Abstract
Background
Apolipoprotein E (apoE) and cholesterol play a critical role in synapse and myelin maintenance and integrity and are thus appealing candidates in the pathogenesis of schizophrenia and bipolar disorder. To explore the role of these 2 molecules, we quantified cholesterol and apoE levels in prefrontal grey and white matter in patients with schizophrenia, bipolar disorder and healthy controls. Furthermore, we investigated the relations between apoE and cholesterol levels and the APOE genotype.
Methods
We obtained dorsolateral prefrontal grey and white matter from the Stanley Medical Research Institute Brain Collection (schizophrenia n = 35, bipolar disorder n = 35 and controls n = 35). Cholesterol levels were quantified using high-pressure liquid chromatography, whereas apoE was measured by enzyme-linked immunosorbent assay.
Results
We found no significant differences in cholesterol or apoE levels among the groups. ApoE levels were higher in grey matter than in white matter in all groups; conversely, levels of cholesterol were higher in white matter than in grey matter. We observed a significant inverse correlation between apoE and cholesterol levels in both grey and white matter. Furthermore, in grey matter, apoE levels were significantly higher in APOE ɛ2 carriers compared with APOE ɛ3 or APOE ɛ4 carriers, with cholesterol levels following the opposite trend.
Limitations
Limitations of our study include our inability to control for potential confounding variables and the small numbers of APOE ɛ2 and ɛ4 carriers in each group.
Conclusion
Although large amounts of cholesterol are present in white matter, apoE expression is limited. The APOE genotype may play a role in the regulation of both cholesterol and apoE levels in grey matter. The impact of APOE polymorphisms on lipid homeostasis in people with psychiatric disorders warrants further investigation.
Introduction
Schizophrenia and bipolar disorder are complex illnesses. Although the molecular and cellular pathology of these disorders has not yet been fully elucidated, alterations in synapses, dendritic spines and myelin have been reported.1–4 Cholesterol and apolipoprotein E (apoE) are both excellent candidates for a pathologic role in schizophrenia and bipolar disorder since cholesterol is an important molecular component of both synapses and myelin, with apoE being the main cholesterol carrier in the brain.
Brain cholesterol is synthesized in situ by astrocytes and oligodendrocytes and is independent of that circulating in plasma. In the brain, essentially all cholesterol (> 99.5%) is un-esterified. Most cholesterol is believed to reside in 2 major pools: 1 in myelin, which represents about 70% of all brain cholesterol, and 1 in plasma membranes of neurons and astrocytes.5 Apolipoprotein E is a 299aa, arginine rich, 2-domain protein encoded by the apolipoprotein E gene (APOE), located on chromosome 19. There are 3 main alleles of the human APOE gene, ɛ2, ɛ3 and ɛ4, which specify 3 protein isoforms, apoE2, apoE3 and apoE4, that differ owing to a cysteine–arginine interchange at residues 112 and 158.6,7 Synthesized by astrocytes and activated microglia, apoE is the major apolipoprotein in the brain, where it forms high-density lipoprotein (HDL)–like particles that distribute cholesterol among neurons and their supporting cells.5,8 Both apoE and cholesterol play a critical role in synaptogenesis, neurite outgrowth and membrane repair and maintenance, including that of the myelin sheath.9–15 Specifically, when cocultured with glial cells, neurons develop more synapses, and those synapses are more efficient compared with neurons cultured without glial cells.9 The factors implicated in this process were determined to be apoE and cholesterol. Cholesterol and apoE also play a role in the regulation of neurite outgrowth,10,11 with apoE3 increasing neurite outgrowth but apoE4 having the opposite effect.10 In addition, apoE plays a critical role in collecting freed cholesterol from damaged myelin and recycling that cholesterol by incorporating it back into repaired membranes.12
Whereas altered lipid levels have been identified in blood and skin fibroblasts in people with schizophrenia and bipolar disorder,16–18 to date there have been few studies in brain tissue. We previously reported reduced cholesterol levels in visual association cortex in people with major depressive disorder, with a similar trend in people with bipolar disorder.3 A second study investigating a heterogeneous diagnostic sample, including people with affective as well as psychotic disorders, found that cholesterol levels were lower in Brodmann areas (BAs) 11 and 47 in individuals who had completed suicide by violent means.19 Evidence also suggests that brain apoE levels are altered in people with schizophrenia and bipolar disorder. Specifically, Dean and colleagues20 found increased apoE in BA 9 and 46, with no difference in BA 10 or the striatum in people with schizophrenia, whereas apoE was lower in BA 10 but higher in BA 9 and in the striatum in people with bipolar disorder.21 Finally, a recent meta-analysis identified the APOE ɛ4 polymorphism as significantly associated with schizophrenia,22 whereas the ɛ4 allele has also been linked to early onset bipolar disorder with psychotic symptoms.23
Whereas cholesterol and apoE appear to be good candidates for a role in the pathophysiology of schizophrenia and bipolar disorder, few studies have assessed cholesterol and apoE abundance in postmortem brain tissues from people with these disorders. Furthermore, despite differences in lipid composition between grey and white matter, neither apoE nor cholesterol levels have yet been quantified in white matter from people with schizophrenia or bipolar disorder. In light of the reported association between the presence of APOE ɛ4 alleles and schizophrenia and bipolar disorder, investigation of the relation between APOE genotype, apoE protein levels and cholesterol concentrations is warranted. Whereas studies involving people with Alzheimer disease24–26 and transgenic mice27 have reported a relation between apoE protein levels and genotype (ɛ2/2 > ɛ3/3 > ɛ4/4), this has not been investigated in populations with other disorders. Based on findings of reduced synaptic and neuritic density and altered myelin in people with schizophrenia and bipolar disorder, we propose that levels of cholesterol and its transporter apoE are reduced in grey and white matter in these populations. In addition, we suggest that the APOE genotype may play a role in the regulation of cholesterol and apoE levels in both brain regions. The aim of this study was to quantify cholesterol and apoE levels in dorsolateral prefrontal grey and white matter in patients with schizophrenia, patients with bipolar disorder and healthy controls. Furthermore, we investigated the relation between apoE, cholesterol and the APOE genotype.
