Abstract
Background
Obsessive–compulsive disorder (OCD) is a common neuropsychiatric disorder that is characterized by recurrent intrusive thoughts, ideas or images and repetitive ritualistic behaviours. Although focal structural and functional abnormalities in specific brain regions have been widely studied in populations with OCD, changes in the functional relations among them remain poorly understood. This study examined OCD–related alterations in functional connectivity patterns in the brain’s top–down control network.
Methods
We applied resting-state functional magnetic resonance imaging to investigate the correlation patterns of intrinsic or spontaneous blood oxygen level–dependent signal fluctuations in 18 patients with OCD and 16 healthy controls. The brain control networks were first constructed by thresholding temporal correlation matrices of 39 brain regions associated with top–down control and then analyzed using graph theory-based approaches.
Results
Compared with healthy controls, the patients with OCD showed decreased functional connectivity in the posterior temporal regions and increased connectivity in various control regions such as the cingulate, precuneus, thalamus and cerebellum. Furthermore, the brain’s control networks in the healthy controls showed small-world architecture (high clustering coefficients and short path lengths), suggesting an optimal balance between modularized and distributed information processing. In contrast, the patients with OCD showed significantly higher local clustering, implying abnormal functional organization in the control network. Further analysis revealed that the changes in network properties occurred in regions of increased functional connectivity strength in patients with OCD.
Limitations
The patient group in the present study was heterogeneous in terms of symptom clusters, and most of the patients with OCD were medicated.
Conclusion
Our preliminary results suggest that the organizational patterns of intrinsic brain activity in the control networks are altered in patients with OCD and thus provide empirical evidence for aberrant functional connectivity in the large-scale brain systems in people with this disorder.
Introduction
Obsessive–compulsive disorder (OCD) is a relatively common, chronically disabling neuropsychiatric disorder with an average lifetime prevalence of 2%–3%.1 This disorder is characterized by thoughts and ideas that are recurrent, intense and intrusive, which are often accompanied by urges to perform repetitive, ritualistic behaviours.2 Cognitive behavioural models incorporating the symptoms and psychology of OCD posit a dysfunction of inhibitory control systems.3 Neuropsychological studies have demonstrated OCD–related abnormalities during various control tasks, such as motor inhibition and cognitive flexibility.4,5 Neuroimaging studies of OCD have also reported focal abnormalities in many brain regions associated with the top–down control system, such as the anterior prefrontal cortex (APFC),6 dorsolateral prefrontal cortex (DLPFC),6–8 cingulate cortex,9,10 insula,6 parietal regions10,11 and thalamus.12–14 Despite these advances, however, very few studies directly examined OCD–related changes in functional integrity of the brain’s control system.
In this study, we used resting-state functional connectivity magnetic resonance imaging (rs-fcMRI)15 to investigate changes in topological organization in intrinsic or spontaneous brain activities in top–down control networks in patients with OCD. This imaging method measures the synchronization in slow blood oxygen level–dependent (BOLD) signal fluctuations (typically < 0.1 Hz)15 between different brain regions during rest. These low-frequency oscillations in BOLD signal are vital for the study of brain function since they are generally thought to reflect intrinsic or spontaneous neuronal activity in the absence of specific external stimuli.16 Recent advances in graph theoretical analysis of rs-fcMRI data have allowed us to map the topological organization of these spontaneous brain functional networks under normal and pathologic conditions.17,18 It is worthy to mention that Dosenbach and colleagues19 conducted 10 different control tasks on 183 human participants and identified a large set of brain regions (n = 39), mainly involving the frontal and parietal regions, cingulate cortex, thalamus and cerebellum (Appendix 1, available at cma.ca/jpn). Using rs-fcMRI and graph theoretical analysis, Dosenbach and colleagues20 further demonstrated that the top–down control network of the healthy brain follows a small-world network structure21 characterized by dense local clustering and short path lengths between regions, which is suggestive of a highly segregated and integrative connectivity pattern in the large-scale brain system. Very recently, 2 rs-fcMRI studies have demonstrated that resting-state functional connectivity pattern in the brain’s control networks changes with normal development22 and during pediatric Tourette syndrome.23 Yet no studies reported OCD–related changes in topological organization of functional connectivity in the top–down control network.
We hypothesized that patients with OCD would show abnormal functional connectivity and small-world attributes in the brain’s top–down control networks. To test our hypothesis we used rs-fcMRI to construct the brain’s control networks in patients with OCD and healthy controls and then conducted a graph theoretical analysis. Using these approaches, we sought to determine whether patients with OCD show alterations in functional connectivity among the brain regions within the control network and in small-world network parameters in the top–down control network.
Methods
Participants
We recruited patients with OCD from the Mental Health Center, West China Hospital, Sichuan University, Chengdu, China. They fulfilled DSM-IV criteria for OCD, as diagnosed using the Structured Clinical Interview for DSM-IV Axis I disorders.24 Patients were diagnosed by 2 experienced psychiatrists (Y.Y. and B.L.) and had no relevant neurologic diseases (e.g., brain tumour, epilepsy), history of significant head injury, alcohol or drug abuse, mental retardation or other major psychiatric diseases. Specifically, no patients in this study met the criteria for major depressive disorder, Tourette syndrome or other tic-related conditions. We used the Yale–Brown Obsessive Compulsive Scale (Y–BOCS) and a clinician-rated Yale-Brown Obsessive Compulsive Scale symptom checklist to assess disease severity and to characterize the clinical expression of OCD.25 In addition, we used the 17-item Hamilton Rating Scale for Depression (HAM-D26) and the Hamilton Anxiety Rating Scale (HARS27) to rate the severity of depressive and anxiety symptoms in the patients with OCD.
