Abstract
Therapeutic interventions that incorporate training in mindfulness meditation have become increasingly popular, but to date, little is known about neural mechanisms associated with these interventions. Mindfulness-Based Stress Reduction (MBSR), one of the most widely used mindfulness training programs, has been reported to produce positive effects on psychological well-being and to ameliorate symptoms of a number of disorders. Here, we report a controlled longitudinal study to investigate pre-post changes in brain gray matter concentration attributable to participation in an MBSR program. Anatomical MRI images from sixteen healthy, meditation-naïve participants were obtained before and after they underwent the eight-week program. Changes in gray matter concentration were investigated using voxel-based morphometry, and compared to a wait-list control group of 17 individuals. Analyses in a priori regions of interest confirmed increases in gray matter concentration within the left hippocampus. Whole brain analyses identified increases in the posterior cingulate cortex, the temporo-parietal junction, and the cerebellum in the MBSR group compared to the controls. The results suggest that participation in MBSR is associated with changes in gray matter concentration in brain regions involved in learning and memory processes, emotion regulation, self-referential processing, and perspective taking.
Keywords: meditation, mindfulness, voxel based morphometry, gray matter, longitudinal, hippocampus, posterior cingulate
1. Introduction
Mindfulness meditation has been reported to produce positive effects on psychological well-being that extend beyond the time the individual is formally meditating. Over the last three decades mindfulness meditation practices have been increasingly incorporated into psychotherapeutic programs, to take advantage of these benefits (cf., Baer, 2003; Grossman et al., 2004). A large body of research has established the efficacy of these mindfulness-based interventions in reducing symptoms of a number of disorders, including anxiety (Roemer et al., 2008), depression (Teasdale et al., 2000), substance abuse (Bowen et al., 2006), eating disorders (Tapper et al., 2009), and chronic pain (Grossman et al., 2007), as well as improving well-being and quality of life (e.g., Carmody and Baer, 2008). Mindfulness meditation involves the development of awareness of present-moment experience with a compassionate, non-judgmental stance (Kabat-Zinn, 1990). It has been suggested that this process is associated with a perceptual shift (Carmody, 2009), in which one’s thoughts and feelings are recognized as events occurring in the broader field of awareness.
Neuroimaging studies have begun to explore the neural mechanisms underlying mindfulness meditation practice with techniques such as EEG (Davidson et al., 2003; Slagter et al., 2007) and functional MRI (Farb et al., 2007; Lutz et al., 2008; Farb et al., 2010; Goldin and Gross, 2010). Recently, several cross-sectional anatomical MRI studies have demonstrated that experienced meditators exhibit a different gray matter morphometry in multiple brain regions when compared to non-meditating individuals (Lazar et al., 2005; Pagnoni and Cekic, 2007; Hölzel et al., 2008; Luders et al., 2009; Vestergaard-Poulsen et al., 2009; Grant et al., 2010;). While most of the brain regions identified have been reported in only one of these studies, the divergent results are likely due to differences in participant characteristics, type of meditation, and data analysis methods (see Table 1). Group differences in the hippocampus and the right anterior insula, however, have each been identified in at least two of the studies. Furthermore, activation in both regions has been reported during meditative states (hippocampus (Lazar et al., 2000; Hölzel et al., 2007); insula (Farb et al., 2007; Lutz et al., 2008)). The hippocampus is known to be critically involved in learning and memory processes (Squire, 1992), and in the modulation of emotional control (Corcoran et al., 2005; Milad et al., 2007), while the insula has been postulated to play a key role in the process of awareness (Craig, 2009) - functions which have been shown to be important in the process and outcomes of mindfulness training (Bishop et al., 2004; Shapiro et al., 2006; Ortner et al., 2007).
Table 1.
Overview of morphometric studies on meditation
| Study | Meditation tradition |
N Meditators/ Controls |
Morphological measures |
Regions identified greater in meditators than controls |
|---|---|---|---|---|
| Lazar et al. (2005) | Insight | 20 / 15 | Cortical thickness |
Right anterior insula and right middle and superior frontal sulci |
|
| ||||
| Pagnoni & Cekic (2007) | Zen | 13 / 13 | Gray matter volume (VBM in SPM5) |
Meditators showed no age- related decline in the left putamen as compared to controls |
|
| ||||
| Hölzel et al. (2008) | Insight | 20 / 20 | Gray matter density (VBM in SPM2) |
Left inferior temporal lobe, right insula, and right hippocampus |
|
| ||||
| Vestergaard-Poulsen et al., (2009) | Tibetan Buddhist |
10 / 10 | Gray matter density & volume (VBM in SPM5) |
Medulla oblongata, left superior and inferior frontal gyri, anterior lobe of the cerebellum and left fusiform gyrus |
|
| ||||
| Luders et al. (2009) | Zazen, Vipassana, Samatha & others |
22 / 22 | Gray matter volume (VBM in SPM5) |
Right orbito-frontal cortex, right thalamus, left inferior temporal lobe, right hippocampus |
|
| ||||
| Grant et al. (2010) | Zen | 19/20 | Cortical thickness |
Right dorsal anterior cingulate cortex, secondary somatosensory cortex |
VBM: voxel-based morphometry (Gaser), SPM: Statistical Parametric Mapping, (Wellcome Department of Cognitive Neurology, London)
A growing body of literature has demonstrated that neural systems are modifiable networks and changes in the neural structure can occur in adults as a result of training. For example, longitudinal studies have shown task-specific increases in brain gray matter as an effect of acquisition of abstract information (Draganski et al., 2006), motor skills (Draganski et al., 2004), aerobic training (Colcombe et al., 2006), and cognitive skills (Ilg et al., 2008). Cross-sectional studies have established that differences in regional gray matter are associated with performance abilities (Mechelli et al., 2004; Milad et al., 2005), suggesting that an increase in gray matter corresponds to improved functioning in the relevant area. Studies of experienced meditators have also suggested the possibility of structural plasticity, but their cross-sectional designs did not exclude the possibility of pre-existing group differences, precluding causal conclusions. Here we report a longitudinal study of gray matter changes associated with a mindfulness-based intervention. The focus of the study was to identify brain regions that changed in association with participation in an eight-week Mindfulness-Based Stress Reduction course (MBSR; Kabat-Zinn, 1990). This group program aims to improve participants’ mindfulness and well-being, and reduce their levels of perceived stress. The study was an attempt to find objectively measurable neurological changes that could underlie the trait-changes associated with mindfulness practice. Changes in gray matter concentration were investigated using voxel-based morphometry. Focused analyses were conducted for the hippocampus and insula as our predefined regions of interest. Exploratory analyses were then performed on the entire brain and compared to a control group.