Methods
Brain tissue
We obtained frozen samples of the dorsolateral prefrontal region (BA 9) from the Stanley Foundation Neuropathology Consortium. Tissue was available from 1 hemisphere of each brain, with about equal numbers sampled in a random manner for each side, and was carefully dissected out into grey matter and adjacent white matter. Our sample consisted of tissue from 105 brains (35 from controls with no known psychiatric or neurologic disorder, 35 from patients with schizophrenia and 35 from patients with bipolar disorder). Diagnoses were made according to DSM-IV criteria.28 All brains underwent clinical neuropathologic examination, and none demonstrated evidence of neurodegenerative changes or other pathological lesions. We excluded the tissue sample from 1 patient with bipolar disorder from this study owing to a change in diagnosis. Investigators were blind to diagnosis and genotype when measuring cholesterol and apoE levels. This study was approved by the University of British Columbia Clinical Research Ethics Board.
Free cholesterol quantification: high-performance liquid chromatography
Cholesterol was separated from other lipids and quantified using a Waters Alliance 2695 high-performance liquid chromatography (HPLC) system equipped with an autosampler and evaporative light scattering mass detector (ESLD) as previously described.29 Briefly, tissue was homogenized in 15 volumes of ice-cold tris buffered saline (TBS) and protein quantified using a Lowry-based method (DC assay; Bio-Rad). Total lipids were extracted from grey and white matter (wet weight, 13.33 and 6.67 mg, respectively) using a modification of the Folch method.30 Sample homogenate was made up to a total of 2.5 mL with NaCl/EDTA in water (9 g/L/1.14 g/L), 3 mL methanol was added and the sample vortexed. A further 6 mL chloroform was added, and the sample vortexed and centrifuged to enable separation of the organic and inorganic phases. The organic phase was then recovered and transferred to a clean tube. The remaining inorganic phase was extracted twice more to ensure complete recovery of all lipids, with organic phases combined. The solvent was then evaporated under nitrogen and resuspended in 50 mL of hexane/acetone/methanol/chloroform, 1/1/6/4 by volume, containing 75 μg of betulin as the internal standard. Lipids classes, including unesterified cholesterol, were separated using a YMC diol 4.6 mm × 250 mm column at 35°C with a quarternay gradient of hexane, methanol, 1.7% triethylamine in acetone and 0.5% acetic acid in isopropanol. The quantity of cholesterol was determined from the area ratio of cholesterol to betulin, which was constant in all sample injections. The detector response is linear for unesterified cholesterol over the range of 0.1–2.4 g/L with a relative response of cholesterol to betulin of 1.24:1. Data were expressed as μg cholesterol per mg protein.
ApoE quantification: enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISA) were per-formed as previously described,1,3 with slight modifications. Briefly, tissue homogenates were diluted to 240 μg protein/mL in TBS. Duplicate samples were then serially diluted over a 128-fold range and incubated overnight at 4°C in 384 well Maxisorp plates (Nunc). Nonspecific binding was blocked using TBS containing 5% milk. Plates were then incubated with primary antibody (WU E-14, obtained from ATCC, 1:10) overnight at 4°C. Each plate also contained negative control wells in which tissue culture-conditioned media was substituted for the primary antibody. The plates were further incubated with peroxidase-conjugated secondary antibody (goat anti-mouse 1:1000, Jackson Immunoresearch Laboratories). Finally, 3,3′,5,5′-tetramentylbenzidine (KPL) was added, and after 30 minutes the reaction was stopped with 1 M phosphoric acid and the optical density determined at 450 nm. The optical density of each well was plotted against the protein concentration and the linear portion of the curve assessed for each sample. The assay was linear over an average 22- and 14-fold-range for grey and white matter, respectively. Samples were run twice, on different days, and mean values used for analyses. Between-run correlations were greater than 0.90. A serial dilution of a reference brain sample was run on each plate to compute a between-plate coefficient of variation. This coefficient of variation was calculated to be 5.1% and 5.7%. To compare immunoreactivity between samples and regions, the amount of protein required to give an optical density reading of 0.4 was used for both grey and white matter. We excluded 2 tissue samples from the white matter analysis and 1 from the grey matter analysis because an optical density reading of 0.4 did not fall within the linear range. The amount of protein required to give an optical density of 0.4 is inversely related to the amount of target antigen present; therefore, low values indicate high amounts of apoE.
ApoE Western blot
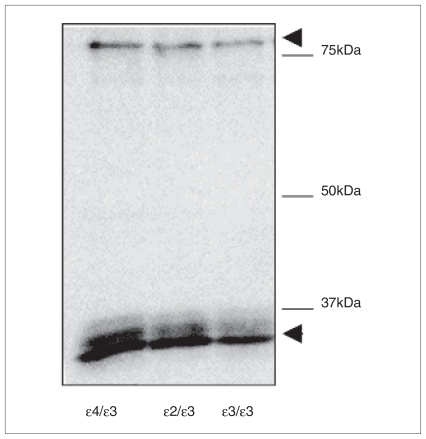
We conducted an immunoblot experiment to confirm the specificity of the antibody. Briefly, 20 μg of brain homogenate from 3 samples (ɛ2/ɛ3, ɛ3/ɛ3, ɛ4/ɛ3) was separated on a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel. After transfer to polyvinylidine fluoride (PVDF) membrane (Bio-Rad), the blot was incubated with primary antibody (WU E-14, 1:10) overnight. The blot was further incubated with peroxidase-conjugated secondary antibody (goat anti-mouse 1:2000, Jackson Immunoresearch Laboratories) for 1 hour. Then ECL reagent (GE Healthcare) was added, and blots imaged using a LAS-3000 imager (Fuji-film). Using this antibody, we were able to detect a doublet at the expected molecular weight of about 36 kDa (Fig. 1), as well as a heavier band at about 80kDa. Doublets at 36kDa have been described previously and are thought to result from apoE sialylation.8,31 In addition, apoE complexes that are not dissociated by SDS have been reported at a molecular weight of about 80kDa.31,32
Fig. 1.
Western blot of apoE immunoreactivity in tissue samples of human dorsolateral prefrontal cortex (APOE genotypes ɛ4/ɛ3, ɛ2/ɛ3, ɛ3/ɛ3). Homogenates (20 mg protein) were diluted in reducing Laemmli buffer and run on a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel. A doublet can be observed at about 36kDa, in addition to a second band at about 80kDa (arrowheads). It has been suggested that doublets may reflect apoE sialylation,8,31 whereas the band at about 80kDa represents an apoE complex not dissociated by SDS.31,32 No qualitative differences in staining were observed among the 3 genotypes.
