Abstract
Background
Imaging studies of pain processing in primary psychiatric disorders are just emerging. This study explored the neural correlates of stress-induced analgesia in individuals with posttraumatic stress disorder (PTSD). It combined functional magnetic resonance imaging (fMRI) and the traumatic script-driven imagery symptom provocation paradigm to examine the effects of trauma-related cues on pain perception in individuals with PTSD.
Methods
The study included 17 patients with PTSD and 26 healthy, trauma-exposed controls. Participants received warm (nonpainful) or hot (painful) thermal stimuli after listening to a neutral or a traumatic script while they were undergoing an fMRI scan at a 4.0 T field strength.
Results
Between-group analyses revealed that after exposure to the traumatic scripts, the blood oxygen level–dependent (BOLD) signal during pain perception was greater in the PTSD group than the control group in the head of the caudate. In the PTSD group, strong positive correlations resulted between BOLD signal and symptom severity in a number of brain regions previously implicated in stress-induced analgesia, such as the thalamus and the head of the caudate nucleus. Trait dissociation as measured by the Dissociative Experiences Scale correlated negatively with the right amygdala and the left putamen.
Limitations
This study included heterogeneous traumatic experiences, a different proportion of military trauma in the PTSD versus the control group and medicated patients with PTSD.
Conclusion
These data indicate that in patients with PTSD trauma recall will lead in a state-dependent manner to greater activation in brain regions implicated in stress-induced analgesia. Correlational analyses lend support to cortical hyperinhibition of the amygdala as a function of dissociation.
Introduction
Despite evidence that pain preferentially recruits affective pain systems in patients with chronic pain1–3 and strong epidemiological links between disorders of mood, anxiety and chronic pain,4 imaging studies of pain processing in primary psychiatric disorders are just emerging.5–7 Posttraumatic stress disorder (PTSD) is associated with significantly elevated prevalence of chronic pain,5 ranging from 25%–80% in veterans8,9 and up to 50% in motor-vehicle collision survivors.10 Conversely, rates of PTSD in patients attending tertiary pain clinics range from 10% to 33%.2 Comparing patients with combat-related PTSD to patients with other anxiety disorders and healthy controls, Defrin and colleagues11 report higher rates of chronic pain and more intense chronic pain in the PTSD group than the anxiety and the healthy control groups. In the PTSD group, the chronic pain started immediately to a few months after exposure to the traumatic incident, whereas no specific onset could be determined in the anxiety group. The most frequent pain-aggravating factors were psychological distress (80%) and tension (32%). Whereas PTSD severity correlated significantly with chronic pain severity, the participants with PTSD exhibited significantly higher pain thresholds.
Alterations in pain threshold in PTSD
The finding of increased thresholds for pain has been corroborated in samples of patients with PTSD but without comorbid chronic pain. Significantly higher pain thresholds were reported by veterans with PTSD than combat-exposed veterans without PTSD at baseline9 and after exposure to a trauma reminder.12,13 However, this difference could not be replicated in the study by Kraus and colleagues,14 who established elevated thresholds in combat-exposed groups both with and without PTSD compared with healthy controls without combat experience but reported significantly lower pain ratings in the PTSD sample compared with the healthy control groups when using long-lasting (30 s) pain stimuli. Interestingly, pain thresholds were also comparable for patients with borderline personality disorder with and without comorbid PTSD,15 a disorder that has been linked to prior trauma experience and is characterized by frequent dissociative coping.
In the only functional magnetic resonance imaging (fMRI) investigation of pain processing in PTSD to date, the PTSD sample exhibited increased activation in the putamen and bilateral anterior insula and decreased activation in the right amygdala compared with combat-exposed veterans without PTSD.9 Interestingly, the PTSD group exhibited significantly higher state dissociation and aversive inner tension scores before the scan than the control group, but no correlations with blood oxygen level–dependent (BOLD) signal were reported.
Neither the neurobiological mechanisms underlying the alterations in pain perception among patients with PTSD nor the comorbidity of PTSD with chronic pain are well understood. Sharp and Harvey16 presented a model of mutual maintenance in which pain serves as a reminder of the traumatic event and the resulting arousal exacerbates the pain. Whereas the aggravating effects of psychological distress reported by Defrin and colleagues11 support this hypothesis, the higher pain thresholds commonly reported in PTSD samples cannot be directly explained by this model. Therefore stress-induced analgesia has been put forward as a possible mediator variable.9
Stress-induced analgesia
Experimental evidence suggests that whereas induced negative emotions can exacerbate pain perception,17,18 acute stress can induce analgesia.19–21 Stress-induced analgesia is a pain suppression response that occurs during or after exposure to a stressful or fearful stimulus. As the release of endogenous opiates in the thalamus, extended amygdala, insula, medial prefrontal cortex, anterior cingulate cortex and dorsal striatum diminishes pain sensation,22 opiates are thought to be key agents in stress-induced analgesia.23,24 In patients with PTSD, stress-induced analgesia is a key component of the broader phenomenon of dissociation, which also entails depersonalization and derealization.25 Dissociation may reflect a compensatory response to greater distress involving a complex corticolimbic network, possibly mediated by alterations in thalamic activation. During dissociative states, the connectivity between subcortical and cortical structures seems to be altered, with greater covariation between the thalamus, right insula and middle frontal regions.26 A recent study revealed a direct link between script-induced dissociative states and increased insula activations in conjunction with reduced pain sensitivity.27
Taken together, these results lead to the hypothesis that elevated pain thresholds reported in studies of people with PTSD with and without comorbid chronic pain could result from stress-induced analgesia in patients with elevated levels of dissociation.