We recruited age-and sex-matched heathy controls from the local community by poster advertisement, and we screened them using the Structured Clinical Interview for the DSM-Non-Patient edition (SCID-NP)28 to confirm the lifetime absence of psychiatric and neurologic illness. There was no history of psychiatric illness in the controls or any of their first-degree relatives. No gross abnormalities were observed for any of the participants when brain magnetic resonance images (T1-and T2-weighted images) were inspected by an experienced neuroradiologist (Q.Y. and S.L.). All participants gave written informed consent, and the institutional ethical review board of West China Hospital, Sichuan University approved this study.
Image acquisition and preprocessing
We acquired all images using a 3.0 T GE scanner (EXCITE, General Electric) with an 8-channel phase array head coil. Resting state BOLD images of the whole brain were acquired using a gradient-echo echo-planar imaging (EPI) sequence in 30 axial slices: repetition time (TR) 2000 ms, echo time (TE) 30 ms, flip angle 90°, slice thickness 5 mm (no slice gap), matrix 64 × 64, field of view (FOV) 240 × 240 mm2. We obtained T1-weighted structural images using a 3-dimensional spoiled gradient echo recall (SPGR) sequence for each participant: TR 8.5 ms, TE 3.4 ms, flip angle 12°, slice thickness 1.0 mm, FOV 240 × 240 mm2. During the MRI scan, we instructed all participants to lie quietly awake with their eyes closed (confirmed by participants immediately after the experiment) and to move as little as possible. Each functional run contained 200 image volumes. The rs-fcMRI time was 6 minutes and 40 seconds, and the total acquisition time of the MRI scan was about 20 minutes.
All rs-fcMRI preprocessing was carried out using the SPM5 package (www.fil.ion.ucl.ac.uk/spm). To allow for magnetization equilibrium, we discarded the first 10 images. For each participant, the remaining 190 EPI images were subjected to slice-timing correction, realigned to the first image in the first series and then subsequently unwarped to correct for susceptibility-by-movement interactions. We obtained the time course of head motions by estimating the translations in each direction and the rotations in angular motion about each axis for each of the 190 consecutive volumes. All the participants included in this study exhibited a maximum displacement of less than 1.5 mm at each axis and an angular motion of less than 1.5° for each axis. All the realigned images were further spatially normalized to the Montreal Neurological Institute (MNI) EPI template in SPM5, resampled to 3 × 3 × 3 mm3 and smoothed with a 4-mm full-width at half-maximum Gaussian kernel. Finally, the data were temporally band-pass filtered (0.01–0.1 Hz; http//resting-fmri.sourceforge.net) to reduce the effects of low-frequency drift and high-frequency noise.
Definition of region of interest
Thirty-nine regions of interest (ROIs) with 12-mm diameters19 were created around centre of mass coordinates previously identified from cross-study analysis of task-control signals. These ROIs have been recently used in several rs-fcMRI studies.20,22,23
Measurement of interregional functional connectivity
For each of the 39 ROIs, we obtained a representative time series by averaging the time series of all voxels within that ROI. Several sources of spurious variances arising from estimated head-motion profiles and global signal activity were further removed by multiple linear regression analysis.29 To measure functional connectivity among regions, we computed the Pearson correlation coefficients between every possible pair of regional residual time series, yielding a correlation matrix (39 × 39) for each participant (Appendix 1).
Graph theoretical approaches
Network construction
To construct the brain’s control networks, we thresholded individual correlation matrices (obtained above) into undirected binarized graphs (networks). To avoid the differences in correlation levels between the OCD and control groups, we employed sparsity, (S; defined as the existing number of edges in a graph divided by the maximum possible number of edges) instead of correlation coefficients as threshold metrics. Setting a sparsity-specific threshold ensures that the graphs of both groups have the same number of edges or wiring cost.30–32 In this study, we computed the network properties over a wide range of sparsity (0.10 ≤ S ≤ 0.50) in which small-world parameters could be properly estimated and the number of spurious edges was minimized, as indicated in previous studies.33,34
Small-world analysis
Small-world models can be characterized by the clustering coefficient Cp and the characteristic path length Lp,21 which quantify the extent of interconnectivity of a network at local and global levels, respectively. Here, we used a “harmonic mean” distance measure to compute the Lp since it considers more than one component in a graph.35 To test the small-world properties of the control networks, the values of Cp and Lp were compared with those of 100 degree-matched random networks (γ = Cp/Cprand and γ = Lp/Lprand with Cprand and Lprand denoting the mean Cp and Lp of 100 random networks). Typically, a network could be considered small-world if it met the following conditions: > 1 and ~1.21
Statistical analysis
Differences in functional connectivity
To determine whether the correlations between regions were significantly nonzero across participants, we performed 1-sample t tests in an element-by-element manner on Fisher’s transformed correlation matrices in both the OCD and control groups. To account for the multiple comparisons, a false discovery rate (FDR) procedure36 was performed at a q value of 0.05. Furthermore, we analyzed functional connectivity differences between the OCD and control groups in 2 different ways. First, as in previous studies,20,22 we selected the 75 strongest positive connections from each group and visualized these in graphs. This process makes the 2 groups of networks have the exact same number of nodes and connections, ensuring that any differences among graphs would reflect topological changes rather than low-level correlation differences. Second, we performed further direct comparisons using 2-tailed 2-sample t tests on the significant nonzero connections in the OCD or control groups.