APOE genotyping: polymerase chain reaction–restriction fragment length polymorphism
We performed APOE genotyping using polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP), as described previously,33 but with slight modifications. Genomic DNA was isolated from 15–25 mg of brain tissue using a DNA purification kit (DNeasy Blood and Tissue Kit; Qiagen). A 318-bp fragment from the APOE gene was PCR–amplified in 50 μL containing 10 μL (0.1–0.4 ng) purified genomic DNA, 1× Qiagen PCR buffer, 0.35 μM each primer, 200 μM each dNTP, 2× Q-Solution and 1.5 U Qiagen Taq DNA polymerase. Two primers were used in the amplification: upstream primer E2mut (5′ ACT GAC CCC GGT GGC GGA GGA GAC GCG TGC) and downstream primer E3 (5′ TGT TCC ACC AGG GGC CCC AGG CGC TCG CGG). Primer E2mut differs from the genomic sequence at 1 position, which creates an additional AflIII recognition site in the amplified fragment. Reaction mixtures were incubated at 94°C for 3 minutes, subjected to 40 cycles of amplification (95°C, 20 s; 65°C, 40 s; 72°C, 45 s) and incubated at 72°C for 7 minutes. Restriction digests containing 15 μL amplification reaction (2.1 μL H2O, 0.15 μL BSA and either 2 μL Qiagen buffer #3 and 0.75 U Afl III or 2 μL Qiagen buffer #4 and 0.125 U Hae II) were incubated at 37°C overnight. The digested product was run on 4% agarose gels, stained with ethidium bromide and visualized under ultraviolet light. We deter-mined genotype by comparison with standards run on the same gel.
Statistical analysis
We assessed differences in age, postmortem interval (PMI) and brain pH among groups using a 1-way analysis of variance (ANOVA), with a significance level of α = 0.05. We used Shapiro-Wilk tests to determine whether ApoE and cholesterol measures conformed to a normal distribution. As data were not normally distributed (apoE grey matter W103 = 0.67, p < 0.001; apoE white matter W102 = 0.89, p < 0.001; cholesterol grey matter W104 = 0.88, p < 0.001; cholesterol white matter W104 = 0.93, p < 0.001), we compared cholesterol and apoE levels among groups using the nonparametric Kruskal–Wallis H test. We further compared ApoE and cholesterol levels between grey and white matter in the whole-brain series using Wilcoxon signed ranks test. Spearman rank correlations were performed to assess the influence of age, PMI and brain pH on apoE and cholesterol levels, whereas Mann–Whitney tests were used to investigate the relations between apoE and cholesterol levels and sex or brain hemisphere. In addition, in the 2 patient groups, we assessed correlations between apoE and cholesterol levels and age at onset, duration of illness and lifetime antipsychotic dose using Spearman rank analyses. Finally, we used Mann–Whitney or Kruskal–Wallis H tests to examine whether alcohol use (none or social v. past or present moderate or heavy use) or illicit drug use (none or social v. past or present moderate or heavy use) was related to cholesterol or apoE levels, to assess the effect of death by suicide and to compare the effect of different treatment conditions (i.e., typical antipsychotic, atypical antipsychotic, no antipsychotic) on cholesterol and apoE levels.
We assessed differences in APOE genotype among groups using the χ2 test. Additionally, to investigate how apoE and cholesterol levels varied as a function of genotype, we compared cholesterol and apoE levels among an ɛ2+ group (comprising ɛ2/ɛ2 and ɛ2/ɛ3 genotypes) an ɛ3+ group (comprising ɛ3/ɛ3 genotype) and an ɛ4+ group (comprising ɛ3/ɛ4 and ɛ4/ɛ4 genotypes) using the nonparametric Kruskal–Wallis H test with post-hoc Mann–Whitney tests. The 1 tissue sample with APOE ɛ2/ɛ4 genotype was not included in this analysis. We computed Spearman rank correlations to assess the relation between apoE and cholesterol, both in the whole sample and after stratification by diagnosis and genotype. We performed all statistical analyses using SPSS 17.0.
As the ELISA data values (amount of protein required to give an optical density reading of 0.4) are inversely related to the amount of target antigen present in a sample, for graphing purposes, we employed a simple algebraic transformation to plot the data in the intuitively simpler fashion where greater values represent greater amounts of the target antigen, as previously described.34
Results
Association between apoE and cholesterol levels and clinical and demographic variables
Detailed demographic, postmortem and clinical information is reported in Table 1. Groups did not differ in age or PMI, although there were a higher proportion of tissue samples from women in the bipolar disorder group compared with the schizophrenia and control groups (χ22= 7.5, p = 0.024, Table 1). Brain pH also differed among groups (F2,103 = 4.174, p = 0.018), being slightly lower in both the bipolar disorder and schizophrenia groups.
Table 1.
Summary of demographic, postmortem and medication variables in the control and psychiatric groups
| Variable | Control, n = 35 | Schizophrenia, n = 35 | Bipolar disorder, n = 34 |
|---|---|---|---|
| Demographic | |||
| Age at death, mean (SD) yr | 44.2 (7.6) | 42.6 (8.5) | 45.4 (10.7) |
| Sex, male:female | 26:9 | 26:9 | 16:18† |
| Postmortem characteristic | |||
| Postmortem interval, mean (SD) h | 29.4 (12.9) | 31.4 (15.5) | 37.9 (18.6) |
| Brain pH, mean (SD) | 6.6 (0.3) | 6.5 (0.2) | 6.4 (0.3)† |
| Brain hemisphere, right:left | 16:19 | 17:18 | 19:15 |
| Clinical | |||
| Cause of death | |||
| Cardiopulmonary | 33 | 21 | 7 |
| Suicide | 7 | 15 | |
| Other | 2 | 7 | 12 |
| Age at onset, mean (SD) yr | — | 21.3 (6.1) | 25.3 (9.2) |
| Duration of disorder, mean (SD) yr | — | 21.29 (10.15) | 20.15 (9.462) |
| Antipsychotic dose, median (range) mg* | — | 48000 (50–400000) | 3000 (0–13000) |
| Alcohol history, none/mild:moderate or heavy past or present use | 30:5 | 17:18 | 12:21 (1 missing) |
| Drug history, none/mild:moderate or heavy past or present use | 35:0 | 18:15 (2 missing) | 15:19 |
SD = standard deviation.
Antipsychotic dose is lifetime dose in chlorpromazine milligram equivalents.
p < 0.05, statistically significant differences between the psychiatric and control groups.
