliliter) and fasting glucose (milligrams per deciliter) concentrations using the formula (insulin × glucose)/405 according to Matthews et al. (22).
Statistics
The nonparametric Mann-Whitney U test was performed to determine statistical significance among the patient groups. Multiple linear regression analysis was used to estimate associations between measures of C-peptide and antibody and T-cell status while adjusting for BMI, HOMA-IR, and duration of diabetes. Adjusted means were computed from these models using Stata 10.1 (StataCorp, College Station, TX). The Bonferroni correction was used to account for multiple testing. P < 0.02 was considered significant for data adjusted for BMI, HOMA-IR, and duration of diabetes.
RESULTS
Patients (n = 36) were assayed for their T-cell and autoantibody responses and subsequently divided into groups based on their islet autoimmune status. The Ab+T+ patients were all male Caucasians, whereas the other patient groups were distributed among different ethnicities. No women were found to be Ab+T+, whereas the other groups were closely split between men and women. The only significant difference (P < 0.05) in diabetes duration among the groups was between the Ab−T+ group (2.7 ± 1.6 years) compared to the Ab+T− (1.0 ± 0.7 years). Therefore, we corrected the C-peptide data for disease duration along with BMI and insulin resistance. We screened patients two to three times and were able to classify 75% (27 of 36) of the patients using two blood draws. Only nine patients required a third blood draw for classification. Of the patients requiring a third blood draw, six of nine demonstrated fluctuating autoantibody responses, whereas three patients who were initially negative for T cells demonstrated T-cell responses to islet antigens during the second and third blood draws. Low-titer ICA (10 JDF units) and IA-2 were the autoantibodies demonstrated to fluctuate. The patient initially identified as Ab−T− who became Ab+T+ was observed to be positive for IA-2 at the second blood draw and GAD at the third blood draw. After screening, 10 of the patients were determined to be negative for both islet autoantibodies and islet reactive T cells (Ab−T−), 15 were Ab−T+, 7 were Ab+T+, and 4 were Ab−T+.
Confirmatory autoantibody responses
All Ab−T− and Ab−T+ patients were confirmed to be autoantibody-negative for ICA, IAA, GAD, IA-2, and ZnT8A. The Ab−T+ patients were limited to positivity for either ICA alone (one patient) or IA-2 alone (two patients) or IA-2 with ICA (one patient). Three of the Ab+T+ patients were positive for both GADA and ICA, two patients were positive for ICA alone, one patient was positive for GAD alone, and one patient was positive for IA-2 alone. Confirmatory autoantibody results were consistent with screening results for the samples tested.
T-cell reactivity to islet proteins
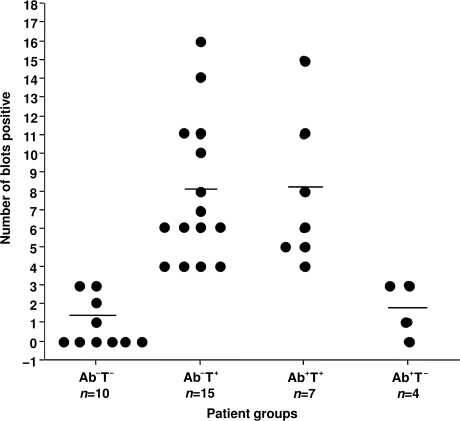
The number of blots stimulatory to T cells from the patients in each of the four groups is shown in Fig. 1. There was no significant difference in the number of blots stimulatory to T cells between the Ab−T+ and Ab+T+ groups. T-cell responses to islet proteins from a subset of patients in each group were confirmed by Dr. Michael Dosch's laboratory (data not shown).
Figure 1.
Number of blot sections stimulatory to T cells responding for each of the patient categories. The number of positive blot sections are demonstrated on the y-axis. Patient groups are shown on the x-axis. A positive response to 4–18 blot sections is similar to responses of type 1 diabetic patients (9).