In the present study, we examined the neural circuitry underlying pain processing in patients with PTSD after traumatic symptom provocation using fMRI at a 4.0 T field strength. Alterations in brain activation were measured during application of warm (nonpainful) versus hot (painful) stimuli. To test the hypothesis that trauma recall does not lead to the expected exacerbation in pain perception in patients with PTSD, we compared activations after neutral versus traumatic memories both within and between study groups. We expected that the traumatic script would exacerbate pain perception in trauma-exposed controls owing to induced negative emotions, but that for the patients with PTSD, the traumatic script would induce stress-induced analgesia. We thus hypothesized that the controls would report increased pain after the trauma script, whereas the patients with PTSD would report decreased pain. In addition, we expected trauma recall in participants with PTSD to lead to differential activation of brain regions involved in stress-induced analgesia and dissociation (dorsal striatum, thalamus, insula, anterior and midcingulate cortices, and extended amygdala) in a state-dependent manner. More precisely, we hypothesized that dissociation and PTSD symptom severity would be closely correlated to activation in these areas after trauma recall in the patients with PTSD.
Methods
Participants
The study involved patients with PTSD and healthy controls who had experienced a criterion A traumatic event but never developed PTSD. We included participants who were right-handed, aged 20–50 years and matched for age and sex. Participants gave written informed consent for inclusion and the study was approved by the Office of Research Ethics at the University of Western Ontario.
All participants met criterion A for PTSD, but it never developed in the control group. Individuals were included in the PTSD group if they met PTSD DSM-IV criteria, as assessed by the Structured Clinical Interview for DSM-IV (SCID28) and the Clinician Administered PTSD Scale (CAPS29). The PTSD group exhibited current DSM-IV comorbid diagnoses (major depression, dysthymia, panic disorder, anorexia, generalized anxiety disorder and social phobia). We also rated participants based on the Beck Depression Inventory-II30 and the Dissociative Experiences Scale (DES31). To examine dissociative symptoms during the scanning session, we used the Clinician-Administered Dissociative State Scale (CADSS32), a measure of state dissociation. The CADSS was scored as symptoms being present or absent. All healthy participants and most of the patients with PTSD were medication-free for at least 2 weeks before scanning, and none of the participants had received antipsychotic agents before the drug washout. We excluded participants if they met the criteria for pain disorder, had any history (current or within the last 6 mo) of drug or alcohol abuse, history of psychotic disorders and bipolar disorder, history of head injury (unconsciousness for any length of time) or any other neurologic disorder or presence of metallic or electronic implants that would preclude fMRI. Specific clinical characteristics of participants, including the nature of the trauma experienced, DSM-IV comorbid diagnoses and prescribed medications, are reported in Results.
Pain thresholding
We carried out thresholding for individual pain temperatures in the scanning room before scanning according to standard procedures.33 The thermal stimulation was applied to the area superior to the lateral malleolus (above the inner ankle) and was produced by the Neurosensory Analyzer TSA-II (Medoc Ltd. Advanced Medical Systems). The Neurosensory Analyzer TSA-II is a computer-controlled device that produces a rapid reproducible onset and offset of nontraumatic, individually titrated thermal nociceptive stimulation, a method that is well established in the pain literature.34 Heat was applied beginning at 38ºC for 12 seconds. One full minute was allowed to pass between stimulations, and the participant verbally rated the stimulus for “intensity of pain” and “unpleasantness” on a scale of 0 (no pain) to 100 (intense pain) according to standard methods.35 The stimulations increased by 1ºC until the participant deemed that the temperature he or she was receiving “painful, but tolerable.” A nonpainful warm temperature (1° higher than the initial temperature the participant was able to detect on his or her skin) was also identified during thresholding. The 2 stimuli were again tested on the participant, but this time for 25 seconds (the length of stimuli used in the imaging paradigm), to make sure that the participant still identified them as warm (non-painful) and hot (painful), but tolerable.
Functional imaging paradigm
The functional paradigm began with 60 seconds of baseline, when participants were instructed to “focus on your breathing,” followed by a 30-second prerecorded script (neutral or trauma). Participants were asked to focus on the script and imagine all the feelings and sensations associated with the memory while listening to the script and for 30 seconds after the script ended. A bell-tone indicated the end of this section, and the participant was instructed to “focus on the stimulation on your leg.” After a 25-second stimulation (warm or hot), a researcher asked the participant to rate pain intensity and unpleasantness (each on 0–100 scales); 120 seconds passed between stimulation and the beginning of the next script.
Participants heard 6 neutral scripts followed by 6 traumatic scripts (played in blocks of 3) prepared according to reported methods.36 The neutral scripts always preceded the trauma scripts, because anxiety elicited by trauma cues has been seen to persist into subsequent neutral conditions.37 This type of paradigm has been well established in the PTSD literature.26,36,38 For each script type, there were 3 warm (non-painful) and 3 hot (painful) stimulations. The warm and painful stimuli were presented in a pseudorandomized order, counterbalanced across groups, because anticipation can affect pain ratings and related activations.39 The participant was then removed from the scanner, completed postscan questionnaires and was debriefed.