The apoE and cholesterol levels are presented in Table 2. We observed no significant differences in either apoE or cholesterol levels among the groups in grey or white matter. Examining the whole series, levels of apoE or cholesterol did not correlate with age or pH, although when stratified by diagnosis, apoE levels in white matter correlated with brain pH in the schizophrenia group (rho = −0.397, p = 0.020) but not in the control or bipolar disorder groups. Postmortem interval correlated weakly with apoE levels in white matter (rho = −0.207, p = 0.037), but not in grey matter. Neither apoE nor cholesterol levels differed as a function of sex or brain hemisphere. However, when stratified by diagnosis, we observed a significant effect of hemisphere on grey matter cholesterol levels in the schizophrenia group (Z = −2.442, p = 0.014) but not in the control or bipolar disorder groups.
Table 2.
Summary of apoE and cholesterol levels in grey matter and white matter in the control and psychiatric groups
| Control |
Schizophrenia |
Bipolar disorder |
||||
|---|---|---|---|---|---|---|
| Molecule, median (IQR) level in brain | Grey matter | White matter | Grey matter | White matter | Grey matter | White matter |
| ApoE, arbitrary units* | 40.4 (54.4) | 406.3 (169.4) | 47.5 (40.3) | 391.3 (376.1) | 36.6 (50.9) | 499.6 (376.5) |
| Cholesterol, μg/mg protein | 124.6 (95.5) | 337.8 (53.8) | 117.5 (80.1) | 332.0 (58.2) | 125.3 (94.0) | 332.3 (48.9) |
ApoE = apolipoprotein E; IQR = interquartile range.
Enzyme-linked immunosorbent assay (ELISA) data values (protein amount required to produce a fixed optical density of 0.4) are inversely related to the amount of antigen present; therefore, lower values represent higher apoE concentrations. Median and interquartile range values are presented as data do not conform to a normal distribution. The ELISA data values (protein amount required to produce a fixed optical density of 0.4) are inversely related to the amount of antigen present; therefore, lower values represent higher apoE concentrations.
In the psychiatric groups, we found no relations between apoE or cholesterol levels and age at illness onset or duration of illness. Death by suicide had no influence on cholesterol or apoE levels, with this finding remaining when cases were stratified into violent and nonviolent suicides. In grey matter, cholesterol concentration did not differ between individuals with a history of no alcohol use or only social alcohol use (n = 29) and those with moderate or heavy past or present alcohol use (n = 39). However, in white matter we found significantly lower white matter cholesterol levels in the moderate/heavy use group (Mann–Whitney Z = −2.139, p = 0.032). Neither cholesterol nor apoE levels differed between individuals with history of no illicit drug use or only social use (n = 33) and those with moderate or heavy past or present drug use (n = 34) in either grey or white matter. With regards to the effect of medication on levels of apoE or cholesterol, lifetime antipsychotic dose did not correlate with either apoE or cholesterol levels in grey or white matter. Furthermore, neither the type of antipsychotic prescribed at the time of death, nor the presence of antipsychotics at death was related to apoE or cholesterol levels.
ApoE and cholesterol levels in grey versus white matter
Taking the 3 study groups as a whole, we found significantly higher levels of apoE in grey matter compared with that in white matter of the same region (Wilcoxon Z = −8.741, p < 0.001). Conversely, cholesterol levels were significantly higher in white matter compared with grey matter (Wilcoxon Z = −8.704, p < 0.001). As reported in Table 2, we observed a grey to white matter ratio of 10:1 for apoE and 0.37:1 for cholesterol.
APOE allele effect on apoE and cholesterol levels
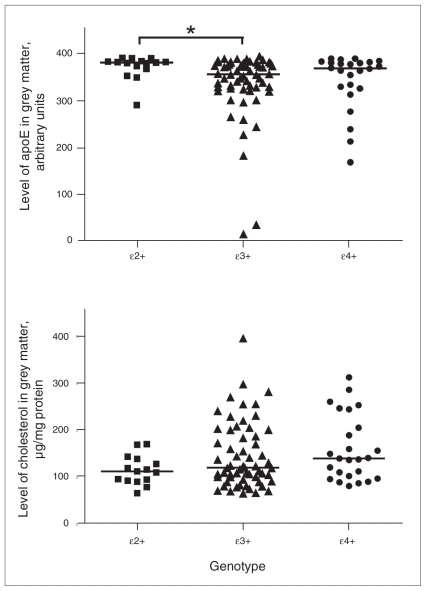
We observed no significant differences in APOE genotypic or allelic frequency among the 3 groups (Table 3). Stratifying the whole sample according to presence of APOE alleles (ɛ2+, n = 15; ɛ3+, n = 60; ɛ4+, n = 24), significant differences in apoE expression in grey matter were identified among the 3 genotypes (χ22= 7.880, p = 0.019, Fig. 2A). Post-hoc analyses revealed that levels of apoE were 105% higher in the ɛ2+ group compared with the ɛ3+ group and 52% greater compared with the ɛ4+ group (ɛ2+ v. ɛ3+ Z = −2.815, p = 0.005; ɛ2+ v. ɛ4+ Z = −1.848, p = 0.066). There were no significant differences between the ɛ3+ and ɛ4+ groups. Conversely, we observed no significant relation between genotype and cholesterol levels. Whereas cholesterol levels were 19% lower in the ɛ2+ carriers compared with ɛ4+ carriers, this did not reach statistical significance (Z = −1.914, p = 0.057, Fig. 2B). In white matter, no relation was identified between genotype and apoE or cholesterol levels.
Table 3.
Summary of genotype and allele frequencies in the control and psychiatric groups
|
APOE genotype;* no. (%) |
Allele frequency; no. (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 2/2 | 2/3 | 3/3 | 3/4 | 4/4 | 2/4 | Total no. | ɛ2 | ɛ3 | ɛ4 | Total no. |
| All | 2 (2) | 13 (12.8) | 60 (59.4) | 24 (23.8) | 1 (1) | 1 (1) | 101 | 18 (8.9) | 157 (77.7) | 27 (13.4) | 202 |
| Control | 0 (0) | 8 (22.9) | 19 (54.3) | 7 (20.0) | 1 (2.9) | 0 (0) | 35 | 8 (11.4) | 53 (75.7) | 9 (12.9) | 70 |
| Schizophrenia | 2 (5.7) | 3 (8.6) | 19 (54.3) | 8 (22.9) | 0 (0) | 1 (2.9) | 33 | 8 (12.1) | 49 (74.2) | 9 (13.6) | 66 |
| Bipolar disorder | 0 (0) | 2 (5.9) | 22 (64.7) | 9 (26.5) | 0 (0) | 0 (0) | 33 | 2 (5.9) | 55 (80.9) | 9 (13.2) | 66 |
APOE = apolipoprotein E genotype.