β-cell function
There were no significant differences among the patient groups in BMI, A1C, or age of onset of diabetes (data not shown). Mean data for C-peptide responses (fasting, glucagon-stimulated, and C-peptide difference) were adjusted for BMI, HOMA-IR, and duration of diabetes. Fasting C-peptide was not calculated for the data adjusted for HOMA-IR because the outcomes of fasting C-peptide and HOMA-IR are going to be highly correlated as both calculations reflect insulin resistance. Results are shown in Table 1. For fasting C-peptide, both the Ab+T+ and Ab−T+ patients demonstrated a significantly lower response (P < 0.02) compared to the Ab+T− patients when the data were adjusted for BMI and diabetes duration. The Ab+T+ patients demonstrated a significantly lower fasting C-peptide than the Ab−T− patients when the data were adjusted for disease duration but not BMI. For glucagon-stimulated C-peptide, the Ab+T+ and Ab−T+ groups demonstrated significantly lower responses (P < 0.02) compared to both the Ab−T− and Ab+T− groups when the data was adjusted for all three variables. There were no differences observed in fasting C-peptide or stimulated C-peptide between the T− (Ab−T− and Ab+T−) or T+ (Ab−T+ and Ab+T+) groups.
Table 1.
Mean C-peptide data for four subgroups of phenotypic type 2 diabetic patients adjusted for BMI, HOMA-IR, and disease duration
| Data adjustment | Patient groups |
|||
|---|---|---|---|---|
| Ab−T− | Ab+T− | Ab−T+ | Ab+T+ | |
| n | 10 | 4 | 15 | 7 |
| BMI | ||||
| Fasting C-peptide | 3.9 + 1.45 | 5.8 + 1.38 | 3.1 + 1.39† | 2.52 + 1.4† |
| Glucagon-stimulated C-peptide | 7.25 + 2.28 | 10.7 + 2.14 | 5.61 + 2.13*† | 4.06 + 2.17*† |
| Change in C-peptide | 3.34 + 1.3 | 4.91 + 1.24 | 2.51 + 1.24† | 1.55 + 1.27*† |
| HOMA-IR | ||||
| Fasting C-peptide | ND | ND | ND | ND |
| Glucagon-stimulated C-peptide | 6.71 + 1.93 | 9.99 + 1.80 | 5.98 + 1.86*† | 4.46 + 1.83*† |
| Change in C-peptide | 3.27 + 1.33† | 4.6 + 1.22 | 2.61 + 1.24† | 1.6 + 1.24*† |
| Diabetes duration | ||||
| Fasting C-peptide | 4.01 + 1.39 | 5.5 + 1.46 | 3.2 + 1.43† | 2.35 + 1.40*† |
| Glucagon-stimulated C-peptide | 7.68 + 2.21 | 10.3 + 2.32 | 5.61 + 2.29*† | 3.7 + 2.25*† |
| Change in C-peptide | 3.6 + 1.3 | 4.72 + 1.36 | 2.48 + 1.36*† | 1.35 + 1.30*† |
Data are means ± SD. ND, not determined.
*P < 0.02 compared with Ab−T−.
†P < 0.02 compared with Ab+T−.
HLA genotyping
The DQB1 and DRB1 genotypes of the patients were categorized as either “risk-associated” if the HLA genotypes were commonly associated with development of type 1 diabetes (0201/0301, 0302/04, or 0502/1601), “protective” if the HLA genotypes were commonly associated with protection from type 1 diabetes (0602/1501 or 0303/0701), or “other” (0501/X, 503/1401, or 0301/0401). There were no significant differences in the presence of HLA genotypes among the groups of patients (data not shown).