Imaging protocol
We scanned participants on a 4.0 T Varian/Siemens UNITYINOVA whole-body imaging system at the Robarts Research Institute. We used a hybrid birdcage radiofrequency coil40 placed around the participant’s head for magnetic resonance signal transmission and reception, packed with foam to reduce head motion. We performed manual and automated shimming procedures using first- and second-order shims to optimize the magnetic field homogeneity over the imaging volume of interest. Using sagittal localizing images, we prescribed 21 contiguous, transversely orientated, 5-mm functional slices and acquired BOLD functional brain volumes with a navigator echo corrected, interleaved, multishot T2*-weighted pulse sequence using an outwardly spiralling k-space trajectory (64 × 64 matrix size, volume acquisition time 2.5 s, echo time [TE] 15 ms, flip angle 30º, field of view [FOV] 22.0 cm). We acquired high-resolution T1-weighted anatomic images using a 3-dimensional spiral sequence using the same FOV and orientation as the functional images (256 × 256 matrix size, TE 3.0 ms, flip angle 20º, repetition time [TR] 50 ms, inversion time [TI] 1.3 s). This acquisition produced 64 contiguous 1.25 mm–thick structural images with excellent grey/white matter contrast for the purpose of BOLD activation registration.
Image processing
We performed image processing and statistical analyses with Statistical Parametric Mapping (SPM 2; Wellcome Department of Neurology, London, UK, www.fil.ion.ucl.ac.uk/spm). For each series, we aligned all volumes to the first volume of series to reduce the effects of head motion and determined normalization parameters from the mean functional image. We normalized the realigned images to an echoplanar imaging template supplied by SPM 2 and smoothed the data with an 8-mm full-width at half-maximum isotropic Gaussian kernel.
Statistical analysis
Group statistics were calculated as Pearson correlations and 2-tailed 2-sample t tests. In case of unequal variances, we used Satterthwaite’s approximation to estimate the degrees of freedom. In all statistics, we considered p < 0.05 to be significant. We carried out our statistical analyses using SPSS version 15.0.
We employed a 2-stage random-effects analysis for the neuroimaging data. At the first level, we analyzed each participant’s functional data separately by modelling the evoked BOLD responses for each task epoch of interest as basis functions (i.e., a boxcar function convolved with a hemodynamic response function). For each participant, 2 contrasts were created: painful-warm after the neutral script and painful-warm after the trauma script. These contrasts were entered into a second-level analysis to make inferences about regionally specific correlates. Analyses examined activations related to the hot and warm stimuli, contrasting the thermal stimulation minus baseline for each, as generally reported for pain neuroimaging studies.41 We examined correlations between participants’ ratings on the DES, CAPS and CADSS and the BOLD response to quantify the influence of PTSD symptom severity as well as state and trait dissociation. Results were converted to Talairach coordinates42 using the program Talairach Client (www.talairach.org).
We set the threshold for statistical analyses at a cluster size of κ > 5 and an α-level of p = 0.001 with a family-wise error correction using 10-mm spheres around regions of interest (ROI; i.e., dorsal striatum, thalamus, insula, anterior and midcingulate and extended amygdala), which were identified on the basis of previous studies examining stress-induced analgesia and pain processing in healthy participants and patients with PTSD.7,14,21,43–45 All analyses were covaried for use of medication. We did not include BDI-II scores as covariates owing to their high correlation with the CAPS scores (r = 0.76, p < 0.001).
Results
Participants
The study included 17 patients with PTSD (assault n = 5, childhood abuse n = 3, military trauma n = 2, workplace trauma n = 2, motor vehicle collision n = 1, other n = 4) and 26 healthy controls who had experienced a criterion A traumatic event (military trauma n = 10, motor vehicle collision n = 8, assault n = 2, other n = 6) but never had PTSD. The participants were matched for age (mean age 36.8, standard deviation [SD] 8.2 yr in the control group v. 36.7, SD 9.7 yr in the PTSD group; t40 = 0.043, p = 0.97) and sex (female controls, n = 11; PTSD n = 9; Fisher exact test p = 0.54). Participants in the PTSD group exhibited the following current DSM-IV comorbid diagnoses: major depression (n = 5), dysthymia (n = 1), panic disorder (n = 2), anorexia (n = 1), generalized anxiety disorder (n = 1) and social phobia (n = 1). The 3 medicated patients with PTSD received fluoxetine (n = 1), quetiapine and bupropion (n = 1), and citalopram and olanzapine (n = 1).
Clinical rating scales and pain ratings
Significant group differences were established for all clinical rating scales (Table 1), including trait dissociation and state dissociation after exposure to the trauma script. There was no significant difference between hot (painful) and warm (nonpainful) temperatures chosen during thresholding by patients with PTSD and controls. After the traumatic script as compared with the neutral script, the painful stimulus was rated as significantly more painful (t25 = 2.502, p = 0.019) and unpleasant (t25 = 3.233, p = 0.003) in the control group (Table 1). We noted a tendency for lower pain ratings after the trauma script as compared with the neutral script in the PTSD group, although it failed to reach statistical significance. In a direct group comparison, patients with PTSD reported significantly lower pain intensity and unpleasantness after the trauma script than the control group.
Table 1.