Differences in network parameters
We performed statistical comparisons between groups using 2-tailed 2-sample t tests over a wide range of S (0.10 ≤ S ≤ 0.50). In addition, we evaluated the areas under the curves of these metrics as the summarized measures of the networks over a preselected range of S and then conducted Student t tests.
Whole-brain network analysis
In addition to the specific top–down control network, we also investigated the topological organization of the whole-brain network in patients with OCD and healthy controls. The network construction and characteristics involved have been described previously.37 Briefly, the brain was first parcellated into 90 ROIs (45 for each hemisphere) using Automated Anatomical Labeling.38 The mean time series of each region was then acquired by averaging the time series of all voxels within that region. The following preprocessing and graph theoretical analyses were similar to those previously described for the control networks.
Results
Demographic and clinical characteristics
Eighteen patients with OCD and 16 age-and sex-matched healthy controls participated in this study. According to clinical dimensions, we found that the patients with OCD were categorized as follows: symmetry/ordering, n = 2; hoarding, n = 3; contamination/cleaning, n = 5; aggressive/checking, n = 7; and sexual/religious, n = 1. Five of the patients were drug-naïve, 10 were prescribed selective serotonin reuptake inhibitors and 3 were prescribed clomipramine.
Table 1 summarizes the demographic and clinical characteristics of both the OCD and control groups. There were no significant differences in the distributions of age (t32 = −0.48, p = 0.64), sex (χ12 = 0.036, p = 0.85), years of education (t32 = −0.28, p = 0.78) and handedness between the groups. The mean Y-BOCS total score for the OCD group indicated that the patients were in the moderate range of OCD severity.
Table 1.
Demographic and clinical characteristics of OCD and control groups
| Group; mean (SD)* |
||
|---|---|---|
| Characteristic | OCD, n = 18 | Control, n = 16 |
| Age, yr | 23.3 (5.0) | 24.1 (5.4) |
| Sex, no. male:female | 14:4 | 12:4 |
| Handedness, no. right:left | 18:0 | 16:0 |
| Education, yr | 13.6 (3.0) | 13.9 (2.6) |
| Illness duration, yr | 4.7 (3.7) | |
| Total Y–BOCS severity score (range 16–33) | 24.0 (5.7) | |
| HARS score | 7.5 (1.9) | |
| HAM-D score | 9.2 (2.2) | |
Interregional functional connectivity in the control networks
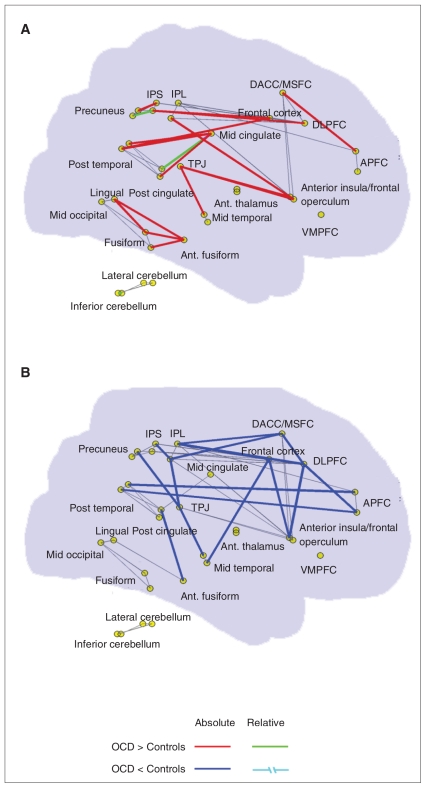
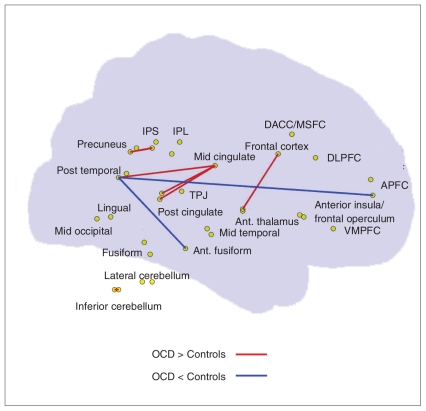
Among the 75 strongest positive connections in both groups, there were 58 overlapping edges and 17 group-specific connections, as shown in Figure 1. Visual examination indicated that a considerable proportion of the OCD–related increases in functional connections were short-range, whereas the OCD–related decreases in connections were mainly associated with different lobes (i.e., long-range connections, especially between the frontal and temporal lobes, and the frontal and parietal lobes). Further comparisons of connections with nonzero connectivity strength revealed significant between-group differences in the connections between various regions (Table 2 and Fig. 2). For instance, compared with the controls, patients with OCD showed decreased positive correlations between the left APFC and left posttemporal and between the left posttemporal and left anterior fusiform, and increased positive correlations between many pairs of brain regions, including the midcingulate–postcingulate, fronto–thalamus, intraparietal sulcus–precuneus, cingulate–temporal and cerebellum–cerebellum. Additionally, we noted several abnormal negative correlations in patients with OCD (Appendix 2, available at cma.ca/jpn).