2. Methods
2.1 Participants
MBSR participants were recruited among individuals enrolled in four MBSR courses held at the Center for Mindfulness at the University of Massachusetts Medical School. The courses included physician- and self-referred individuals from across New England who were seeking stress reduction. Individuals were included in the study if they self-reported as physically and psychologically healthy and not taking any medications. Further inclusion criteria were: no meditation classes in the past six months, no more than four classes in the past five years, or ten classes in their lifetime; 25 to 55 years old; no contra-indications for MRI scanning (i.e., metallic implants, claustrophobia); commitment to attend all eight classes and perform the prescribed daily homework. Eighteen healthy, right-handed individuals were enrolled in the study, 8 male and 10 female, with a mean age of: 37.89 years (SD: 4.04 years). Due to discomforts during the first MRI scanning session, two participants did not return for the second session. The resulting sample consisted of 6 male and 10 female participants with a mean age of 38.0 years (SD: 4.1 years). Ethnicities were: 13 Caucasians, one Asian, one African American, and one multi-ethnic. Participants had an average of 17.7 years of education (SD: 1.9 years). Reimbursement for study participation was a discounted MBSR course fee.
The control sample consisted of 17 participants (11 male and 6 female) with a mean age of 39.0 years (SD: 9.2 years) and an average of 17.3 years of education (SD: 1.8 years). Ethnicities were: 13 Caucasians, two Asians, two African American, and one Hispanic. The groups did not differ in age (t (22.3) = .56; p = .58), or education (t (30) = −.56, p = .58). The study protocol was approved by the IRBs of Massachusetts General Hospital and the University of Massachusetts Medical School, and written informed consent was obtained from all participants. A previous publication that investigated neural correlates of changes in perceived stress (Hölzel et al., 2009) included data from this sample.
2.2 Intervention
The MBSR program has been described extensively elsewhere (Kabat-Zinn, 1990). Briefly, it consists of eight weekly group meetings lasting two and a half hours each, plus one full day (6.5 hours) during the sixth week of the course. Formal mindfulness training exercises aim at developing the capacity for mindfulness (awareness of present-moment experiences with a compassionate, non-judgmental stance) and include a body scan, mindful yoga, and sitting meditation. During the body scan attention is sequentially guided through the entire body, observing with non-judgmental awareness the sensations in each region and ending with an awareness of the body “as a complete whole”. The mindful yoga typically contains gentle stretching exercises and slow movements that are often coordinated with the breath, with emphasis placed on bringing full awareness to the moment-to-moment experience and a non-harming attitude towards the body. Participants are encouraged to investigate what feels appropriate for themselves and to honor their body’s limitations. Sitting meditation practices typically begin with awareness of the sensations of breathing, then evolve to include awareness of different modalities (such as sounds, sight, taste, other body sensations, thoughts and emotions). Later, emphasis is given to open awareness meditation, where the field of awareness is expanded to include anything that appears in consciousness, or a simple awareness of one’s presence in the here and now.
Participants received audio recordings containing 45-minute guided mindfulness exercises (body scan, yoga, and sitting meditation) that they were instructed to practice daily at home. To facilitate the integration of mindfulness into daily life, they were also taught to practice mindfulness informally in everyday activities such as eating, walking, washing the dishes, taking a shower etc. During classes the formal mindfulness exercises were practiced, questions relating to the practice of mindfulness in everyday life were clarified and didactic instruction given on using mindfulness for coping with stress in daily life. Historically, MBSR participants have reported a wide range of home practice compliance and for this reason study participants recorded the amount of time they spent engaged in mindfulness exercises each day.
2.3 Five facet mindfulness questionnaire
The Five Facet Mindfulness Questionnaire (FFMQ; Baer et al., 2006) is a 39-item scale to measure five factors of mindfulness: Observing (attending to or noticing internal and external stimuli, such as sensations, emotions, cognitions, sights, sounds, and smells), describing (noting or mentally labeling these stimuli with words), acting with awareness (attending to one’s current actions, as opposed to behaving automatically or absent-mindedly), non-judging of inner experience (refraining from evaluation of one’s sensations, cognitions, and emotions) and non-reactivity to inner experience (allowing thoughts and feeling to come and go, without attention getting caught up in them). Responses to the items are given on a 5-point Likert-type scale (1 = never or very rarely true, 5 = very often or always true). The five subscales have shown adequate to good internal consistency (Baer et al., 2006). Useable data from both time-points was obtained from 14 MBSR and 14 control participants.