Pain ratings, clinical rating scales and mean temperatures* of patients with PTSD and controls
| Group; mean (SD) |
Group comparison |
|||
|---|---|---|---|---|
| Variable | PTSD, n = 17 | Control, n = 26 | t value | p value |
| Clinician Administered PTSD Scale29 score | 70.1 (19.4) | 2.8 (6.2) | t18.4 = 13.7 | < 0.001‡ |
| Beck Depression Inventory-II30 score | 10.6 (2.6) | 2.0 (0.4) | t16.8 = 8.4 | < 0.001‡ |
| Dissociative Experiences Scale31 score | 9.3 (8.0) | 2.1 (1.8) | t17.3 = 3.7 | < 0.001‡ |
| Clinician-Administered Dissociative State Scale32 score after scan† | 4.0 (5.1) | 0.31 (0.62) | U = 77.0 | < 0.001‡ |
| Hot (painful) stimulus, °C | 45.8 (1.5) | 46.2 (1.6) | t39 = 0.759 | 0.45 |
| Warm (nonpainful) stimulus, °C | 42.2 (1.8) | 42.1 (2.2) | t39 = 0.241 | 0.81 |
| Intensity, neutral script, hot stimulus | 73.7 (21.5) | 78.9 (15.9) | t40 = 0.912 | 0.37 |
| Intensity, neutral script, warm stimulus | 14.4 (18.9) | 24.5 (20.3) | t40 = 1.600 | 0.12 |
| Intensity, trauma script, hot stimulus | 70.8 (24.1) | 83.8 (15.7) | t40 = 2.122 | 0.040§ |
| Intensity, trauma script, warm stimulus | 8.9 (14.9) | 17.3 (14.9) | t40 = 1.767 | 0.08 |
| Unpleasantness, neutral script, hot stimulus | 61.8 (25.7) | 73.0 (16.9) | t40 = 1.720 | 0.09 |
| Unpleasantness, neutral script, warm stimulus | 9.1 (17.2) | 15.5 (20.1) | t40 = 1.057 | 0.30 |
| Unpleasantness, trauma script, hot stimulus | 62.9 (23.6) | 79.1 (17.4) | t40 = 2.556 | 0.015§ |
| Unpleasantness, trauma script, warm stimulus | 6.7 (15.5) | 11.4 (15.7) | t40 = 0.931 | 0.36 |
PTSD = posttraumatic stress disorder; SD = standard deviation.
Within-group differences are presented in the main text of the article.
The Mann–Whitney U test was used owing to skewed scores.
Significant at p = 0.01 (2-sided).
Significant at p = 0.05 (2-sided).
Neuroimaging statistical parametric mapping analyses
All analyses presented refer to the comparison of hot (painful) stimulation with warm (nonpainful) stimulation and were covaried for use of medication.
Within-group analyses of pain perception after the traumatic script
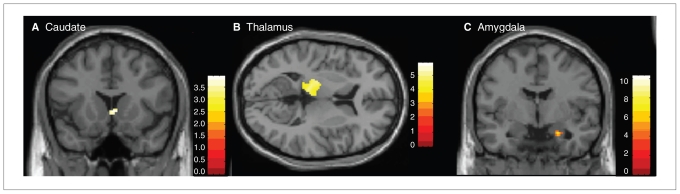
Comparing pain perception after traumatic and neutral scripts, no significant differences in BOLD signal emerged for the control group. The PTSD group exhibited greater activations in the head of the left caudate (Fig. 1 and Table 2).
Fig. 1.
(A) Caudate: between-group results show greater activation in the posttramatic stress disorder (PTSD) group than the control group. (B) Thalamus: positive correlation with the Clinician Administered PTSD Scale29 in the PTSD group. (C) Amygdala: negative correlation with Dissociative Experiences Scale31 in the PTSD group.
Table 2.
Blood oxygen level–dependent activations
| Patient group comparison; MNI coordinate | z score | Cluster size | p value* | Brain region |
|---|---|---|---|---|
| PTSD > controls, trauma > neutral | ||||
| 4, 16, 0 | 3.60 | 40 | 0.008 | Caudate head |
| PTSD, trauma > neutral | ||||
| −8, 22, 0 | 3.47 | 25 | 0.020 | Caudate head |
| −6, 18, 2 | 3.36 | 0.026 | Caudate head | |
MNI = Montreal Neurological Insitute; PTSD = posttraumatic stress disorder.
Family-wise error–corrected.
Between-group analyses of pain perception
No significant group differences emerged after exposure to the neutral script.
Between-group analyses revealed that after exposure to the trauma script compared with the neutral script, activation of the head of the right caudate was significantly greater in the PTSD group than the control group during pain perception (Table 2).
Correlation analyses
The CAPS scores correlated positively with the BOLD signal after the trauma script in a number of areas implicated in stress-induced analgesia, such as the bilateral insulae, left thalamus, left caudate and left putamen. Activity in the right medial frontal gyrus (Brodmann area [BA] 9) was negatively correlated with CAPS scores (Table 3).
Table 3.
Correlations with CAPS scores in the PTSD group
| Correlation; MNI coordinate | z score | Cluster size | p value* | Brain region |
|---|---|---|---|---|
| Positive correlations | ||||
| −34, −20, 12 | 4.41 | 293 | 0.001 | Insula, BA 13 |
| −34, 18, 18 | 3.62 | 29 | 0.014 | Insula, BA 13 |
| 38, −2, 20 | 4.07 | 162 | 0.004 | Insula, BA 13 |
| −4, 14, 4 | 3.25 | 10 | 0.038 | Caudate head |
| −14, 24, −2 | 3.64 | 18 | 0.013 | Caudate head |
| −18, −16, 4 | 4.12 | 295 | 0.003 | Thalamus |
| −26, −20, 8 | 3.82 | 0.008 | Putamen | |
| −18, −24, 10 | 3.70 | 0.011 | Thalamus, pulvinar | |
| −14, −24, 8 | 3.66 | 0.013 | Thalamus, pulvinar | |
| Negative correlations | ||||
| 28, 38, 22 | 3.35 | 15 | 0.029 | Medial frontal gyrus, BA 9 |
BA = Brodmann area; CAPS = Clinician Administered PTSD Scale;29 MNI = Montreal Neurological Institute; PTSD = posttraumatic stress disorder.