Fig. 1.
An anatomic representation of the 75 strongest positive connections in (A) patients with obesessive–compulsive disorder (OCD) and (B) control groups mapped onto sagittal views of a transparent brain. The red and blue lines highlight those connections only existing in either OCD or control groups (i.e., absolute difference). Green lines highlight those connections present in both groups but with significant between-group differences in connection strengths (i.e., relative differences). Gray lines represent those connections present in both groups but without significant between-group differences. APFC = anterior prefrontal cortex; DACC/MSFC = dorsolateral anterior cingulate cortex/medial superior frontal cortex; DLPFC = dorsolateral prefrontal cortex; IPL = inferior parietal lobule; IPS = intraparietal sulcus; TPJ = temporo-parietal conjunction; VMPFC = ventral medial prefrontal cortex.
Table 2.
Abnormal functional connectivity in patients with OCD*
| Group; mean (SD) correlation r |
|||||
|---|---|---|---|---|---|
| Correlation; brain regions | Control | OCD | t value | p value | Distance |
| Decreased positive correlation in OCD | |||||
| Left posttemporal and left anterior fusiform | 0.193 (0.166) | 0.037 (0.206) | 2.306 | 0.028 | 51.740 |
| Left posttemporal and left anterior prefrontal cortex | 0.293 (0.267) | 0.087 (0.294) | 2.114 | 0.042 | 129.869 |
| Increased positive correlation in OCD | |||||
| Midcingulate and left postcingulate | 0.247 (0.189) | 0.469 (0.193) | −3.395 | 0.002 | 34.554 |
| Right frontal cortex and right anterior thalamus | 0.010 (0.206) | 0.193 (0.211) | −2.586 | 0.014 | 45.486 |
| Midcingulate and right postcingulate | 0.278 (0.219) | 0.432 (0.179) | −2.289 | 0.029 | 32.016 |
| Right intraparietal sulcus and left precuneus | 0.271 (0.163) | 0.404 (0.212) | −2.115 | 0.042 | 40.571 |
| Midcingulate and left posttemporal | 0.135 (0.272) | 0.320 (0.238) | −2.096 | 0.044 | 63.537 |
| Right inferior cerebellum and left inferior cerebellum | 0.337 (0.310) | 0.545 (0.204) | −2.079 | 0.046 | 37.054 |
OCD = obsessive–compulsive disorder; SD = standard deviation.
The r values indicate Pearson correlation coefficients of the resting state blood oxygen level–dependent (BOLD) signal between regions in either the patients with OCD or controls. Correlation coefficients in bold indicate significant interregional association of resting state BOLD signal within group (p < 0.05, false discovery rate [FDR]–corrected). The t and p values showed significant differences in correlation between the 2 groups (p < 0.05, uncorrected). Note that pairs of regions were only listed if they met the following criteria: they were significantly nonzero in either the OCD group, control group, or both at p < 0.05 (FDR–corrected), and they showed significant between-group differences at p < 0.05 (uncorrected). The table shows the changes in positive correlations in the OCD group. For changes in negative correlations, see Appendix 2, available online at cma.ca/jpn.
Fig. 2.
Significant differences in interregional positive correlations between patients with obsessive–compulsive disorder (OCD) and controls. Compared with the controls, the patients with OCD showed increased (red lines) and decreased (blue lines) positive correlations in several brain regions associated with top–down control. Both left-and right-hemispheric regions are placed on a transparent brain to aid with visualization. APFC = anterior prefrontal cortex; DACC/MSFC = dorsolateral anterior cingulate cortex/medial superior frontal cortex; DLPFC = dorsolateral prefrontal cortex; IPL = inferior parietal lobule; IPS = intraparietal sulcus; TPJ = temporo-parietal conjunction; VMPFC = ventral medial prefrontal cortex.
Small-world top–down control networks
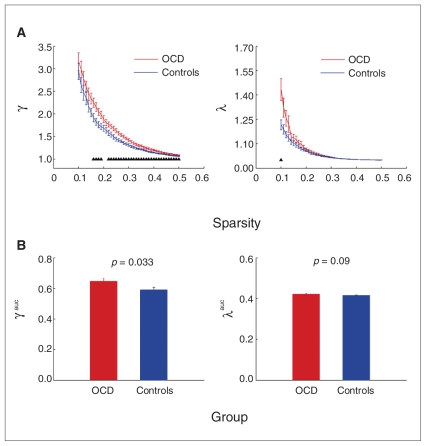
Using graph theoretical approaches, we further investigated the topological properties of the brain top–down control networks in patients with OCD and healthy controls. The small-world network parameters (normalized clustering coefficient, γ, and normalized shortest path length, λ) were computed as a function of network sparsity, S. As expected, both groups demonstrated small-world network architecture over a wide range of sparsity (0.10 ≤ S ≤ 0.50) as they had an almost identical path length (λ ≈ 1) but were more locally clustered (γ > 1) in comparison with the matched random networks (Fig. 3).