Three genotypes are missing owing to unclear resolution of amplified DNA fragments.
Fig. 2.
Levels of apoE and cholesterol in grey matter: effect of APOE allele. The ɛ2+ group comprised ɛ2/ɛ2 and ɛ2/ɛ3 genotypes; ɛ3+ group comprised ɛ3/ɛ3 genotype; e4+ group comprised ɛ4/ɛ4 and ɛ4/ɛ4 genotypes; ɛ2/ɛ4 genotype was excluded. (A) ApoE levels (arbitrary units) stratified by APOE allele status (ɛ2+, n = 15; ɛ3+, n = 60; ɛ4+, n = 24) in grey matter in the total sample series. Bars represent median values. Results of the Kruskal–Wallis H test indicate that apoE levels differ among genotypes (χ22 = 7.880, p = 0.019), with higher levels in the ɛ2+ group compared with ɛ3+ or ɛ4+ groups (ɛ2+ v. ɛ3+ Z = −2.815, p = 0.005, as indicated by the asterisk; ɛ2+ v. ɛ4+ Z = −1.848, p = 0.07). Note: As the enzyme-linked immunosorbent assay data values (amount of protein required to give an optical density reading of 0.4) are inversely related to the amount of target antigen present in a sample, for graphing purposes we employed a simple algebraic transformation (raw value x [−1] + constant) to plot the data in the intuitively simpler fashion where greater values represent greater amounts of the target antigen, as previously described.34 (B) Cholesterol levels (μg/mg of protein) stratified by APOE allele status (ɛ2+, n = 15; ɛ3+, n = 60; ɛ4+, n = 25) in grey matter in the total sample. Bars represent median values. Results of Kruskal–Wallis H tests indicate that cholesterol does not differ significantly between genotypes.
Correlation between apoE and cholesterol: effect of diagnosis and APOE allele
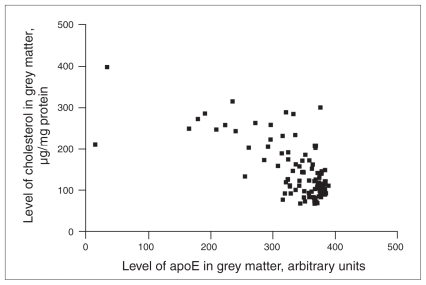
Cholesterol and apoE levels were inversely correlated in grey matter (rho = −0.500, p < 0.001 Fig. 3). Correlation analyses were computed after stratification by study group and by APOE allele status. The control group showed the strongest correlation (rho = −0.625, p < 0.001), followed by the bipolar disorder group (rho = −0.521, p = 0.002) and the schizophrenia group (rho = −0.339, p = 0.05). The APOE ɛ4 carriers showed the strongest correlation between levels of cholesterol and apoE protein in grey matter (rho = −0.715, p < 0.001), followed by the APOE ɛ3/ɛ3 group (rho = −0.494, p < 0.001). The APOE ɛ2 carriers showed no statistically significant correlation. We also observed an inverse correlation between cholesterol and apoE in white matter (rho = −0.269, p = 0.006). This did not reach statistical significance for any individual psychiatric group. We observed significant correlations in the APOE ɛ3/ɛ3 (rho = −0.321, p = 0.013) and the APOE ɛ4 carriers (rho = −0.391, p = 0.059), but again the APOE ɛ2 carriers showed no statistically significant relation between cholesterol and apoE levels.
Fig. 3.
Correlation between apoE (arbitrary units) and cholesterol levels (μg/mg of protein) in grey matter in the total sample (n = 103). As the enzyme-linked immunosorbent assay data values (amount of protein required to give an optical density reading of 0.4) are inversely related to the amount of target antigen present in a sample, for graphing purposes we employed a simple algebraic transformation to plot the data in the intuitively simpler fashion where greater values represent greater amounts of the target antigen, as previously described.34
Discussion
ApoE and cholesterol levels in people with schizophrenia and bipolar disorder
Median levels of apoE and cholesterol did not differ significantly in the schizophrenia or bipolar disorder groups relative to the control group in either grey or white matter, although we do report a 15% decrease in apoE levels in grey matter in the schizophrenia group and a 19% decrease in apoE in white matter in the bipolar disorder group. In 2 previous studies of apoE expression in the major psychiatric disorders, apoE levels were higher in the prefrontal cortex (BA 9 and 46) in people with schizophrenia,20,21 whereas apoE levels were increased in BA9 but decreased in BA 10 in a small series involving people with bipolar disorder.21 Several possible reasons might account for the discrepancy between our data and that of the 2 previous studies. First, clinical and demographic characteristics were different; for example, our tissue samples came from younger individuals and included both bipolar I and II disorders. Furthermore, in the previous studies, apoE was only measured in the left hemisphere, thus raising questions about the presence of a laterality effect. In addition, the methodology used to measure apoE differed between the studies. We used an ELISA assay, which targets the protein of interest in its native conformational state, whereas the previous studies used Western blotting, which targets the protein’s epitope in an unfolded state. In agreement with our previous study of visual association cortex,3 we found that cholesterol levels were not significantly different in people with either bipolar disorder or schizophrenia.
ApoE and cholesterol levels in grey versus white matter
Our data indicate that cholesterol levels are higher in white matter than in grey matter, whereas apoE levels are higher in grey matter than in white matter. Within the brain, about 70% of cholesterol is estimated to be present within myelin.5 Therefore, high levels of cholesterol in white matter are to be expected. We found a grey to white matter ratio of about 0.37:1 for cholesterol and 10:1 for apoE. Previous studies have reported grey to white matter cholesterol ratios of about 0.44:1.19,35
To our knowledge, this is the first study to assess the relation between cholesterol and apoE in the major psychiatric disorders. The brain depends on a highly efficient apolipoprotein-dependent recycling mechanism to maintain cholesterol homeostasis. Our results show that apoE levels are substantially lower in white matter, which may be explained by the fact that cholesterol turnover in the myelin pool is extremely low in the nonpathologic adult brain.5 On the other hand, high levels of apoE in grey matter may point to a more dynamic pool of cholesterol. This is consistent with the wide array of dynamic cellular events occurring in grey matter that require cholesterol trafficking such as synaptic transmission, synaptic plasticity or neuronal maintenance. Alternatively, other apolipoproteins may be involved in cholesterol transport in white matter.36 In grey, and to a lesser extent white matter, we observed an inverse relation between apoE and cholesterol levels. The inverse association may seem counterintuitive. However, we measured free cholesterol (i.e., that bound to membranes), whereas cholesterol is transformed into the esterified form once loaded into the core of apolipoproteins.37 Therefore it seems plausible that when apoE levels are high, more cholesterol is in the esterified form rather than the free form. Nonetheless, this remains to be resolved. Of note, the control and bipolar disorder groups showed a strong correlation between grey matter cholesterol and apoE levels, whereas a much weaker correlation was observed in the schizophrenia group. Although the exploratory nature of the analysis prevents any causal interpretation, this finding may reflect a pathological process occurring in people with schizophrenia that interferes with the lipidation of apoE. Alterations in levels of ApoA1, ApoL and ApoD have been reported in patents with schizophrenia38,39 and may indicate a general abnormality of lipid transport in people with this disorder.