Family-wise error–corrected.
The DES scores, a measure of trait dissociation, correlated negatively with the right amygdala, the left putamen, the right anterior cingluate cortex (BA 32) and the left superior frontal gyrus (BA 9, 10) in the PTSD group after the trauma script (Table 4). We detected no positive correlations. The CADSS scores, a measure of state dissociation, did not reveal any significant correlations with the BOLD signal in the ROIs in the PTSD group.
Table 4.
Negative correlations with DES scores in the PTSD group
| MNI coordinate | z score | Cluster size | p value* | Brain region |
|---|---|---|---|---|
| 24, −4, −22 | 4.62 | 33 | 0.001 | Amygdala |
| −26, 12, 0 | 3.54 | 48 | 0.018 | Putamen |
| −26, 10, −6 | 3.52 | 0.018 | Putamen | |
| 20, 46, 6 | 3.92 | 48 | 0.006 | Anterior cingulate, BA 32 |
| −20, 56, 2 | 3.52 | 23 | 0.018 | Superior frontal gyrus, BA 10 |
| −10, 58, 28 | 3.81 | 17 | 0.008 | Superior frontal gyrus, BA 9 |
BA = Brodmann area; DES = Dissociative Experiences Scale;31 MNI = Montreal Neurological Institute; PTSD = posttraumatic stress disorder.
Family-wise error–corrected.
Discussion
To our knowledge, this is the first study that has combined fMRI and the script-driven imagery symptom provocation paradigm to examine the effects of trauma-related cues on pain perception in individuals with PTSD.
Patients with PTSD reported significantly lower pain intensity and unpleasantness after the trauma script than the trauma-exposed control group. No significant group differences in brain activations emerged during pain application after the neutral script, indicating that the group differences observed after the trauma script are not merely baseline differences in pain processing, but are related to the script-induced trauma recall.
Within-group analysis of patients with PTSD and the between-group analysis revealed higher activation of the head of the caudate during pain perception after the trauma script as compared with the neutral script. The caudate is known to be more active after exposure to aversive stimuli46 and has previously been associated with stress-induced analgesia.22
In the PTSD group, strong positive correlations resulted between BOLD signal and symptom severity in a number of brain regions previously implicated in stress-induced analgesia, including the caudate, the insula and the thalamus.22
Trait dissociation was negatively correlated with areas implicated in emotion and pain processing, including the amygdala and the putamen.
Pain ratings
With respect to the stimuli, neither the warm (nonpainful) nor the hot (painful) temperatures chosen during thresholding differed significantly between groups. This replicates findings by Kraus and colleagues,14 who also reported comparable thresholds in trauma-exposed groups with and without PTSD.
Significantly greater pain ratings after the trauma script than the neutral script in the control group indicate that the script-driven imagery paradigm succeeded in exacerbating pain perception in this group. In contrast, the ratings in the PTSD group show a trend toward lower-intensity ratings after the trauma script as compared with the neutral script. Consequently, significantly lower ratings in the PTSD than the control group for pain intensity and unpleasantness were established after the trauma script. A potential design limitation of the present study may be that patients received only 2 specific stimulus levels (warm and hot), so that in their cognitive response set they may in some way appreciate the test as a 2-choice response task. This could therefore restrict the range of pain ratings participants endorsed during the scripts.
Stress-induced analgesia
In a direct group comparison between patients with PTSD and controls, activation of the head of the right caudate was the only significant difference in BOLD signal intensity and showed greater activation in the PTSD group compared with the control group. The caudate receives input from 3 brain regions implicated in emotion processing, namely the ventro-medial prefrontal cortex,47 the insula48 and the amygdala.49 The caudate nucleus is part of the opiate-based pain modulation system, and the opiate-antagonist naloxone reverses induced analgesia in mammals when administered into the an-terodorsal head of the caudate.50 In addition, placebo-induced activation of the dopamine-based pain modulation system in the ventral caudate nucleus has been previously reported.51
Electrical stimulation of the caudate has been shown to provide relief from pain in patients with chronic pain, possibly by inhibiting the activity of the medial thalamus.52 The specific area in the caudate head identified by the between-group contrast (Montreal Neurological Institutes [MNI] coordinates 4, 16, 0) has previously also been implicated in modulation of pain intensity through expectancy53 and at the onset of effortful suppression of thermally induced pain,54 indicating that top–down modulation may be at play. However, the brain regions that have previously been implicated in top–down modulation, namely the anterior cingulate cortex and the medial prefrontal cortex,46,55,56 did not show increased activations.
In addition, the pseudorandomized order of the stimuli rules out expectancy as the main factor. Therefore, these findings may instead indicate that the PTSD group experienced a greater degree of relative, stress-induced analgesia than the control group, mediated by the caudate nucleus.22
We could also establish a dimensional relation between PTSD symptom severity as measured with the CAPS and activity in the head of the left caudate, the left putamen, the left thalamus and bilateral insulae as indicated by significant positive correlations. The activation cluster comprising the thalamus can be interpreted as a key region of opioid pain modulation.22,57,58 The thalamus has been implicated in dissociation and has previously been reported to be more active during pain processing in patients with borderline personality disorder, a patient group equally characterized by higher pain thresholds, levels of relative, stress-induced analgesia and dissociation.6 It has been hypothesized that direct pathways from the thalamus to the amygdala exist that bypass cortical modulation and thus directly mediate effects of emotion on pain perception.59
It is interesting to note that chronic clinical pain conditions are often associated with decreased stimulus-related activity in the thalamus. Thalamic activation is correlated with time since onset of the chronic pain, with hyperperfusion in short-term and hypoperfusion in long-term chronic pain, indicating adaptive changes during the development of chronic pain.60 Future studies examining patients with PTSD with and without chronic pain will be necessary to gain more insight into the role of the thalamus in developing chronic pain as part of a posttraumatic syndrome.