Fig. 3.
Small-world top–down control networks in healthy controls and patients with obsessive–compulsive disorder (OCD). (A) The graph shows significant between-group differences in the normalized clustering coefficient (γ, Cpreal/Cprand; left panel) as a function of sparsity thresholds. Blue lines represent the controls and red lines represent the OCD group. Black triangles indicate significant (t test, p < 0.05) differences between the 2 groups. There were no significant between-group differences in the normalized shortest path length (λ, Lpreal/Lprand; right panel) at most sparsity values (besides at a sparsity of 0.10). Of note, both networks have γ > 1 and λ ≈ 1, which implies prominent small-world properties in the top–down control networks. (B) The bars show significant differences (p = 0.033) in the area under the γ curves (γauc) (left panel) between the controls (blue bars) and OCD patients (red bars). There were no significant differences (p = 0.09) in the area under the λ curves (λauc; right panel) between the 2 groups.
Despite the regime of small-worldness in both groups, patients with OCD showed significant changes in the small-world network parameters compared with controls. As shown in Figure 3A, we found that both γ and λ of patients with OCD were larger than those of the controls. Further statistical comparisons revealed significant between-group differences in the γ values over a wide range of sparsity (0.16 ≤ S ≤ 0.50), and differences in the λ values at S = 0.10 (p = 0.008). Moreover, patients with OCD exhibited a significantly (t32 = 2.22, p = 0.033) larger area under the γ curves. There were no significant differences in area under the λ curves between the groups, although there was a smaller increased trend (t32 = 1.73, p = 0.09; Fig. 3B). This combination of significantly increased γ with slightly changed λ indicates a tendency for the large-scale brain control system in patients with OCD to shift toward a more regular organization, implying abnormalities of small-world network characteristics. Finally, we demonstrated that the increase in the area under the γ curve is significantly correlated (r = 0.46, p = 0.006) with increases in mean functional connectivity that are mainly associated with short-range functional connections (Appendix 3, available at cma.ca/jpn).
Small-world whole-brain functional networks
Several previous studies of rs-fcMRI have shown that the small-world topology demonstrated in the whole-brain functional network in healthy participants33,39 is significantly altered in conditions such as Alzheimer disease40 and schizophrenia.41 We found no significant between-group differences in the small-world parameters for the whole brain networks, even though both groups displayed significant small-world properties. This result suggests that OCD may be mainly associated with disruptive integrity of the specific brain control system rather than of the whole-brain network.
Discussion
To our knowledge, this is the first study to demonstrate OCD–related changes in functional connectivity patterns in the brain’s control network using rs-fcMRI. Compared with controls, the patients with OCD not only showed abnormalities in functional connectivity among the brain’s control regions, but also exhibited topological alterations in the control network characterized by abnormal small-world parameters. Taken together, these results strongly suggest that OCD is associated with disruptive functional integrity in the large-scale brain control system, thus providing new insights into the understanding of the pathophysiology of this disorder.
Abnormal small-world architecture in the control networks in OCD
Both the OCD and control groups displayed a small-world topology in the brain’s control networks, which was consistent with a previous fMRI study.20 The small-world model was introduced by Watts and Strogatz,21 and has made a tremendous impact on the studies of complex brain networks (for a review, see Bullmore and Sporns17). The 2 main features of a small-world topology, high clustering and short paths, refer to a dense local clustering or cliquishness and a small number of connections between any pair of regions. High clustering ensures functionally segregated processing, whereas short paths ensure effective integrity and the rapid transfers of information between distant brain regions that are thought to constitute the basis of cognitive processes. Thus, when the human brain is taking part in a top–down control task, the coexistence of functional segregation and integration facilitates the effective integration of multiple segregated sources of information.42,43
Although both groups exhibited small-world topology in their brains’ control networks, those in the OCD group had significantly larger clustering coefficients (γ), suggesting a disturbance in the organization of the control network and a shift toward a more regular organization. This result was similar to our recent finding in patients with attention-deficit/hyperactivity disorder.44 It has been suggested that regular lattices reduce signal propagation speed and synchronizability across distant regions compared with small-world networks.45 Therefore, the alterations in the small-world attributes observed here may reflect a less optimal topological brain network organization in patients with OCD. Interestingly, we also found that the changes in γ were correlated with increased positive correlations in connections that are mainly short-ranged. The abnormalities in the short-range connections could be the predominant contributors to the alterations of topological organization in the top–down control network. Of note, Vul and colleagues46 suggest that the correlations between brain activation and personality measures could be greatly inflated because of nonindependent analysis error in fMRI studies, such as cognitive neuroscience and clinical neuroscience. However, there is larger debate about the effect size of correlation. Lieberman and colleagues47 argued that whatever inflation does exist may be far more modest. Such correlations in neuroscience may reflect meaningful biological relations when valid multiple comparisons procedures are applied in a reasonable sample size. In this study, we did not observe significant changes in the small-world organization in the whole-brain system in patients with OCD, suggesting that the disorder could mainly be associated with disruptive integrity in the brain control network. This result provides further support for our recent finding that the topological organization of specific brain functional clusters cannot be captured by computing network properties on a whole-brain scale.48
Abnormal intrinsic functional connectivity in OCD
In addition to the above-mentioned abnormalities in network attributes in patients with OCD, We also observed inter-regional correlation changes. Decreased positive correlations in the OCD group were located in the APFC/posttemporal and posttemporal/fusiform connections. The APFC plays a vital role in integrating the outcomes of 2 or more separate higher cognitive tasks.49 Using fMRI, Remijnse and colleagues6 reported reduced APFC activity in patients with OCD during affective switching. Using structural MRI, Narayan and colleagues50 showed a thicker posterior temporal gyrus in patients with OCD. They argued that the structural abnormalities might underlie poor neuronal connectivity, which in turn could affect information integration and cognitive functions in patients with OCD.