CONCLUSIONS
In 2006–2007, our group was investigating the relevance of screening for autoantibodies versus islet reactive T cells to identify type 2 diabetic patients with varying degrees of β-cell function. We observed the potential existence of a group of phenotypic type 2 diabetic patients negative for autoantibodies but positive for islet reactive T cells (12). At that time, it was decided that a study to investigate the potential existence of this new subgroup of type 2 diabetic patients (Ab−T+) along with their demographic and β-cell functional status was needed. Thus, the research presented in this report was initiated. We recruited other laboratories recognized for their expertise in assaying either autoantibodies or T cells in diabetic patients for confirmation of both our positive and negative results. We observed that 11 of 36 (31%) of the type 2 diabetic patients were Ab+ with only 4 of 11 (36%) of the Ab+ patients being positive for GADA. If we had defined autoreactivity in this study as GADA+ alone, we would have missed 22 of 26 (85%) of the autoimmune patients because 0 of 4 Ab−T+ patients were GADA+ and only 4 of 7 (57%) Ab+T+ patients were GADA+. Our data demonstrate that autoantibody reactivity may also be more prevalent in type 2 diabetic patients than reported previously if multiple autoantibodies are analyzed. One important issue to keep in mind is that the islet autoantibodies we screened for in our study were autoantibodies that have been identified as being important in type 1 diabetic patients. The autoantibody-negative T-cell positive patients may have autoantibodies that are as yet undefined, which could raise the percentage of type 2 diabetic patients exhibiting islet autoimmunity even further. Therefore, based on our results, we propose that there exists a subgroup of autoantibody-negative islet reactive T-cell–positive adult phenotypic type 2 diabetic patients (Ab−T+) who have diminished β-cell function similar to that in Ab+T+ phenotypic type 2 diabetic patients.
T-cell assays assessing autoimmune T-cell responses have been difficult to perform and validate for use in diabetes research. We were in a unique position to use a T-cell assay (cellular immunoblotting) that has been validated and confirmed to be able to detect islet reactive T cells in type 1 diabetic patients (9,10) with a sensitivity of 94 and 74% and a specificity of 83 and 88% in two validation studies. In the validation study sponsored by Trial Net (10), the sensitivity of any single autoantibody assay ranged from 59 to 67%, whereas in the ITN-sponsored validation study (9), the adjusted sensitivity for anti-GADA was 84% and for ICA-512 was 58%. Therefore, the sensitivity of the cellular immunoblotting assay was comparable to the “gold standard” for detecting autoimmunity in diabetic patients, namely autoantibody assays. We used cellular immunoblotting to detect the presence of islet reactive T cells in adult phenotypic type 2 diabetic patients. Major drawbacks to using the cellular immunoblotting assay for screening patients are the need for a large amount of blood (30 ml) to perform the assay and a source of human islets. We are, however, working to optimize and scale down cellular immunoblotting (23), so that this technique may be more applicable to future clinical trials. The existence of the Ab−T+ type 2 diabetic patients is extremely important because many larger studies, such as the UK Prospective Diabetes Study (UKPDS), define autoimmunity in type 2 diabetic subjects based solely on autoantibody positivity (24). Therefore, it is important that future studies of type 2 diabetes consider redefining islet autoimmunity to include islet reactive T cells, thus potentially increasing the percentage of type 2 diabetic patients exhibiting islet autoimmunity.
Autoantibody negativity in type 1 diabetes has been linked to increasing age at diagnosis of patients as well as increasing time from diagnosis (7). We observed that the autoantibody responses seem to fluctuate more commonly than the islet reactive T-cell responses. We also observed the development of islet autoimmunity (development of autoantibodies and/or T cells) in a number of patients screened two or three times within a short period of time (3 months). This occurrence may also add to the misclassification of patients in studies if they are initiated using only one screening result to classify patients.
Comparing the C-peptide responses among the patient groups, we observed that fasting C-peptide was only different between the Ab+T+ and Ab−T− groups if the data were adjusted for diabetes duration but not BMI. In contrast, both the Ab+T+ and Ab−T+ groups had a significantly lower fasting C-peptide response compared with that for the Ab−T+ group. For stimulated C-peptide, a significantly lower response was observed in both T+ groups compared with the T− groups when adjusted for BMI, insulin resistance, and diabetes duration. These data suggest there are most likely differences among the different groups, which could have an influence on outcomes of clinical trials if the patients are misclassified. Unfortunately, this study was initiated to screen only patients with newly diagnosed type 2 diabetes to determine whether the Ab−T+ patient population existed as a substantial subpopulation of type 2 diabetic patients. The lack of longitudinal follow-up in this study precluded us from determining whether the presence of the islet reactive T cells correlated with a decline in β-cell function or whether the patients who were singularly positive for either autoantibodies or T cells would become positive for both with long-term follow-up. Although the sample size in our study was sufficient to identify the four subgroups of type 2 diabetic patients, in future studies to determine the prevalence of these subgroups a much larger patient sample should be used. Based on results published by Greenbaum et al. (25), the mixed-meal tolerance test was demonstrated to be superior to the glucagon stimulation test and thus should replace the glucagon stimulation test for future studies. However, the study outlined in this report was underway before the results of the comparison of mixed-meal tolerance test and glucagon-stimulation tests were available (25).