The positive correlation between PTSD symptom severity and bilateral insula activation is in line with the results presented by Ludäscher and colleagues,27 who reported increased insula activations during pain application and reduced pain sensitivity in patients with borderline personality disorder with and without comorbid PTSD. It also replicates findings reported by Geuze and colleagues,7 who identified greater insula activations during pain perception in patients with PTSD as compared with a healthy, trauma-exposed control group. As the insula is implicated in pain modulation and has direct afferents to the caudate, it is likely that this positive correlation indicates its role in relative, stress-induced analgesia.
Interestingly, PTSD symptom severity was also negatively correlated with BOLD signal intensity in the medial frontal gyrus (BA 9), indicating less cortical modulation in more severe PTSD.43 This finding could possibly point to a direct relation between PTSD severity and the development of chronic pain syndrome as it is in line with a reported decrease in connectivity between medial prefrontal cortex structures and basal ganglia in patients with chronic pain.61
Trait dissociation
Interestingly, we found differential results for state and trait dissociation. Whereas the state dissociation measure did not correlate significantly with activations in the ROIs, correlation analyses with the DES, a measure of trait dissociation, revealed a significant negative correlation with the right amygdala after the trauma script. This finding is consistent with amygdala deactivation in response to thermal pain stimuli reported for patients with PTSD as well as with borderline personality disorder who generally show high levels of dissociation.6,7,14 A negative correlation between the analgesic effect and neuronal activity in the amygdala was observed during tests with electroacupuncture.43 As decreased amygdala activity in response to painful stimulation has also been shown to result from hypnosis-induced states of depersonalization, this finding supports the notion that dissociative processing is the underlying reason for this deactivation.62
The putamen, which is thought to play a crucial role in pain processing by representing somatotopic nociceptive information,63 was negatively correlated with trait dissociation. This finding supports the notion that relative, stress-induced analgesia is a key component of dissociation in individuals with PTSD and is readily induced by exposure to traumatic reminders.
Trait dissociation also correlated negatively with activation in the anterior cingulate cortex (BA 32) and the superior frontal gyrus (BA 9,10), which have previously been implicated in the modulation of negative affect and pain.56 These results are more difficult to interpret, as the correlated areas are also known to be activated during dissociation.25 To elucidate the specific role that these brain regions play during pain perception in clinical populations, future studies should combine pain processing tasks with both active emotion regulation tasks and dissociation induction paradigms.
Limitations
There are several limitations to this study. The 2 trauma-exposed groups differed concerning the type of traumatic experiences they had and the percentage of female participants, with a higher percentage of female participants in the PTSD group and a higher rate of military trauma in the control group. As previous studies on sex differences in neural responses to painful stimuli yielded both increased and decreased brain activations in several brain regions, future studies should aim for a balanced sex distribution. Although chronic pain in individuals with PTSD has been shown to have very little overlap with physical injuries resulting from the trauma, future studies should also match participants for trauma type and physical injuries to avoid confounded variables.
Different methodological approaches to establish stimulus temperatures complicate direct comparisons between studies. As in the study by Kraus and colleagues,14 the 2 trauma-exposed groups with and without PTSD did not differ concerning the temperatures considered painful, but reported higher pain thresholds than a healthy control group who had not been exposed to a traumatic event. As we did not investigate such a healthy, nonexposed control group, we cannot ascertain whether our participants exhibited elevated pain thresholds.
Finally, we failed to establish significant correlations between state dissociation and BOLD signal. The differential findings for state and trait dissociation are likely a result of the paradigm employed in this study, which allowed 60 seconds between onset of the script and onset of the heat stimulus. As state dissociation is thought to be a transient phenomenon, the accompanying changes in BOLD activity might not have been detectable during the subsequent processing of the heat stimulus. Future studies should therefore consider using brief trauma cues to elicit dissociative responses instead of scripts to administer the pain stimulus shortly after the induction of dissociation.
Conclusion
To our knowledge, this is the first study that has combined neuroimaging and the script-driven imagery symptom provocation paradigm to examine the effects of trauma-related cues on pain perception in patients with PTSD. The findings point toward altered pain processing after traumatic script-driven imagery in PTSD. Patients with PTSD reported significantly lower pain intensity and unpleasantness after listening to the trauma script than the control group, which was associated with greater activation in the head of the caudate nucleus. The BOLD signal intensity in the caudate nucleus was also positively correlated with PTSD symptom severity. As the caudate nucleus is known to be involved in stress-induced analgesia, the current findings may shed light on the mechanisms underlying stress-induced analgesia and other dissociative states often associated with PTSD. Furthermore, these results may lay the groundwork for future studies examining the mechanisms underlying chronic pain in PTSD.
Acknowledgements
This work was supported by grants from the Department of National Defence, the Canadian Institute of Health Research and Psychiatry Seed Funding from the University of Western Ontario Psychiatry Department. We thank Stephanie Nevill and Suzy Southwell for screening and assessing the participants and Nancy Mazza for preparation of the manuscript.
Footnotes
Competing interests: None declared.