We found increased positive correlations in patients with OCD mainly in the cingulate cortex (middle cingulate cortex MCC/posterior cingulate cortex [PCC]), frontal–thalamic circuit and cerebellum. The MCC is vital in response selection during fear avoidance, whereas the PCC plays a role in personal visuospatial orientation, self-relevance assessment and episodic memory processes.51 Recent studies have demonstrated that patients with OCD exhibit excessive activation in the PCC during errors of commission and high-conflict trials9 and increased grey matter concentration in both the MCC and PCC that was positively correlated with variability in stop-signal task performance (response inhibition).10 A diffusion tensor imaging study has also shown OCD-associated increases in fractional anisotropy (a measure of the directional diffusivity of water) in the cingulum bundles linking the PCC and MCC,52 which provides support for our finding of increased functional correlations between the 2 regions. Other studies have shown that patients with OCD have greater activation in the frontal cortex during planning tasks,53 higher glucose metabolism and grey matter concentration in the thalamus13,14 and higher fractional anisotropy in the anterior limb of the internal capsule (the final pathway for all higher cognitive feedback loops connecting the frontal lobe and the thalamus).54 Thus, increased frontal–thalamus connectivity in patients with OCD observed here are consistent with previous studies, which provides further support for the presence of abnormal frontal–striatal–thalamic–cortical circuitry in patients with OCD.55,56 We also found increased positive correlations in 2 parietal regions (intraparietal sulcus and precuneus) and in the cerebellum, compatible with previous studies.12,14,57
An interesting finding was that the increased positive correlations in patients with OCD were mainly associated with short-range connections (i.e., segregation), whereas decreases in correlations were associated with long-range connections (i.e., integration). Previous studies have suggested that OCD is a developmental disorder that is usually accompanied by recurrent, intense and intrusive thoughts or ideas accompanied by a delay or inefficiency of synaptic pruning in brain regions.2,58 Using rs-fcMRI, Fair and colleagues22 showed that children have a larger proportion of short-range functional connections in the brain’s control system, which decreases with age. In contrast, long-range connections tend to increase in strength with age. Another developmental disorder study by Church and colleagues23 showed that in adolescents (10–15 years of age) with Tourette syndrome, the patterns of resting state functional connectivity in the brain’s control network resembled those in children (7–9 years of age). Thus, our preliminary results of increased short-range connections and decreased long-range connections in patients with OCD may provide new evidence for the delay or disruption of segregated and integrative processing in the brain’s control system, which might help to understand the defect in the inhibition of unwanted thoughts and behaviours observed in populations with this disorder. Further study with larger samples and more representative younger patients is necessary to confirm these preliminary findings.
In this study, we also observed altered negative correlations in patients with OCD, which were predominately involved in the frontal, inferior parietal lobule, dorsolateral anterior cingulate cortex/medial superior frontal cortex and anterior insula/frontal operculum regions. Although the phenomenon of negative correlations of spontaneous brain activity has been frequently observed in healthy participants,29 it remains unclear as to what they precisely reflect. It is worthy to note that there has been controversy regarding a definitive source of negative correlations. Murphy and colleagues59 suggested that global signal regression during rs-fcMRI data preprocessing is most likely the cause of negative correlations. However, Fox and colleagues60 showed that various characteristics of negative correlations are not determined by global regression. Moreover, the removal of global signals could reduce the effect of physiologic artifacts and facilitate the observation of true physiologic relations. Of note, a previous structural MRI study57 showed that patients with OCD had abnormal negative correlations between the grey matter volumes of cortical and subcortical structures. Based on these findings, we speculated that the abnormal negative correlations in patients with OCD might be of physiologic significance. Further studies still need to be conducted to clarify biological mechanisms of altered negative correlations in populations with OCD.