As mentioned earlier, the prevalence of autoimmunity as demonstrated by testing for multiple autoantibodies and islet reactive T cells among adult phenotypic type 2 diabetic patients is unknown at this time. Further studies investigating the prevalence of autoimmunity in type 2 diabetic patients are needed. Our data suggest that islet autoimmunity may be more common than previously thought and may be an important contributor to the progressive decline in β-cell function observed in phenotypic type 2 diabetic patients. This is the first study identifying a group of phenotypic type 2 diabetic patients demonstrating autoimmunity to islet proteins without the presence of islet autoantibodies.
Acknowledgments
This work was supported in part by the Medical Research Service of the Department of Veterans Affairs and the National Institutes of Health (grants P01-DK-053004 and P30-DK-017047).
This work was also supported in part by GlaxoSmithKline. No other potential conflicts of interest relevant to this article were reported.
B.M.B.-W., J.L.R., A.G., H.I., and J.P.P researched data, contributed to discussion, wrote the manuscript, and revised/edited the manuscript.
We extend our sincere thanks to Pam Mansfield, RN (University of Washington, Seattle, WA) for her assistance in recruitment and scheduling of the study subjects and to Drs. Yu and Eisenbarth (Barbara Davis Center, Denver, CO) and Dr. Winter (University of Florida, Gainesville, FL) for confirmatory autoantibody results. We also thank Dr. Trucco (University of Pittsburgh, Pittsburgh, PA) for performing HLA typing on our patients, Dr. Dosch's laboratory (Hospital for Sick Children, Toronto, Canada) for performing the T-cell proliferation assay, and Dr. Ed Boyko (University of Washington, Puget Sound Health Care System, Seattle, WA) and the Clinical Research Core of the Diabetes Endocrinology research group at the University of Washington for helping with statistical analysis.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Lampeter EF, Homberg M, Quabeck K, Schaefer UW, Wernet P, Bertrams J, Grosse-Wilde H, Gries FA, Kolb H: Transfer of insulin-dependent diabetes between HLA-identical siblings by bone marrow transplantation. Lancet 1993;341:1243–1244 [DOI] [PubMed] [Google Scholar]
- 2.Ehses JA, Ellingsgaard H, Böni-Schnetzler M, Donath MY: Pancreatic islet inflammation in type 2 diabetes: from α and β cell compensation to dysfunction. Arch Physiol Biochem 2009;115:240–147 [DOI] [PubMed] [Google Scholar]
- 3.Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA: Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care 2008;31:S161–S164 [DOI] [PubMed] [Google Scholar]
- 4.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG: Islet-associated macrophages in type 2 diabetes. Diabetologia 2009;52:1686–1688 [DOI] [PubMed] [Google Scholar]
- 5.Juneja R, Hirsch IB, Naik RG, Brooks-Worrell BM, Greenbaum CJ, Palmer JP: Islet cell antibodies and glutamic acid decarboxylase antibodies, but not the clinical phenotype, help to identify type 1(1/2) diabetes in patients presenting with type 2 diabetes. Metabolism 2001;50:1008–1013 [DOI] [PubMed] [Google Scholar]
- 6.Lohmann T, Sessler J, Verlohren HJ, Schröder S, Rötger J, Dãhn K, Morgenthaler N, Scherbaum WA: Distinct genetic and immunological features in patients with onset of IDDM before and after age 40. Diabetes Care 1997;20:524–529 [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Miao D, Babu S, Yu J, Barker J, Klingensmith G, Rewers M, Eisenbarth GS, Yu L: Prevalence of autoantibody-negative diabetes is not rare at all ages and increases with older age and obesity. J Clin Endocrinol Metab 2007;92:88–92 [DOI] [PubMed] [Google Scholar]
- 8.Nagata M, Moriyama H, Kotani R, Yasuda H, Kishi M, Kurohara M, Hara K, Yokono K: Immunological aspects of ‘fulminant type 1 diabetes.’ Diabetes Res Clin Pract 2007(Suppl.);77:S99–S103 [DOI] [PubMed] [Google Scholar]