2.4 MRI data collection and analysis
All participants were scanned at the Martinos Center for Biomedical Imaging in Charlestown, MA. MBSR participants were scanned during the 2 weeks before (Pre) and after (Post) participation in the program. Control participants were also scanned twice, approximately two months apart. There was an average time of 56.25 days (SD: 4.5 days) in between scanning sessions for the MBSR group and 65.67 days (SD: 11.22 days) for the control group. High-resolution MRI data were acquired with a Siemens Magnetom Avanto 1.5 T scanner with standard head coil. Data sets of the whole brain were collected using a T1 weighted MPRAGE-sequence, consisting of 128 sagittal slices (1.0 × 1.0 × 1.3 mm, TI = 1000 ms; TE = 3.39 ms; TR = 2730 ms). Image analysis was performed with voxel-based morphometry (VBM) tools within the SPM5 neuroimaging statistical software (Wellcome Department of Cognitive Neurology, London, www.fil.ion.ucl.ac.uk/spm/software/spm5/) based in MATLAB 7.1, release 14 (Mathworks Inc., Natick, MA, USA), using default settings unless otherwise specified. VBM permits an automated voxel-wise whole-brain statistical comparison of MRI scan (Ashburner and Friston, 2000). Images were manually aligned to the anterior commissure then segmented into gray and white matter in native space (i.e., before normalization, using the ‘Native Space’ segmentation option implemented in SPM5). For each individual, the (unmodulated) gray matter segmentations of the Pre and Post images were spatially co-registered. Normalization parameters were calculated for the Pre scan and were applied to both time points (trilinear interpolation, 2 × 2 × 2 mm), to make sure that regional differences between the images were not removed by scan-specific spatial normalization (Driemeyer et al., 2008; Ilg et al., 2008). Images were smoothed using an 8 mm full width at half maximum Isotropic Gaussian Kernel.
We computed exploratory whole brain analyses as well as region of interest (ROI) analyses. The ROI contained the bilateral hippocampi and bilateral insulae and was created using the WFU Pickatlas software (Maldjian et al., 2003) and based on the parcellation of Tzourio-Mazoyer et al. (Tzourio-Mazoyer et al., 2002). A paired t-test within the MBSR group was first performed in SPM5, in order to identify those brain regions with significantly increased gray matter concentration following participation in the MBSR program. Since our ROI analysis was spatially focused, we chose to correct for multiple comparisons within the ROI (bilateral hippocampi and insulae) using the voxel-wise method implemented in SPM5 . Given the very large number of voxels in the whole brain analysis, a voxel-wise method for preventing false positives seemed too conservative and leads to a substantial loss of statistical power (Forman et al., 1995; Friston et al., 1996). We therefore chose to use a cluster-wise method for the exploratory whole-brain analysis and corrected for multiple comparisons across the entire brain using the method implemented in SPM5 (Friston et al., 1994). In order to exceed the threshold of p <.05, clusters had to exceed a size of 250 voxels. Statistical parametric maps were initially thresholded with p = .01, uncorrected. P-values < .05, corrected for multiple comparisons were considered significant for both the exploratory whole brain analysis as well as the ROI analysis.
Following the paired t-test within the MBSR group, follow-up tests were then conducted within the identified regions to test for significance compared to the control group. Values from the identified clusters were extracted for each person and each time point using the Marsbar toolbox (Brett et al., 2002). A repeated measures ANOVA was then performed for each cluster in SPSS, with group (MBSR and control group) as between-subjects factor and time-point (Pre and Post) as within-subjects factor. Since groups were not identical in age and gender, these variables were controlled by entering them as nuisance variables.
3. Results
3.1 Amount of mindfulness practice
MBSR participants reported spending an average 22.6 hours (SD: 6.3 hours) engaged in formal homework exercises over the 8-week course (average = 27 minutes per day). In detail, the amount of body scan practice ranged between 335 to 1002 minutes (mean: 699 min, SD: 217 min), yoga between 103 and 775 minutes (mean: 327 min, SD: 194 min), and sitting meditation between 0 and 755 minutes (mean: 332 min, SD: 211 min). The three measures were not significantly correlated with each other: body scan and yoga: r = −0.042, P = 0.87; body scan and sitting: r = −0.26, P = 0.33; yoga and sitting: r = 0.49, P = 0.06, N = 16.
3.2 Improvements in mindfulness
Repeated measures ANOVAs confirmed significant group-by-time interactions for three of the five mindfulness subscales (acting with awareness: F (1,26) = 16.87, P < 0.001; observing: F (1,26) = 7.09, P = 0.013; non-judging: F (1,26) = 4.61, P = 0.041; describing: F (1,26) = 1.95, P = 0.175; non-reactivity: F (1,26) = 2.79, P = 0.107). Paired t-tests confirmed significant increases in the MBSR group (acting with awareness: t (13) = 3.665, P = 0.003; observing: t (13) = 4.218, P = 0.001; non-judging: t (13) = 3.580, P = 0.003), but not the control group (observing: t (13) = −0.698, P = 0.498; acting with awareness: t (13) = −1.991, P = 0.068; non-judging: t (13) = 0.657, P = 0.523; two-tailed). That is, MBSR participants significantly increased their mindfulness scores on these three scales.
3.3 Gray matter changes in a priori regions of interest
The paired t-test within the MBSR group identified a small cluster in the left hippocampus with increased gray matter concentration (peak voxel MNI coordinates x, y, z: −36, −34, −8; t (15) = 6.89; voxel level P = 0.014, corrected for multiple comparisons with FWE correction; cluster size k = 30; Figure 1). The averaged gray matter concentration within this cluster was then extracted for each individual at each time-point using the Marsbar toolbox and further analyses were performed in SPSS. A repeated measures ANOVA (2 groups × 2 time-points; age and gender as nuisance variables) showed a significant group x time interaction (F (1,29) = 4.92; P = .035). There was no difference in gray matter concentration within this cluster between the two groups at the Pre time-point (2-sample t-test for equal variances; t (31) = .06; P = .956) and the control group did not show a change in gray matter concentration from the Pre to Post time-point (paired t-test; t (16) = .343; P = .736). Pre to Post changes in the other regions of interest were not significant, and change in the a priori regions were also not correlated with the amount of mindfulness homework practice or with changes in the FFMQ. Furthermore, we performed a paired t-test within the control group in SPM5 and applied the same thresholds. No significant voxels were identified to increases or decreases in gray matter concentration from Pre to Post in the control group. To summarize, analyses of gray matter concentration changes in the regions of interest analysis supported significant increases in the left hippocampus in the MBSR group, confirming that structural changes in this region are detectable within eight weeks following the participation in this mindfulness training program.
Figure 1.