APOE allele effect on apoE and cholesterol levels
We also observed a relation between genotype, apoE and cholesterol levels in grey matter. In this study, apoE levels were higher in APOE ɛ2 carriers compared with APOE ɛ3 carriers and to a lesser extent APOE ɛ4 carriers. Conversely, cholesterol levels were lower in APOE ɛ2 carriers compared with APOE ɛ4 carriers. This result points to a genotypic regulation of apoE and cholesterol levels and is consistent with a recent animal study reporting that apoE levels in the brain differ depending on genotype, with ɛ2/2 > ɛ3/3 > ɛ4/4.27 It is not clear what mechanisms may be behind this, although apoE4 is more prone to remain in a partially unfolded tertiary conformation, making it more susceptible to protease digestion.40
In addition, genotype also influenced the strength of the relation between cholesterol and apoE levels. The APOE ɛ4 carriers had the strongest negative correlation followed by APOE ɛ3 carriers, with APOE ɛ2 carriers having no statistically significant correlation. This differential strength of association may be related to the different lipidation capacity of the different isoforms.41
APOE ɛ4 genotype and risk for schizophrenia and bipolar disorder
Our study found allele and genotype frequencies similar to those present in the general population.42 Whereas the number of ɛ2 carriers was low in the bipolar disorder group, this was not statistically significant, although we may be under-powered to detect such an effect. Whereas initial reports identified an increase in ɛ4 allele frequency in patients with schizophrenia,43 later studies have failed to replicate this.44–47 However, a recent meta-analysis did find that the APOE ɛ4 genotype is a risk factor for schizophrenia, albeit of small effect.22 The ɛ4 allele has also been associated with early onset bipolar disorder with psychotic symptoms.23 Although, APOE ɛ4 is a major genetic risk factor for Alzheimer disease,48 the mechanism by which carrying the APOE ɛ4 allele translates to neuropathology remains elusive. It has been suggested that the APOE ɛ4 allele results in a lower protein expression, which in turn could impair cholesterol homeostasis and synaptic plasticity. Since abnormalities in synaptic proteins have been reported in people with schizophrenia and bipolar disorder,1 further investigation of the relations between synaptic plasticity, genotype and cholesterol homeostasis is warranted.
Limitations
Several limitations of the present study need to be addressed. First, as our primary variables of interest were not normally distributed, our statistical analyses relied on nonparametric tests. Thus, it was not possible to control for potential confounding variables. One such important confounding factor is exposure to antipsychotic medication. We observed no significant correlation between apoE or cholesterol levels and life-time antipsychotic dose, and there were no differences in apoE or cholesterol levels among patients treated with atypical antipsychotics, typical antipsychotics or no antipsychotics before death. Our data do not support those of a previous study that reported a significant reduction in apoE levels in grey matter in rats treated with haloperidol.20 Although animal studies are a necessary strategy, it is important to bear in mind differences to human physiology when translating research results. Specifically, rodents do not have the 3 common human allele variants for APOE (i.e., ɛ2, ɛ3, ɛ4), and this difference has an impact on apoE and cholesterol metabolism in rats.49 The effect of antipsychotic treatment on apoE and cholesterol in the human brain remains open to further research.
A second potentially important factor is the presence of lipid-lowering medications in these patients. Risk for cardiovascular disease and dyslipidemia is elevated in patients with schizophrenia relative to the general population.50,51 Unfortunately, information on whether patients were prescribed statins or other lipid-lowering drugs before death is not available to us. However, animal studies indicate that administration of high doses of simvastatin or pravastatin does not change total brain cholesterol levels52,53 Furthermore, a study of human volunteers showed that administration of a high-dose of either simvastatin or pravastatin did not change plasma 24(S)-hydroxycholesterol to cholesterol ratio, a surrogate marker of brain cholesterol homeostasis.54 In addition, moderate or heavy past or present alcohol use was reported in a significant proportion of the psychiatric patients. In this group of patients, we found a significant deficit in cholesterol levels in white matter but not in grey matter relative to patients reported to have no or only social alcohol use. This finding is contrary to that of Olsson and colleagues,55 who reported no change in cholesterol levels in either grey or white matter in a small series of individuals with alcoholism. Finally, the number of APOE ɛ2 and ɛ4 carriers was relatively small and precluded examination of the relation between apoE and cholesterol levels between psychiatric groups stratified by genotype.
Conclusion
To our knowledge, our study provides for the first time apoE and cholesterol measurements in white matter in people with schizophrenia and bipolar disorder. Whereas diagnostic effects were not obvious, our data indicate that white matter is rich in cholesterol but apoE is rather scarce, whereas, conversely, apoE is abundant in grey matter but cholesterol is present at substantially lower amounts. In addition, we identified an inverse relation between these molecules in both grey and white matter. We also provide evidence for genotype-dependent regulation of apoE and cholesterol in human grey matter. The impact of APOE polymorphisms on lipid homeostasis in people with psychiatric disorders requires further investigation.
Acknowledgements
Postmortem brain tissue was donated by The Stanley Medical Research Institute’s brain collection. We would like to acknowledge Jennifer Chan for technical assistance. Funding for this study was provided by a BC Mental Health and Addiction Services Clinical Research Fellowship (to Dr. Vila-Rodriguez), BC Mental Health and Addiction Services, Michael Smith Foundation for Health Research, Canadian Institutes of Health Research (MOP-14037, NET-54013, MOP-81112) and the Stanley Medical Research Institute (to Drs. Honer and Beasley).