Limitations
Several methodologic issues need to be further addressed. First, head motion of participants during the scans may have affected our results. Recently, Salvador and colleagues61 showed that the residuals of head motion were associated with the functional connectivity of brain regions predominantly at high frequency (0.17~0.25 Hz), but there were no strong correlations with that at middle (0.08~0.17 Hz) and low (< 0.08 Hz) frequencies. When analyzing the head motions of participants, we did not observe significant differences between the 2 groups or correlations with network parameters. Second, we reported uncorrected results for between-group analysis in functional connectivity (Table 2). We found that the results were not significant after a multiple comparison correction (p > 0.05, FDR–corrected). However, we still reported the uncorrected results and thus these results could be considered exploratory in nature. To further increase statistical power, future studies need to be conducted by using a large sample of patients with OCD or by selecting a limited number of ROIs. Third, the patient group in the present study is heterogeneous in terms of symptom clusters. A previous fMRI study12 suggested that different OCD symptom dimensions could have distinct neural mechanisms. In the future, it would be interesting to investigate whether patients with different OCD symptoms show distinct connectivity patterns in the top–down brain control network. Fourth, in the present investigation, most of the patients with OCD (13 of 18) were medicated, thus we cannot completely rule out the possibility that our results were influenced by drug effects. However, it has been noted previously that brain abnormalities involving brain structure,54 function62 and metabolism63 in patients with OCD were gradually restored and tended to normalize after treatment with selective serotonin reuptake inhibitors. Further investigations using large, drug-naïve samples or longitudinal studies are warranted to confirm our preliminary results of intrinsic or spontaneous changes in brain function. Fifth, in this study, the participants were scanned during rest. It is generally thought that resting is a natural state in which participants are not performing any specific demanding tasks. However, we cannot completely rule out the possibility that the patients with OCD have experienced pervasive intrusive thoughts during the scan, thereby affecting our results of functional analysis. Of note, this is a common question for rs-fcMRI studies in populations with neuropsychiatric diseases. Finally, the top–down brain control network studied here includes only 39 regions. It could be further extended to contain more regions, such as the orbitofrontal cortex and striatal regions, which have been found to exhibit OCD–related abnormalities.6,11
Conclusion
In the present study, we used rs-fcMRI and graph theoretical approaches to show that patients with OCD have abnormal intrinsic functional connectivity in the brain’s control network, thus providing novel insights into the understanding of the underlying pathophysiology of OCD. Future studies combining rs-fcMRI with structural MRI and diffusion MRI techniques will be vital to explore how the functional changes shown here are associated with structural alterations, and whether these changes could be used to characterize different clinical subtypes, disease progressions and medication effects in patients with OCD.
Acknowledgements
This study was supported by National Natural Science Foundation of China (Grant Nos. 30625024, 81030027, 81030028, 30870667, 30728017 and 30960099), National Basic Research Program (973 Program No: 2007CB512305/2) and National High Technology Program of China (863 Program No: 2008AA02Z408). We thank Zhang John Chen for English editing and proofreading. Dr. Gong also acknowledges his appointment as an Honorary Fellow of the Faculty of Medicine, University of Liverpool, Liverpool, United Kingdom.
Footnotes
Competing interests: None declared.
Contributors: Drs. Zhang, Gong and He contributed to the conception and design of the study and the acquisition, analysis and interpretation of data. Ms. Chen and Tang and Drs. Yang, Wu, Li, Yue, Lui, Huang contributed to the conception and design of the study and the acquisition of data. Ms. Wang and Yan and Drs. Zang and Chan contributed to the conception and design of the study and the analysis of data. Drs. Zhang and He wrote the article, which all other authors reviewed. All authors gave final approval for publication.
References
- 1.Rasmussen SA, Eisen JL. The epidemiology and differential diagnosis of obsessive compulsive disorder. J Clin Psychiatry. 1992;53 (Suppl):4–10. [PubMed] [Google Scholar]
- 2.Weissman MM, Bland RC, Canino GJ, et al. The cross national epidemiology of obsessive compulsive disorder. The Cross National Collaborative Group. J Clin Psychiatry. 1994;55(Suppl):5–10. [PubMed] [Google Scholar]
- 3.Chamberlain SR, Blackwell AD, Fineberg NA, et al. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain SR, Fineberg NA, Menzies LA, et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164:335–8. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penades R, Catalan R, Rubia K, et al. Impaired response inhibition in obsessive compulsive disorder. Eur Psychiatry. 2007;22:404–10. doi: 10.1016/j.eurpsy.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Remijnse PL, Nielen MM, van Balkom AJ, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:1225–36. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- 7.van den Heuvel OA, Remijnse PL, Mataix-Cols D, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–68. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- 8.van den Heuvel OA, Veltman DJ, Groenewegen HJ, et al. Frontalstriatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry. 2005;62:301–9. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- 9.Maltby N, Tolin DF, Worhunsky P, et al. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Menzies L, Achard S, Chamberlain SR, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–36. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain SR, Menzies L, Hampshire A, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–2. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- 12.Mataix-Cols D, Wooderson S, Lawrence N, et al. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–76. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 13.Saxena S, Brody AL, Maidment KM, et al. Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry. 2004;161:1038–48. doi: 10.1176/appi.ajp.161.6.1038. [DOI] [PubMed] [Google Scholar]