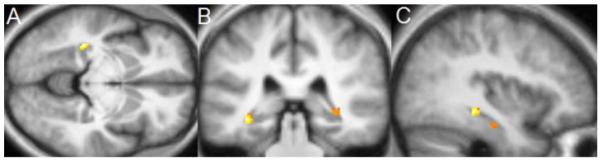
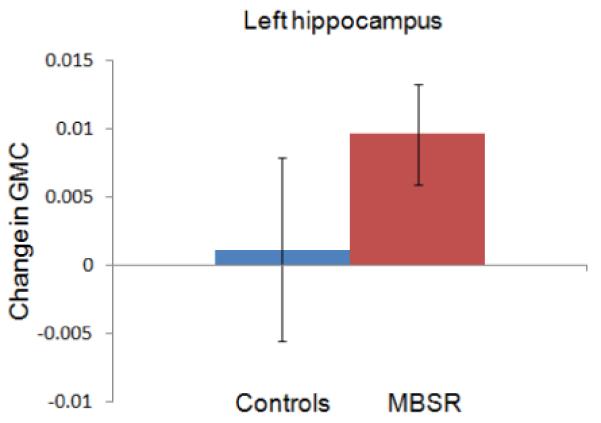
Region of interest analysis identifies gray matter concentration increases in the left hippocampus (MNI coordinates x = −36 (1A), y = −34 (1B), z = −8 (1C)) in the MBSR group. Voxels (thresholded at P = 0.01 and masked for the regions of interest) are overlaid over the group-averaged brain. 1D: Change in gray matter concentration (GMC) within the cluster in the left hippocampus from the Pre to the Post time-point in the MBSR and the control group; error bars show 95% confidence interval.
3.4 Whole brain analysis
Exploratory analysis of the entire brain (paired t-test in SPM5) revealed four clusters with significantly greater gray matter concentration at the Post compared to the Pre time-point in the MBSR group (Table 2). One cluster was located in the posterior cingulate cortex (PCC; Figure 2A, 3A), one in the left temporo-parietal junction (TPJ; Figure 2B, 3B), and two clusters were located in the cerebellum (Figures 2A and C, 3 C and D). One of the two clusters identified in the cerebellum was centered in the vermis and extended into the brainstem, encompassing several pontine nuclei in the brainstem. The second cerebellar cluster was located more laterally, including parts of the left lobule X and VIII, i.e., lateral parts of the posterior and flocculonodular lobe. No regions showed a significant decrease in gray matter concentration following the MBSR intervention.
Table 2.
Increase in gray matter concentration from Pre- to Post-MBSR training in the exploratory whole brain analysis within the MBSR group
| Region (peak of cluster) |
Cluster size k |
Cluster- level P- value |
MNI coordinates of the peak voxel (x, y, z) |
T of peak voxel |
|---|---|---|---|---|
| Posterior cingulate gyrus |
418 | 0.004 | −4, −34, 32 | 5.07 |
|
| ||||
| Cerebellum Lobule 8 - L |
329 | 0.018 | −28, −38, −48 | 5.31 |
|
| ||||
| Cerebellum, Vermis, Lobule 1- 2 |
499 | 0.001 | 4, −40, −24 | 5.03 |
|
| ||||
| Temporo-parietal junction (peak in middle temporal gyrus) |
291 | 0.036 | −50, −48, 20 | 5.08 |
P-values are corrected for multiple comparisons for the whole brain.
Figure 2.
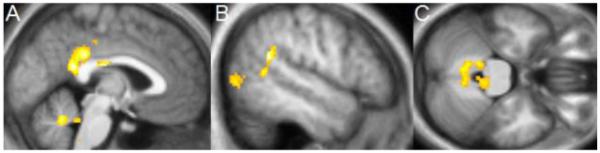
Increase in gray matter concentration in the MBSR group from Pre- to Post-intervention in the exploratory whole brain analysis. A: cluster in the posterior cingulate cortex and cerebellum (sagittal slice at x = −2); B: cluster in the left temporo-parietal junction (peak in the middle temporal gyrus; sagittal slice at x = −52); C: clusters in the cerebellum and brainstem (axial slice at z = −28). Significant clusters within the whole brain (clusters with P < .05, corrected for multiple comparisons across the entire brain, initial voxel-level threshold of P = 0.01) are overlaid over the group averaged normalized structural MPRAGE image.
Figure 3.