Contributors: Ms. Mickleborough and Drs. Coupland, Williamson, U.F. Lanius, Hegadoren and R.A. Lanius designed the study. Ms. Mickleborough and Drs. Kao, Hegadoren, Stevens and R.A. Lanius acquired the data, which Ms. Mickleborough and Densmore and Drs. Daniels, Coupland, Kao, Schore and R.A. Lanius analyzed. Ms. Mickleborough, Ms. Densmore and Drs. Daniels, Coupland and R.A. Lanius wrote the article, which Ms. Mickleborough and Drs. Coupland, Kao, Williamson, U.F. Lanius, Hegadoren, Schore, Stevens and R.A. Lanius reviewed. All authors approved the article for publication.
References
- 1.Gundel H, Valet M, Sorg C, et al. Altered cerebral response to noxious heat stimulation in patients with somatoform pain disorder. Pain. 2008;137:413–21. doi: 10.1016/j.pain.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Schweinhardt P, Kalk N, Wartolowska K, et al. Investigation into the neural correlates of emotional augmentation of clinical pain. Neuroimage. 2008;40:759–66. doi: 10.1016/j.neuroimage.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–33. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- 5.Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 6.Schmahl C, Bohus M, Esposito F, et al. Neural correlates of antinociception in borderline personality disorder. Arch Gen Psychiatry. 2006;63:659–667. doi: 10.1001/archpsyc.63.6.659. [DOI] [PubMed] [Google Scholar]
- 7.Geuze E, Westenberg HG, Jochims A, et al. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry. 2007;64:76–85. doi: 10.1001/archpsyc.64.1.76. [DOI] [PubMed] [Google Scholar]
- 8.Beckham JC, Crawford AL, Feldman ME, et al. Chronic posttraumatic stress disorder and chronic pain in Vietnam combat veterans. J Psychosom Res. 1997;43:379–89. doi: 10.1016/s0022-3999(97)00129-3. [DOI] [PubMed] [Google Scholar]
- 9.Shipherd JC, Keyes M, Jovanovic T, et al. Veterans seeking treatment for posttraumatic stress disorder: What about comorbid chronic pain? J Rehabil Res Dev. 2007;44:153–66. doi: 10.1682/jrrd.2006.06.0065. [DOI] [PubMed] [Google Scholar]
- 10.Hickling EJ, Blanchard EB, Silverman DJ, et al. Motor vehicle accidents, headaches and post-traumatic stress disorder: assessment findings in a consecutive series. Headache. 1992;32:147–51. doi: 10.1111/j.1526-4610.1992.hed3203147.x. [DOI] [PubMed] [Google Scholar]
- 11.Defrin R, Ginzburg K, Solomon Z, et al. Quantitative testing of pain perception in subjects with PTSD — implications for the mechanisms of the coexistence between PTSD and chronic pain. Pain. 2008;138:450–9. doi: 10.1016/j.pain.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Pitman RK, van der Kolk BA, Orr SP, et al. Naloxone-reversible analgesic response to combat-related stimuli in posttraumatic stress disorder. A pilot study. Arch Gen Psychiatry. 1990;47:541–4. doi: 10.1001/archpsyc.1990.01810180041007. [DOI] [PubMed] [Google Scholar]
- 13.van der Kolk BA, Greenberg MS, Orr SP, et al. Endogenous opiods, stress induced analgesia, and posttraumatic stress disorder. Psychopharmacol Bull. 1989;25:417–21. [PubMed] [Google Scholar]
- 14.Kraus A, Geuze E, Schmahl C, et al. Differentiation of pain ratings in combat-related posttraumatic stress disorder. Pain. 2009;143:179–85. doi: 10.1016/j.pain.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Kraus A, Esposito F, Seifritz E, et al. Amygdala deactivation as a neural correlate of pain processing in patients with borderline personality disorder and co-occurent posttraumatic stress disorder. Biol Psychiatry. 2009;65:819–22. doi: 10.1016/j.biopsych.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: Mutual maintenance? Clin Psychol Rev. 2001;21:857–77. doi: 10.1016/s0272-7358(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 17.Meagher MW, Arnau RC, Rhudy JL. Pain and emotion: effects of affective picture modulation. Psychosom Med. 2001;63:79–90. doi: 10.1097/00006842-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Phillips ML, Gregory LJ, Cullen S, et al. The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain. 2003;126:669–84. doi: 10.1093/brain/awg065. [DOI] [PubMed] [Google Scholar]
- 19.Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84:65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- 20.Ford GK, Finn DP. Clinical correlates of stress-induced analgesia: evidence from pharmacological studies. Pain. 2008;140:3–7. doi: 10.1016/j.pain.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Zubieta JK, Smith YR, Bueller JA, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–5. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 23.Willer JC, Dehen H, Cambier J. Stress-induced analgesia in humans: endogenous opiods and naloxone-reversible depression of pain reflexes. Science. 1981;212:689–91. doi: 10.1126/science.6261330. [DOI] [PubMed] [Google Scholar]
- 24.Wagner KJ, Willoch F, Kochs EF, et al. Dose-dependent regional cerebral blood flow changes during remifentanil infusion in humans: a positron emission tomography study. Anesthesiology. 2001;94:732–9. doi: 10.1097/00000542-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Lanius RA, Vermetten E, Loewenstein RJ, et al. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010;167:640–7. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanius RA, Williamson PC, Bluhm RL, et al. Functional connectivity of dissociative responses in posttraumatic stress disorder: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2005;57:873–84. doi: 10.1016/j.biopsych.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Ludäscher P, Valerius G, Stiglmayr C, et al. Pain sensitivity and neural processing during dissociative states in patients with borderline personality disorder with and without comorbid PTSD: a pilot study. J Psychiatry Neurosci. 2010;35:177–84. doi: 10.1503/jpn.090022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Williams BW, Spitzer RL. Structured clinical interview for DSM-IV Axis II personality disorders. Washington (DC): American Psychiatric Press; 1997. [Google Scholar]