Footnotes
Competing interests: None declared for Drs. Vila-Rodriguez, Innis and Wellington. Dr. Honer is a paid board member of the Alberta Heritage Medical Research Foundation, Janssen, Novartis and Astra-Zeneca and a voluntary board member of In Silico Biosciences; he has received or will receive grants from Janssen, Eli Lilly and Astra-Zeneca; he has received honoraria from Partners in Psychiatry, Hotel Dieu Hospital (Kingston), Rush University, the Capital Mental Health Association (Victoria), Université de Montréal, Janssen and AstraZeneca. Dr. Beasley declared having received an honorarium from the Ontraio Mental Health Foundation and a Winter Conference on Brain Research travel fellowship.
Contributors: Drs. Vila-Rodriguez, Honer and Beasley designed the study. Drs. Vila-Rodriguez, Innis, Wellington and Beasley acquired the data, which Drs. Vila-Rodriguez, Honer and Beasley analyzed. Drs. Vila-Rodriguez and Beasley wrote the article, which Drs. Vila-Rodriguez, Honer, Innis and Wellington reviewed. All authors approved the article’s publication.
References
- 1.Honer WG, Falkai P, Chen C, et al. Synaptic and plasticity-associated proteins in anterior frontal cortex in severe mental illness. Neuroscience. 1999;91:1247–55. doi: 10.1016/s0306-4522(98)00679-4. [DOI] [PubMed] [Google Scholar]
- 2.Rosoklija G, Toomayan G, Ellis SP, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–56. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- 3.Beasley CL, Honer WG, Bergmann K, et al. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7:449–55. doi: 10.1111/j.1399-5618.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 4.Tkachev D, Mimmack ML, Ryan MM, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 5.Björkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24:806–15. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 6.Das HK, McPherson J, Bruns GA, et al. Isolation, characterization, and mapping to chromosome 19 of the human apolipoprotein E gene. J Biol Chem. 1985;260:6240–7. [PubMed] [Google Scholar]
- 7.Paik YK, Chang DJ, Reardon CA, et al. Nucleotide sequence and structure of the human apolipoprotein E gene. Proc Natl Acad Sci U S A. 1985;82:3445–9. doi: 10.1073/pnas.82.10.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitas RE, Boyles JK, Lee SH, et al. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917:148–61. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- 9.Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–7. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 10.Nathan BP, Jiang Y, Wong GK, et al. Apolipoprotein E4 inhibits, and apolipoprotein E3 promotes neurite outgrowth in cultured adult mouse cortical neurons through the low-density lipoprotein receptor-related protein. Brain Res. 2002;928:96–105. doi: 10.1016/s0006-8993(01)03367-4. [DOI] [PubMed] [Google Scholar]
- 11.Handelmann GE, Boyles JK, Weisgraber KH, et al. Effects of apolipoprotein E, beta-very low density lipoproteins, and cholesterol on the extension of neurites by rabbit dorsal root ganglion neurons in vitro. J Lipid Res. 1992;33:1677–88. [PubMed] [Google Scholar]
- 12.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–12. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Boyles JK, Zoellner CD, Anderson LJ, et al. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest. 1989;83:1015–31. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ignatius MJ, Shooter EM, Pitas RE, et al. Lipoprotein uptake by neuronal growth cones in vitro. Science. 1987;236:959–62. doi: 10.1126/science.3576212. [DOI] [PubMed] [Google Scholar]
- 15.Mauch DH, Nagler K, Schumacher S, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–7. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 16.Brandrup E, Randrup A. A controlled investigation of plasma lipids in manic-depressives. Br J Psychiatry. 1967;113:987–92. doi: 10.1192/bjp.113.502.987. [DOI] [PubMed] [Google Scholar]
- 17.Mahadik SP, Mukherjee S, Correnti EE, et al. Plasma membrane phospholipid and cholesterol distribution of skin fibroblasts from drug-naive patients at the onset of psychosis. Schizophr Res. 1994;13:239–47. doi: 10.1016/0920-9964(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 18.Atmaca M, Kuloglu M, Tezcan E, et al. Serum leptin and cholesterol levels in schizophrenic patients with and without suicide attempts. Acta Psychiatr Scand. 2003;108:208–14. doi: 10.1034/j.1600-0447.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 19.Lalovic A, Levy E, Luheshi G, et al. Cholesterol content in brains of suicide completers. Int J Neuropsychopharmacol. 2007;10:159–66. doi: 10.1017/S1461145706006663. [DOI] [PubMed] [Google Scholar]
- 20.Dean B, Laws SM, Hone E, et al. Increased levels of apolipoprotein E in the frontal cortex of subjects with schizophrenia. Biol Psychiatry. 2003;54:616–22. doi: 10.1016/s0006-3223(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 21.Digney A, Keriakous D, Scarr E, et al. Differential changes in apolipoprotein E in schizophrenia and bipolar I disorder. Biol Psychiatry. 2005;57:711–5. doi: 10.1016/j.biopsych.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–34. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 23.Bellivier F, Laplanche JL, Schürhoff F, et al. Apolipoprotein E gene polymorphism in early and late onset bipolar patients. Neurosci Lett. 1997;233:45–8. doi: 10.1016/s0304-3940(97)00624-1. [DOI] [PubMed] [Google Scholar]
- 24.Beffert U, Cohn JS, Petit-Turcotte C, et al. Apolipoprotein E and β-amyloid levels in the hippocampus and frontal cortex of Alzheimer’s disease subjects are disease-related and apolipoprotein E genotype dependent. Brain Res. 1999;843:87–94. doi: 10.1016/s0006-8993(99)01894-6. [DOI] [PubMed] [Google Scholar]
- 25.Bertrand P, Poirier J, Oda T, et al. Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in alzheimer disease. Brain Res Mol Brain Res. 1995;33:174–8. doi: 10.1016/0169-328x(95)00097-c. [DOI] [PubMed] [Google Scholar]
- 26.Glöckner F, Meske V, Ohm TG. Genotype-related differences of hippocampal apolipoprotein E levels only in early stages of neuro-pathological changes in Alzheimer’s disease. Neuroscience. 2002;114:1103–14. doi: 10.1016/s0306-4522(02)00178-1. [DOI] [PubMed] [Google Scholar]