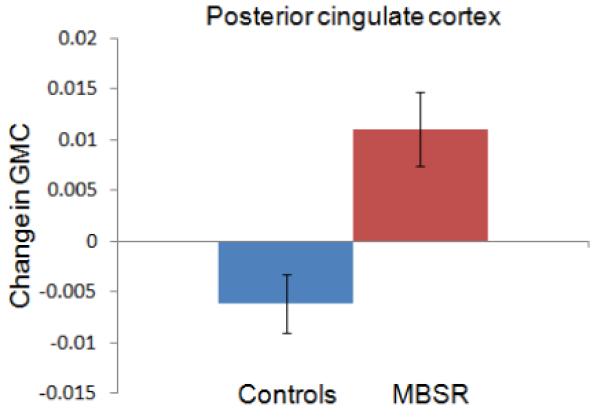
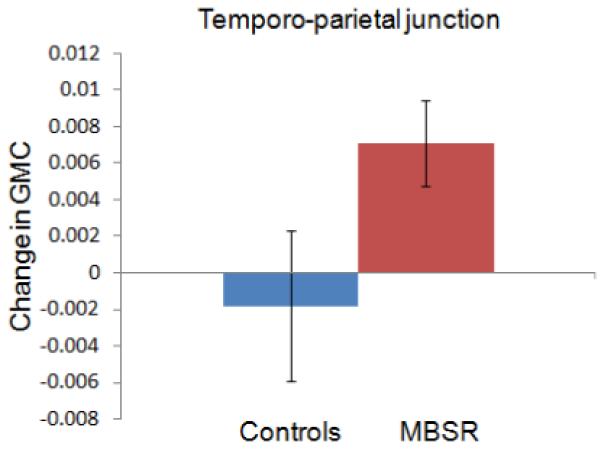
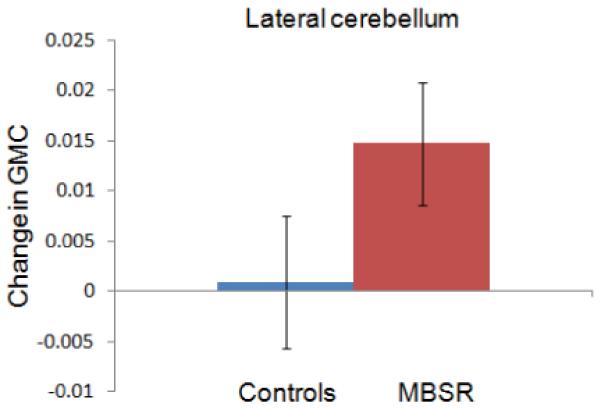
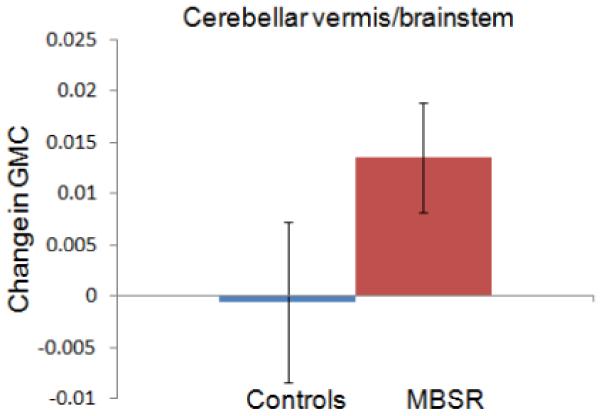
Change in gray matter concentration (GMC) within the clusters in the posterior cingulate cortex (3A), the temporo-parietal junction (3B), the lateral cerebellum (3C) and the cerebellar vermis/brainstem (3D) in the MBSR and control group. Error bars show 95% confidence interval.
For each of the four clusters, the averaged values were then extracted for each individual and each time-point using the Marsbar toolbox (Brett et al., 2002) and repeated measures ANOVAs (2 groups × 2 time-points) with age and gender entered as nuisance variables, were performed in SPSS. Group x time interactions were significant for all four regions, indicating that increases in gray matter concentration were significantly greater in the MBSR than the control group: PCC (F (1,29) = 50.124; P < .001), TPJ (F (1,29) = 11.456; P = .002), cerebellar vermis/brainstem (F (1,29) = 11.292; P = .002), lateral cerebellum (F (1,29) = 9.806; P = .004).
Change in gray matter concentration in the control group was not significant for the clusters in the TPJ (t (16) = −.87; P = .40), cerebellar vermis (t (16) = −.15; P = .88), or lateral cerebellum (t (16) = .273; P = .79), but there was a decrease in the PCC cluster (t (16) = −4.121; P = .001). Independent samples t-tests (with equal variances) at the Pre time-point indicated that the groups did not differ in gray matter concentration in the PCC (t (31) = .24; P = .81), TPJ (t (31) = .85; P = .40) and the lateral cerebellum (t (31) = .−1.41; P = .17), but the control group had greater gray matter concentration in the cerebellar vermis cluster (t (31) = 2.84; P = .008). The amount of homework practice and the change in mindfulness scores (FFMQ) were not correlated with changes in the identified clusters. Furthermore, we performed a paired t-test within the control group in SPM5 and applied the same thresholds. No significant clusters were identified to increases or decreases in gray matter concentration from Pre to Post in the control group. To summarize, exploratory analyses identified increases in gray matter concentration in regions in the PCC, TPJ, and cerebellum in the MBSR, but not the control group over the 8-week period, suggesting that participation in an MBSR course causes structural changes in these brain regions.
4. Discussion
This study demonstrates longitudinal changes in brain gray matter concentration following an eight-week Mindfulness-Based Stress Reduction course compared to a control group. Hypothesized increases in gray matter concentration within the left hippocampus were confirmed. Exploratory whole brain analyses identified significant increases in gray matter concentration in the PCC, TPJ, and the cerebellum.
The hippocampus has been postulated to play a central role in mediating some of the benefits of meditation, due to its involvement in the modulation of cortical arousal and responsiveness (Newberg and Iversen, 2003), and morphological differences between meditators and non-meditators in the hippocampus have previously been reported (Hölzel et al., 2008; Luders et al., 2009). The hippocampus also contributes to the regulation of emotion (Corcoran and Maren, 2001; Corcoran et al., 2005; Milad et al., 2007) and the structural changes in this area following mindfulness practice may reflect improved function in regulating emotional responding. In contrast to these increases, several pathological conditions (e.g., major depression (Sheline, 2000), post-traumatic stress disorder (Kasai et al., 2008)) are associated with decreased density or volume of the hippocampus. And while the precise mechanisms of hippocampal volume decrease are not known, a number of factors such as neuronal loss through chronic hypercortisolemia, glial cell loss, stress-induced reduction in neurotrophic factors, or stress-induced reduction in neurogenesis may contribute to this (Sheline, 2000). Furthermore, smaller hippocampi have also been shown to constitute a risk factor for the development of stress-related psychopathology (Gilbertson et al., 2002). However, the hippocampus is a region well known for its ability to remodel synapses and generate new neurons (Gage, 2002), and volume loss in this region seems to be reversible (Gould et al., 2000; Jacobs et al., 2000). For example, treatment with selective serotonin reuptake inhibitors – aside from improvement of stress disorder symptoms - has been found to lead to an increase in hippocampal volume (Vermetten et al., 2003) and it has been suggested that some of the behavioral effects of antidepressant treatment might depend on neurogenesis in the hippocampus (Santarelli et al., 2003). Future research will be needed to investigate whether similar neural mechanisms contribute to improvements in mental health following a medication-free behavioral intervention. We previously reported that changes in perceived stress were correlated with structural changes in the amygdala in a study that included subjects in the present study, and changes in stress were not correlated with changes in the hippocampus (Hölzel et al., 2009). However, the structural changes in the hippocampus identified here might be related to improvements in one of the other well-being-related variables that have been reported to improve following participation in an MBSR course.