- 14.Kim JJ, Lee MC, Kim J, et al. Grey matter abnormalities in obsessive-compulsive disorder: statistical parametric mapping of segmented magnetic resonance images. Br J Psychiatry. 2001;179:330–4. doi: 10.1192/bjp.179.4.330. [DOI] [PubMed] [Google Scholar]
- 15.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 16.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 17.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 18.He Y, Chen Z, Gong G, et al. Neuronal networks in Alzheimer’s disease. Neuroscientist. 2009;15:333–50. doi: 10.1177/1073858409334423. [DOI] [PubMed] [Google Scholar]
- 19.Dosenbach NU, Visscher KM, Palmer ED, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–8. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–2. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 22.Fair DA, Dosenbach NU, Church JA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Church JA, Fair DA, Dosenbach NU, et al. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain. 2009;132:225–38. doi: 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P, Version 20) New York (NY): Biometrics Research; 1996. [Google Scholar]
- 25.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. The assessment of anxiety state by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Non-patient Edition (SCID-I/NP) New York (NY): Biometrics Research; 2001. [Google Scholar]
- 29.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stam CJ, Jones BF, Nolte G, et al. Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex. 2007;17:92–9. doi: 10.1093/cercor/bhj127. [DOI] [PubMed] [Google Scholar]
- 31.He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s disease. J Neurosci. 2008;28:4756–66. doi: 10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Y, Dagher A, Chen Z, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain. 2009;132:3366–79. doi: 10.1093/brain/awp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achard S, Salvador R, Whitcher B, et al. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 2007;17:2407–19. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- 35.Newman M. The structure and function of complex networks. SIAM Rev. 2003;45:167–256. [Google Scholar]
- 36.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Wang L, Zang Y, et al. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum Brain Mapp. 2009;30:1511–23. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 39.Salvador R, Suckling J, Coleman MR, et al. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 2005;15:1332–42. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- 40.Supekar K, Menon V, Rubin D, et al. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLOS Comput Biol. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Liang M, Zhou Y, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–61. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- 42.Dosenbach NU, Fair DA, Cohen AL, et al. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sporns O, Chialvo DR, Kaiser M, et al. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8:418–25. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Zhu C, He Y, et al. Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2009;30:638–49. doi: 10.1002/hbm.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strogatz SH. Exploring complex networks. Nature. 2001;410:268–76. doi: 10.1038/35065725. [DOI] [PubMed] [Google Scholar]
- 46.Vul E, Harris C, Winkielman P, et al. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009;4:274–90. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 47.Lieberman MD, Berkman ET, Wager TD. Correlations in social neuroscience aren’t voodoo. Commentary on Vul et al. (2009) Perspect Psychol Sci. 2009;4:299–307. doi: 10.1111/j.1745-6924.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y, Wang J, Wang L, et al. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS One. 2009;4:e5226. doi: 10.1371/journal.pone.0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–94. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 50.Narayan VM, Narr KL, Phillips OR, et al. Greater regional cortical gray matter thickness in obsessive-compulsive disorder. Neuroreport. 2008;19:1551–5. doi: 10.1097/WNR.0b013e3283112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cannistraro PA, Makris N, Howard JD, et al. A diffusion tensor imaging study of white matter in obsessive-compulsive disorder. Depress Anxiety. 2007;24:440–6. doi: 10.1002/da.20246. [DOI] [PubMed] [Google Scholar]
- 53.Nakao T, Nakagawa A, Yoshiura T, et al. A functional MRI comparison of patients with obsessive-compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry Res. 2005;139:101–14. doi: 10.1016/j.pscychresns.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Yoo SY, Jang JH, Shin YW, et al. White matter abnormalities in drug-naive patients with obsessive-compulsive disorder: a diffusion tensor study before and after citalopram treatment. Acta Psychiatr Scand. 2007;116:211–9. doi: 10.1111/j.1600-0447.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- 55.Saxena S, Brody AL, Schwartz JM, et al. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998;(35):26–37. [PubMed] [Google Scholar]
- 56.Roth RM, Saykin AJ, Flashman LA, et al. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biol Psychiatry. 2007;62:901–9. doi: 10.1016/j.biopsych.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Pujol J, Soriano-Mas C, Alonso P, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–30. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg DR, Keshavan MS. A.E. Bennett Research Award. Toward a neurodevelopmental model of obsessive–compulsive disorder. Biol Psychiatry. 1998;43:623–40. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- 59.Murphy K, Birn RM, Handwerker DA, et al. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fox MD, Zhang D, Snyder AZ, et al. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–83. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salvador R, Martinez A, Pomarol-Clotet E, et al. A simple view of the brain through a frequency-specific functional connectivity measure. Neuroimage. 2008;39:279–89. doi: 10.1016/j.neuroimage.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 62.Lazaro L, Caldu X, Junque C, et al. Cerebral activation in children and adolescents with obsessive-compulsive disorder before and after treatment: a functional MRI study. J Psychiatr Res. 2008;42:1051–9. doi: 10.1016/j.jpsychires.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 63.Jang JH, Kwon JS, Jang DP, et al. A proton MRSI study of brain N-acetylaspartate level after 12 weeks of citalopram treatment in drug-naive patients with obsessive-compulsive disorder. Am J Psychiatry. 2006;163:1202–7. doi: 10.1176/ajp.2006.163.7.1202. [DOI] [PubMed] [Google Scholar]