- 29.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 30.Beck AT, Ward C, Mendelson M. Beck depression inventory (BDI) Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis. 1986;174:727–35. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS) J Trauma Stress. 1998;11:125–36. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 33.Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A. 2003b;100:8538–42. doi: 10.1073/pnas.1430684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 35.Koyama Y, Koyama T, Kroncke AP, et al. Effects of stimulus duration on heat induced pain: the relationship between real-time and post-stimulus pain ratings. Pain. 2004;107:256–66. doi: 10.1016/j.pain.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Lanius RA, Williamson PC, Boksman K, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52:305–11. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 37.Pissiota A, Frans O, Fernandez M, et al. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur Arch Psychiatry Clin Neurosci. 2002;252:68–75. doi: 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- 38.Bremner JD, Staib LH, Kaloupek D, et al. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–16. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ploghaus A, Tracey I, Gati JS, et al. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–81. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 40.Barberi EA, Gati JS, Rutt BK, et al. A transmit-only/receive-only (TORO) RF system for high-field MRI/MRS applications. Magn Reson Med. 2000;43:284–9. doi: 10.1002/(sici)1522-2594(200002)43:2<284::aid-mrm16>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 41.Tracey I. Imaging pain. Br J Anaesth. 2008;101:32–9. doi: 10.1093/bja/aen102. [DOI] [PubMed] [Google Scholar]
- 42.Talairach J, Tournoux P. [Stereotaxic localization of central gray nuclei] [Article in French] Neurochirurgia (Stuttg) 1958;1:88–93. doi: 10.1055/s-0028-1095515. [DOI] [PubMed] [Google Scholar]
- 43.Zhang WT, Jin Z, Huang J, et al. Modulation of cold pain in human brain by electronic acupoint stimulation: evidence from fMRI. Neuroreport. 2003;14:1591–6. doi: 10.1097/00001756-200308260-00010. [DOI] [PubMed] [Google Scholar]
- 44.Coghill RC, Sang CN, Maisog JM, et al. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–43. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 45.Bingel U, Quante M, Knab R, et al. Subcorticol structures involved in pain processing: evidence from single-trial fMRI. Pain. 2002;99:313–21. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- 46.Carretie L, Rios M, de la Gandara BS, et al. The striatum beyond reward: caudate responds intensely to unpleasant pictures. Neuroscience. 2009;164:1615–22. doi: 10.1016/j.neuroscience.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 47.Cavada C, Company T, Tejedor J, et al. The anatomical connections of the macaque monkey orbitofrontal cortex: a review. Cereb Cortex. 2000;10:220–42. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- 48.Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nat Rev Neurosci. 2001;2:352–63. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- 49.Emery NJ, Amaral DG. The role of the amygdala in primate social cognition. In: Lane RD, Nadel L, editors. Cognitive neuroscience of emotion. New York (NY): Oxford University Press; 2000. pp. 156–91. [Google Scholar]
- 50.He LF, Lue RL, Zhuang SY, et al. Possible involvement of opiod peptides of caudate nucleus in acupuncture analgesia. Pain. 1985;23:83–93. doi: 10.1016/0304-3959(85)90233-7. [DOI] [PubMed] [Google Scholar]
- 51.Scott DJ, Stohler CS, Egnatuk CM, et al. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–31. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 52.Chen G, Jiang C, Li S, et al. The role of the human caudate nucleus in acupuncture analgesia. Acupunct Electrother Res. 1982;7:255–65. doi: 10.3727/036012982816952053. [DOI] [PubMed] [Google Scholar]
- 53.Keltner JR, Furst A, Fan C, et al. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci. 2006;26:4437–43. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freund W, Klug R, Weber F, et al. Perception and suppression of thermally induced pain: a fMRI study. Somatosens Mot Res. 2009;26:1–10. doi: 10.1080/08990220902738243. [DOI] [PubMed] [Google Scholar]
- 55.Phan KL, Fitzgerald DA, Nathan PJ, et al. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 56.Bantick SJ, Wise RG, Ploghaus A, et al. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–9. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- 57.Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27:729–37. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Apkarian AV, Bushnell MC, Treede RD, et al. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Phelps EA, LeDoux JE. Contributions of the amygdale to emotion processing from animal models to human behaviour. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 60.Fukumoto M, Ushida T, Zinchuk VS, et al. Contralateral thalamic perfusion in patients with reflex sympathetic dystrophy syndrome. Lancet. 1999;354:1790–1. doi: 10.1016/S0140-6736(99)03746-0. [DOI] [PubMed] [Google Scholar]
- 61.Geha PY, Baliki MN, Harden RN, et al. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–81. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Röder CH, Michal M, Overbeck G, et al. Pain response in depersonalization: a functional imaging study using hypnosis in healthy subjects. Psychother Psychosom. 2007;76:115–21. doi: 10.1159/000097970. [DOI] [PubMed] [Google Scholar]
- 63.Bingel U, Glascher J, Weiller C, et al. Somatotopic representation of nociceptive information in the putamen: an event related fMRI study. Cereb Cortex. 2004;14:1340–5. doi: 10.1093/cercor/bhh094. [DOI] [PubMed] [Google Scholar]