The insula is known to be impacted in interoceptive/visceral awareness (Critchley et al., 2004) as well as in empathic responses (Singer et al., 2004). More generally, a recent review points to the fundamental role of the insula in human awareness, or consciousness (Craig, 2009). Given that mindfulness meditation constitutes training in interoception and conscious awareness, and based on the findings of previous studies which described functional as well as morphological differences in the insula between meditators and non-meditators (Hölzel et al., 2008; Lazar et al., 2005; Lutz et al., 2008), we hypothesized structural increases in the current study. However, the Pre-Post comparison within the MBSR group was not significant. It is possible that greater amounts of practice are required to produce structural changes in this region. It is also possible that previously identified differences between meditators and non-meditators were unrelated to the meditation training, but rather pre-existing. Furthermore, a recent study revealed that meditators did not show superior performance in an interoceptive task (Khalsa et al., 2008), challenging the assumption that enhanced cortical thickness and functional activation in the insula in meditators are related to better interoceptive awareness. Future studies that include a longer training program and assess interoceptive awareness Pre and Post intervention could help address these contradictory findings.
It has been suggested that the TPJ is a crucial structure for the conscious experience of the self, mediating spatial unity of self and body (Blanke et al., 2005), or embodiment (Arzy et al., 2006), and impaired processing at the TPJ may lead to the pathological experience of the self, such as disembodiment or out-of-body experiences (Blanke et al., 2005). Furthermore, the TPJ is also involved in social cognition, i.e., the ability to infer states such as desires, intentions, and goals of other people (Van Overwalle, 2009) and there is evidence of greater activation of this region during feelings of compassion in meditators (Lutz et al., 2008). Mindfulness training involves both the establishment of an awareness of oneself as a ‘complete whole’ (Kabat-Zinn, 1990), and the cultivation of compassion. The morphological changes in the TPJ might be associated with increases in compassion attributed to meditation training (Shapiro et al., 2005) and the cultivation of an embodied self.
Correspondingly, several studies suggest that the PCC is engaged when assessing the relevance or significance of a stimulus for oneself (Gusnard, 2001; Schmitz and Johnson, 2007) and it has been suggested to be particularly important for the integration of self-referential stimuli in the emotional and autobiographical context of one’s own person (Northoff and Bermpohl, 2004). These functions also are closely related to mindfulness practice, which involves the introspective observation of phenomenal experiences as they are encountered (Kabat-Zinn, 1990). Structural increases might be related to the repeated activation of this region during this process. Interestingly, the hippocampus, TPJ, and PCC (as well as parts of the medial prefrontal cortex not identified in the present study) form a brain network (Vincent et al., 2006) that supports diverse forms of self-projection (Buckner and Carroll, 2007), including remembering the past, thinking about the future (Schacter et al., 2007), and conceiving the viewpoint of others (Saxe and Kanwisher, 2003). These abilities have been suggested to share a common set of processes, by which autobiographical information is used adaptively to enable the perception of alternative perspectives (Buckner and Carroll, 2007). Literature on the mechanisms of mindfulness proposes that the positive benefits of the practice might be mediated by a perceptual shift that modulates the internal representation of the self (Shapiro et al., 2006; Carmody, 2009) and it is possible that structural changes in the brain network involved in the projection of oneself into another perspective may underlie this perceptual shift.
One of the two extensive clusters identified in the cerebellum was located in lateral parts of the posterior and flocculonodular lobe and the other one was located in the vermis, reaching into the brainstem. Aside from the well-known function of the cerebellum in the integration of sensory perception, coordination, and motor control (Marr, 1969), this structure also plays a crucial role in the regulation of emotion and cognition. Lesions of the cerebellum have been found to lead to a constellation of cognitive, affective and behavioral abnormalities, the so-called “cerebellar cognitive affective syndrome” (Schmahmann et al., 2007). It has been suggested that in the same way that the cerebellum regulates the rate, force, rhythm, and accuracy of movements, it also regulates the speed, capacity, consistency, and appropriateness of cognitive and emotional processes (Schmahmann, 2004), i.e., it modulates behavior automatically around a homeostatic baseline. Given the importance that the regulation of emotions and cognition play in healthy psychological functioning, the morphological changes in these regions might contribute to the positive effects of mindfulness meditation on the salutary changes in well-being.
Regions within the brainstem were found to increase in gray matter concentration over the eight weeks. These regions appear to include the area of the locus coeruleus, nucleus raphe pontis, pontine tegmentum, and the sensory trigeminal nucleus (Naidlich et al., 2009). The regions of gray matter differences between meditators and non-meditators in the cerebellum and brainstem identified by Vestergaard-Poulsen et al. (Vestergaard-Poulsen et al., 2009) do not appear to overlap with the ones identified here. The locus coeruleus is the site of synthesis and release of the neurotransmitter norephinephrine, while the raphe nuclei release serotonin. The modulation of the serotonin system has been profoundly effective for the treatment of a wide range of mood and anxiety disorders, as evidenced by the widespread use of SSRIs (Masand and Gupta, 1999). The norephinephrine system of the locus coeruleus is thought to optimize behavioral performance by modulating arousal, regulating the interplay between focused vs. flexible responding to environmental demands, or selective vs. scanning attention (Aston-Jones et al., 2000; Aston-Jones and Cohen, 2005). Considerable evidence exists that the neurons of this system are important in a variety of cognitive, affective, and other behavioral functions, as well as associated clinical dysfunctions (e.g., depression, anxiety, sleep, and circadian disorders; for discussion, see (Aston-Jones, 2002). It is also one of the primary sites for the mediation of the stress response as well as a site of action of antidepressant drugs (Brady, 1994). Several studies have documented the positive impact of mindfulness-based programs on symptoms of anxiety and depression (Baer, 2003; Kuyken et al., 2008; Roemer et al., 2008), as well as improvements in sleep patterns (Carlson and Garland, 2005; Ong et al., 2009) and attention (Jha et al., 2007). The morphological changes reported here might contribute to some of these enhancements.