- 27.Riddell DR, Zhou H, Atchison K, et al. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28:11445–53. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington (DC): The Association; 1994. [Google Scholar]
- 29.Innis SM, Dyer RA. Brain astrocyte synthesis of docosahexaenoic acid from n-3 fatty acids is limited at the elongation of docosapen-taenoic acid. J Lipid Res. 2002;43:1529–36. doi: 10.1194/jlr.m200120-jlr200. [DOI] [PubMed] [Google Scholar]
- 30.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 31.Fagan AM, Holtzman DM, Munson G, et al. Unique lipoproteins secreted by primary astrocytes from wild type, apoE (−/ −), and human apoE transgenic mice. J Biol Chem. 1999;274:30001–7. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- 32.Haas C, Cazorla P, Miguel CD, et al. Apolipoprotein E forms stable complexes with recombinant Alzheimer’s disease beta-amyloid precursor protein. Biochem J. 1997;325:169–75. doi: 10.1042/bj3250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman J, Estupiñan J, Asherov A, et al. A simple efficient method for apolipoprotein E genotype determination. Neurology. 1996;46:1484–5. doi: 10.1212/wnl.46.5.1484-a. [DOI] [PubMed] [Google Scholar]
- 34.Barakauskas VE, Beasley CL, Barr AM, et al. A novel mechanism and treatment target for presynaptic abnormalities in specific striatal regions in schizophrenia. Neuropsychopharmacology. 2010;35:1226–38. doi: 10.1038/npp.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Söderberg M, Edlund C, Kristensson K, et al. Lipid compositions of different regions of the human brain during aging. J Neurochem. 1990;54:415–23. doi: 10.1111/j.1471-4159.1990.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 36.Rickhag M, Deierborg T, Patel S, et al. Apolipoprotein D is elevated in oligodendrocytes in the peri-infarct region after experimental stroke: influence of enriched environment. J Cereb Blood Flow Metab. 2008;28:551–62. doi: 10.1038/sj.jcbfm.9600552. [DOI] [PubMed] [Google Scholar]
- 37.LaDu MJ, Gilligan SM, Lukens JR, et al. Nascent astrocyte particles differ from lipoproteins in CSF. J Neurochem. 1998;70:2070–81. doi: 10.1046/j.1471-4159.1998.70052070.x. [DOI] [PubMed] [Google Scholar]
- 38.Huang JT, Wang L, Prabakaran S, et al. Independent protein-profiling studies show a decrease in apolipoprotein A1 levels in schizophrenia CSF, brain and peripheral tissues. Mol Psychiatry. 2008;13:1118–28. doi: 10.1038/sj.mp.4002108. [DOI] [PubMed] [Google Scholar]
- 39.Thomas EA, Dean B, Pavey G, et al. Increased CNS levels of apolipoprotein D in schizophrenic and bipolar subjects: implications for the pathophysiology of psychiatric disorders. Proc Natl Acad Sci U S A. 2001;98:4066–71. doi: 10.1073/pnas.071056198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrow JA, Segall ML, Lund-Katz S, et al. Differences in stability among the human apolipoprotein E isoforms determined by the amino-terminal domain. Biochemistry. 2000;39:11657–66. doi: 10.1021/bi000099m. [DOI] [PubMed] [Google Scholar]
- 41.Gong JS, Kobayashi M, Hayashi H, et al. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J Biol Chem. 2002;277:29919–26. doi: 10.1074/jbc.M203934200. [DOI] [PubMed] [Google Scholar]
- 42.Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world: Is APOE*4 a ‘thrifty’ allele? Ann Hum Genet. 1999;63:301–10. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- 43.Harrington CR, Roth M, Xuereb JH, et al. Apolipoprotein E type epsilon 4 allele frequency is increased in patients with schizophrenia. Neurosci Lett. 1995;202:101–4. doi: 10.1016/0304-3940(95)12218-4. [DOI] [PubMed] [Google Scholar]
- 44.Lan TH, Hong CJ, Chen JY, et al. Apolipoprotein E-epsilon 4 frequency in patients with schizophrenia. Biol Psychiatry. 1997;42:225–7. doi: 10.1016/s0006-3223(97)00241-2. [DOI] [PubMed] [Google Scholar]
- 45.Martorell L, Virgos C, Valero J, et al. Schizophrenic women with the APOE epsilon 4 allele have a worse prognosis than those without it. Mol Psychiatry. 2001;6:307–10. doi: 10.1038/sj.mp.4000855. [DOI] [PubMed] [Google Scholar]
- 46.Powchik P, Friedman J, Haroutunian V, et al. Apolipoprotein E4 in schizophrenia: a study of one hundred sixteen cases with concomitant neuropathological examination. Biol Psychiatry. 1997;42:296–8. doi: 10.1016/S0006-3223(97)00034-6. [DOI] [PubMed] [Google Scholar]
- 47.Town T, Fallin D, Crawford F, et al. Lack of association between the apolipoprotein E epsilon4 allele (APOE epsilon4) and chronic schizophrenia. Am J Med Genet. 1997;74:451–2. [PubMed] [Google Scholar]
- 48.Strittmatter WJ, Saunders AM, Goedert M, et al. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: Implications for alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:11183–6. doi: 10.1073/pnas.91.23.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Runge MS, Patterson C. Principles of molecular cardiology. Totowa (NJ): Humana Press; 2005. [Google Scholar]
- 50.Henderson DC. Schizophrenia and comorbid metabolic disorders. J Clin Psychiatry. 2005;66(Suppl 6):11–20. [PubMed] [Google Scholar]
- 51.Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70:1–17. doi: 10.1016/j.schres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Lütjohann D, Stroick M, Bertsch T, et al. High doses of simvastatin, pravastatin, and cholesterol reduce brain cholesterol synthesis in guinea pigs. Steroids. 2004;69:431–8. doi: 10.1016/j.steroids.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Thelen KM, Rentsch KM, Gutteck U, et al. Brain cholesterol synthesis in mice is affected by high dose of simvastatin but not of pravastatin. J Pharmacol Exp Ther. 2006;316:1146–52. doi: 10.1124/jpet.105.094136. [DOI] [PubMed] [Google Scholar]
- 54.Thelen KM, Laaksonen R, Päivä H, et al. High-dose statin treatment does not alter plasma marker for brain cholesterol metabolism in patients with moderately elevated plasma cholesterol levels. J Clin Pharmacol. 2006;46:812–6. doi: 10.1177/0091270006289851. [DOI] [PubMed] [Google Scholar]
- 55.Olsson NU, Harding AJ, Harper C, et al. High-performance liquid chromatography method with light-scattering detection for measurements of lipid class composition: analysis of brains from alcoholics. J Chromatogr B Biomed Appl. 1996;681:213–8. doi: 10.1016/0378-4347(95)00576-5. [DOI] [PubMed] [Google Scholar]