- 9.Seyfert-Margolis V, Gisler TD, Asare AL, Wang RS, Dosch HM, Brooks-Worrell B, Eisenbarth GS, Palmer JP, Greenbaum CJ, Gitelman SE, Nepom GT, Bluestone JA, Herold KC: Analysis of T-cell assays to measure autoimmune responses in subjects with type 1 diabetes: results of a blinded controlled study. Diabetes 2006;55:2588–2594 [DOI] [PubMed] [Google Scholar]
- 10.Herold KC, Brooks-Worrell B, Palmer J, Dosch HM, Peakman M, Gottlieb P, Reijonen H, Arif S, Spain LM, Thompson C, Lachin JMType 1 Diabetes TrailNet Research Group Validity and reproducibility of measurement of islet autoreactivity by T-cell assays in subjects with early type 1 diabetes. Diabetes 2009;58:2588–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks-Worrell BM, Starkebaum GA, Greenbaum C, Palmer JP: Peripheral blood mononuclear cells of insulin-dependent diabetic patients respond to multiple islet cell proteins. J Immunol 1996;157:5668–5674 [PubMed] [Google Scholar]
- 12.Goel A, Chiu H, Felton J, Palmer JP, Brooks-Worrell B: T cell responses to islet antigens improves detection of autoimmune diabetes and identifies patients with more severe β-cell lesions in phenotypic type 2 diabetes. Diabetes 2007;56:2110–2115 [DOI] [PubMed] [Google Scholar]
- 13.Brooks-Worrell BM, Juneja R, Minokadeh A, Greenbaum CJ, Palmer JP: Cellular immune responses to human islet proteins in antibody-positive type 2 diabetes patients. Diabetes 1999;48:983–988 [DOI] [PubMed] [Google Scholar]
- 14.Falorni A, Ortqvist E, Persson B, Lernmark A: Radioimmunoassays for glutamic acid decarboxylase (GAD65) and GAD65 autoantibodies using 35S or 3H recombinant human ligands. J Immunol Methods 1995;196:89–99 [DOI] [PubMed] [Google Scholar]
- 15.Törn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ: Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia 2008;51:846–852 [DOI] [PubMed] [Google Scholar]
- 16.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC., Hutton JC: The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 2007;104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowe RE, Leech NJ, Nepom GT, McCulloch DK: High genetic risk for IDDM in the Pacific northwest: first report from the Washington State Diabetes Prediction Study. Diabetes 1994;43:87–94 [DOI] [PubMed] [Google Scholar]
- 18.Schatz D, Krischer J, Horne G, Riley W, Spillar R, Silverstein J, Winter W, Muir A, Derovanesian D, Shah S., Derovanesian D, Shah S, Malone J, MaClaren N: Islet cell antibodies predict insulin-dependent diabetes in United States school age children as powerfully as in unaffected relatives. J Clin Invest 1994;93:2403–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, Chase P, Klingensmith G, Erlich H, Norris J, Eisenbarth GS: Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 1996;81:4264–4267 [DOI] [PubMed] [Google Scholar]
- 20.Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth GS: Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci USA 2000;97:1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ringquist S, Nichol L, Trucco M: Transplantation genetics. In Principles and Practice of Medical Genetics. 5th ed Elsevier, Philadelphia PA, 2007, p. 983–1010 [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 23.Brooks-Worrell B, Warsen A, Palmer JP: Improved T cell assay for identification of type 1 diabetes patients. J Immunol Methods 2009;344:79–83 [DOI] [PubMed] [Google Scholar]
- 24.Desai M, Clark A: Autoimmune diabetes in adults: lessons from the UKPDS. Diabet Med 2008;25:30–34 [DOI] [PubMed] [Google Scholar]
- 25.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, Battelino T, Haastert B, Ludvigsson J, Pozzilli P, Lachin JM, Kolb HType 1 Diabetes Trial Net Research Group, European C-Peptide Trial Study Group Mixed-mean tolerance test versus glucagons stimulation test for the assessment of β-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;10:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]