While significant Pre-Post changes in the TPJ, PCC, and cerebellum have been found in the present study, it is unclear why previous cross-sectional studies of meditators have not identified group differences in these regions. It is possible that small differences existed but were not detected due to the lack of power in the previous small cross-sectional studies, or that structural changes are transient and change might be maximal when a skill is newly acquired (Driemeyer et al., 2008).
It should be noted also that MBSR is a multifaceted group program and some positive effects may result from components not specific to meditation or mindfulness, such as group social interaction, stress education, or gentle stretching exercises. Exercise is know to increase neurogenesis in the hippocampus (van Praag et al., 1999). Since it also plays a crucial role in long-term memory consolidation and learning, structural changes might be related to general learning that occurred during the MBSR course analogous to those found in a study of medical students learning new information (Draganski et al., 2006). Comparing the brain gray matter concentration changes in the MBSR group to those of a waitlist control group, the current study did not allow differentiating between the effects of these different components. Indeed, the absence of a positive correlation between the change in gray matter concentration and the amount of homework suggests that the number of minutes of formal homework exercise are not the primary driving force behind the effects, but that the MBSR program as a whole influences the morphological changes. Future studies employing an active control condition that includes the mindfulness-unspecific components of the program (e.g., MacCoon et al., 2008) would help isolate the specific effects of meditation. Also, the current study investigated physician- and self-referred individuals seeking stress reduction and generalizations should therefore be limited to this population of stress individuals. Future studies will be required to test whether findings extend to non-stressed individuals as well as individuals suffering from mental disorders. Finally, the current study employed a rather small sample size and replication is necessary.
The adult nervous system has the capacity for plasticity, and the structure of the brain can change in response to training (Gage, 2002; Draganski et al., 2004; Colcombe et al., 2006; Driemeyer et al., 2008). It is generally assumed that the increased gray matter results from repeated activation of a brain region (May et al., 2007; Ilg et al., 2008) and previous studies have shown activation during meditation in brain regions identified here (Lou et al., 1999; Lazar et al., 2000; Newberg et al., 2001; Hölzel et al., 2007; Lutz et al., 2008). The cellular mechanisms underlying training-induced neuroanatomical plasticity are not yet understood however. An extensive body of research during the last decade has established that MBSR leads to improvements in psychological health and well-being (Grossman et al., 2004; Carmody et al., 2009). Demonstrating morphological increases in regions associated with mental health, the data presented here suggest a plausible underlying neural mechanism, namely, that such increases represent enduring changes in brain structure that could support improved mental functioning. Knowledge of the neurobiological mechanisms of behavioral interventions is indispensable to their effective and targeted use.
Acknowledgement
We thank our participants for their cooperation and the Center for Mindfulness for conducting the Mindfulness-based stress reduction courses. We thank Daniel McCaffrey and Nik Olendzki for support in data collection, and Douglas Greve, Ulrich Ott, and Julie Bates for helpful discussions. This research was funded by the National Institutes of Health-NCCAM (R21-AT003425-01A2), the British Broadcasting Company, and the Mind and Life Institute (Varela research grant). B.K.H. was supported by a Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme. S.W.L. was supported by National Institutes of Health funding K01AT00694. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arzy S, Thut G, Mohr C, Michel CM, Blanke O. Neural basis of embodiment: distinct contributions of temporoparietal junction and extrastriate body area. Journal of Neuroscience. 2006;26:8074–8081. doi: 10.1523/JNEUROSCI.0745-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6, Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G. Norepinephrine. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. American College of Neuropsychopharmacology; 2002. pp. 47–57. [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Progress in Brain Research. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: A conceptual and empirical review. Clinical Psychology: Science & Practice. 2003;10:125–143. [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson LE, Anderson ND, Carmody J, Segal ZV, Abbey S, Speca M, Velting D, Devins G. Mindfulness: A proposed operational definition. Clinical Psychology: Science & Practice. 2004;11:230–241. [Google Scholar]
- Blanke O, Mohr C, Michel CM, Pascual-Leone A, Brugger P, Seeck M, Landis T, Thut G. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. Journal of Neuroscience. 2005;25:550–557. doi: 10.1523/JNEUROSCI.2612-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Witkiewitz K, Dillworth TM, Chawla N, Simpson TL, Ostafin BD, Larimer ME, Blume AW, Parks GA, Marlatt GA. Mindfulness meditation and substance use in an incarcerated population. Psychology of Addictive Behaviors. 2006;20:343–347. doi: 10.1037/0893-164X.20.3.343. [DOI] [PubMed] [Google Scholar]
- Brady LS. Stress, antidepressant drugs, and the locus coeruleus. Brain Research Bulletin. 1994;35:545–556. doi: 10.1016/0361-9230(94)90168-6. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox [abstract]; 8th International Conference on Functional Mapping of the Human Brain Neuroimage; Sendai, Japan. 2002. [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Garland SN. Impact of mindfulness-based stress reduction (MBSR) on sleep, mood, stress and fatigue symptoms in cancer outpatients. International Journal of Behavioral Medicine. 2005;12:278–285. doi: 10.1207/s15327558ijbm1204_9. [DOI] [PubMed] [Google Scholar]
- Carmody J. Invited Commentary: Evolving Conceptions of Mindfulness in Clinical Settings. Journal of Cognitive Psychotherapy. 2009;23:270–280. [Google Scholar]
- Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. Journal of Behavioral Medicine. 2008;31:23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- Carmody J, Baer RA, E LBL, Olendzki N. An empirical study of the mechanisms of mindfulness in a mindfulness-based stress reduction program. Journal of Clinical Psychology. 2009;65:613–626. doi: 10.1002/jclp.20579. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. Journals of Gerontology Series A - Biological Sciences and Medical Sciences. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. Journal of Neuroscience. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. Journal of Neuroscience. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HG, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural Systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Urbanowski F, Harrington A, Bonus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosomatic Medicine. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Changes in Grey Matter Induced By Training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. Journal of Neuroscience. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Buchel C, May A. Changes in gray matter induced by learning--revisited. PLoS ONE. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one’s emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10:25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline J-B, Price CJ, Frith CD. Detecting activations in PET and fMRI: Levels of Inference and Power. Neuroimage. 1996;40:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. Journal of Neuroscience. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10:83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biological Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- Grant JA, Courtemanche J, Duerden EG, Duncan GH, Rainville P. Cortical thickness and pain sensitivity in zen meditators. Emotion. 2010;10:43–53. doi: 10.1037/a0018334. [DOI] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. Journal of Psychosomatic Research. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Grossman P, Tiefenthaler-Gilmer U, Raysz A, Kesper U. Mindfulness training as an intervention for fibromyalgia: evidence of postintervention and 3-year follow-up benefits in well-being. Psychotherapy and Psychosomatics. 2007;76:226–233. doi: 10.1159/000101501. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle M. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW. Stress reduction correlates with structural changes in the amygdala. Social Cognitive and Affective Neuroscience. 2009 doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive and Affective Neuroscience. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Hempel H, Hackl A, Wolf K, Stark R, Vaitl D. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neuroscience Letters. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Ilg R, Wohlschlager AM, Gaser C, Liebau Y, Dauner R, Woller A, Zimmer C, Zihl J, Muhlau M. Gray matter increase induced by practice correlates with task-specific activation: A combined functional and morphometric magnetic resonance Imaging study. Journal of Neuroscience. 2008;28:4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Molecular Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive Affective and Behavioral Neuroscience. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living. Delta Publishing; New York: 1990. [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological Psychiatry. 2008;63:550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Damasio AR, Davidson RJ, Lutz A, Tranel D. Interoceptive awareness in experienced meditators. Psychophysiology. 2008;45:671–677. doi: 10.1111/j.1469-8986.2008.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, Barrett B, Byng R, Evans A, Mullan E, Teasdale JD. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76:966–978. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11:1581–1585. [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, Rauch SL, Moore CI, Fischl B. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Kjaer TW, Friberg L, Wildschiodtz G, Holm S, Nowak M. A 15O-H2O PET study of meditation and the resting state of normal consciousness. Human Brain Mapping. 1999;7:98–105. doi: 10.1002/(SICI)1097-0193(1999)7:2<98::AID-HBM3>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. Neuroimage. 2009;45:672–678. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PLoS ONE. 2008;3:e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCoon D, Sullivan J, Lutz A, Stoney CM, Johnson LL, Christmas P, Thurlow J, Davidson R. Health-enhancement program (HEP) guidelines. 2008 [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. Journal of Physiology. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masand PS, Gupta S. Selective serotonin-reuptake inhibitors: an update. Harvard Review of Psychiatry. 1999;7:69–84. [PubMed] [Google Scholar]
- May A, Hajak G, Gaenssbauer S, Steffens T, Langguth B, Kleinjung T, Eichhammer P. Structural brain alterations following 5 days of intervention: Dynamic aspects of neuroplasticity. Cerebral Cortex. 2007;17:205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, Price CJ. Structural plasticity in the bilingual brain. Proficiency in a second language and age at acquisition affect grey-matter density. Nature. 2004;431:757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Naidlich TP, Duvernoy HM, Delman BN, Sorensen AG, Kollias SS, Haacke EM. Duvernoy’s Atlas of the Human Brain Stem and Cerebellum. Springer; Wien: 2009. [Google Scholar]
- Newberg A, Alavi A, Baime M, Pourdehnad M, Santanna J, d’Aquili E. The measurement of regional cerebral blood flow during the complex cognitive task of meditation: A preliminary SPECT study. Psychiatry Research. 2001;106:113–122. doi: 10.1016/s0925-4927(01)00074-9. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Iversen J. The neural basis of the complex mental task of meditation: neurotransmitter and neurochemical considerations. Medical Hypotheses. 2003;61:282–291. doi: 10.1016/s0306-9877(03)00175-0. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ong JC, Shapiro SL, Manber R. Mindfulness meditation and cognitive behavioral therapy for insomnia: a naturalistic 12-month follow-up. Explore (NY) 2009;5:30–36. doi: 10.1016/j.explore.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner CNM, Kilner SJ, Zelazo PD. Mindfulness meditation and reduced emotional interference on a cognitive task. Motivation and Emotion. 2007;31:271–283. [Google Scholar]
- Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiology of Aging. 2007;28:1623–1627. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Roemer L, Orsillo SM, Salters-Pedneault K. Efficacy of an acceptance-based behavior therapy for generalized anxiety disorder: evaluation in a randomized controlled trial. Journal of Consulting and Clinical Psychology. 2008;76:1083–1089. doi: 10.1037/a0012720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum. 2007;6:254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neuroscience and Biobehavioral Reviews. 2007;31:585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SL, Astin JA, Bishop SR, Cordova M. Mindfulness-based stress reduction for health care professionals: Results from a randomized trial. International Journal of Stress Management. 2005;12:164–176. [Google Scholar]
- Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of mindfulness. Journal of Clinical Psychology. 2006;62:373–386. doi: 10.1002/jclp.20237. [DOI] [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biological Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Francis AD, Nieuwenhuis S, Davis JM, Davidson RJ. Mental training affects distribution of limited brain resources. PLoS Biology. 2007;5:1228–1235. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Tapper K, Shaw C, Ilsley J, Hill AJ, Bond FW, Moore L. Exploratory randomised controlled trial of a mindfulness-based weight loss intervention for women. Appetite. 2009;52:396–404. doi: 10.1016/j.appet.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Longterm treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biological Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard-Poulsen P, van Beek M, Skewes J, Bjarkam CR, Stubberup M, Bertelsen J, Roepstorff A. Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport. 2009;20:170–174. doi: 10.1097/WNR.0b013e328320012a. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]


