Abstract
PURPOSE
A novel nanomedicine, CYT-6091, constructed by simultaneously binding recombinant human tumor necrosis factor alpha (rhTNF) and thiolyated polyethylene glycol to the surface of 27 nm colloidal gold particles, was tested in a phase I dose escalation clinical trial in advanced-stage cancer patients.
EXPERIMENTAL DESIGN
CYT-6091, whose dosing was based on the amount of rhTNF in the nanomedicine, was injected intravenously, and one cycle of treatment consisted of two treatments administered 14 days apart.
RESULTS
Doses from 50 μg/m2 to 600 μg/m2 were well tolerated, and no MTD was reached, as the highest dose exceeded the target dosage of 1 mg rhTNF per treatment, exceeding the previous MTD for native rhTNF by 3-fold. The first 2 patients on the study, each receiving 50 μg/m2, did not receive any prophylactic anti-pyretics or H2 blockade. A predicted, yet controllable fever occurred in these patients, so all subsequently treated patients received prophylactic anti-pyretics and H2 blockers. However, even at the highest dose rhTNF’s dose limiting toxic (DLT) effect of hypotension was not seen. Using electron microscopy to visualize nanoparticles of gold in patient biopsies of tumor and healthy tissue showed that patient biopsies taken 24-hours after treatment had nanoparticles of gold in tumor tissue.
CONCLUSIONS
These data indicate that rhTNF formulated as CYT-6091 may be administered systemically at doses of rhTNF that were previously shown to be toxic and that CYT-6091 may target to tumors. Future clinical studies will focus on combining CYT-6091 with approved chemotherapies for the systemic treatment of non-resectable cancers.
Translational Relevance.
The following manuscript presents the first-in-man clinical trial of a unique nanomedicine, CYT-6091, which is comprised of recombinant human tumor necrosis factor alpha (rhTNF) bound to the surface of PEGylated 27 nm colloidal gold particles. The significance of this clinical study is multifold. First, we achieved doses of rhTNF formulated as CYT-6091 that exceed previous clinical trials with rhTNF by 3-fold and are equal to that given for the isolated limb perfusion (ILP) for in-transit melanoma or sarcoma, where 85% complete remission rates are routinely observed. Second, the tumor targeting seen in this study suggests that mimicking the ILP protocol with CYT-6091 administered systemically prior to chemotherapy might result in similar response rates. And last, since an intact tumor neovasculature is critical for this nanomedicine to target tumors, then the treatment of solid tumors should begin with nanomedicine administration prior to surgery, even for resectable tumors.
Introduction
Tumor necrosis factor alpha (TNF) became available in recombinant form in the mid-1980s and remarkable anti-tumor effects were observed in experimental animals. However, numerous human clinical trials revealed that humans are exceedingly sensitive to the toxic side effects of rhTNF and contemporaneous research in the 1980s identified this protein as a primary mediator of endotoxic shock and sepsis (1-5). The main dose-limiting toxicities (DLTs) of intravenous recombinant rhTNF were hypotension, hepatotoxicity, and malaise and fatigue (1-5). The results of multiple phase 2 studies using intermittent bolus intravenous injections of rhTNF were reported between 1989 and 1992 (6-10). In over 156 evaluable patients there was only 1 complete and 1 partial response at the MTD. These disappointing results may, in part, be due to the inability to achieve therapeutic doses at the site of disease.
Limiting biodistribution of rhTNF primarily to tumors by hyperthermic limb perfusion for in-transit melanoma or sarcoma was shown to be dramatically successful when used in combination with interferon and melphalan (11-12). When administered via isolation perfusion to the extremity, the overall response rates with rhTNF followed by melphalan ranged from 75 to 100% (1; 13-16). The responses were observed against a range of histologies including melanoma, carcinoma, and sarcoma, and highlight the fact that when rhTNF is administered in a manner that limits its biodistribution primarily to tumor and is administered prior to chemotherapy, the anti-tumor effects are reminiscent of those reported in pre-clinical animal models (17).
This observation serves to underscore the critical importance of balancing efficacy against toxicity and the role of targeting therapy to the site of tumor growth. The maximum safe tolerated rhTNF doses administered in isolation perfusion of the limb is 4 mg, which is 10-fold higher than that tolerated by intravenous administration (11-16). However, lowering the perfused rhTNF dose from 4 mg to 1 mg resulted in local response rates that are equivalent to that seen with 4 mg, indicating the benefit of limiting rhTNF’s biodistribution to the tumor site (18-19).
But, rhTNF alone has minimal anti-tumor effects in regional limb perfusion. Rather, it’s the combination of rhTNF followed by chemotherapy where dramatic anti-tumor effects have been observed. The temporal sequence of treatment in this protocol is critical, as rhTNF has been shown to target tumor-associated vasculature, inducing hyper-permeability of the tumor blood vessels, leading to extravasation of erythrocytes and massive hemorrhagic necrosis of the tumor (20-21). However, this hemorrhagic necrosis alone has not been shown to result in significant and sustained tumor reduction. Rather, it’s been proposed that the rhTNF induction of hyper-permeability in the tumor is what enhances the efficacy of subsequently administered chemotherapy, resulting in such significant response rates with regional perfusion.
For tumors that are not able to be surgically isolated, improved response rates to chemotherapy with prior treatment with rhTNF might be accomplished by nanoparticle delivery systems capable of escaping phagocytic clearance by the reticuloendothelial system (RES; 22-23). By avoiding the RES, nanotherapeutics can circulate in the blood for longer periods of time. Under these conditions, such delivery systems permit preferential extravasation into the inherently leaky tumor-associated vasculature and facilitate drug accumulation within the tumor (24-26). By design, nanoparticles capable of sequestering a cancer drug within a tumor may also reduce the accumulation of the drug in healthy organs, increasing the relative efficacy or safety of a cancer therapy and increasing its therapeutic index.
In this study we used colloidal gold, as the core of a delivery system for tumor targeted drug delivery (27). Colloidal gold is a nanoparticle that is ideally suited for the delivery of protein biologics, such as rhTNF, since it binds these molecules very avidly and preferentially through available thiol groups (27). The long history of clinical use of colloidal gold in the treatment of rheumatoid arthritis and the prior use of radio-gold colloids in various human therapeutics, indicate that colloidal gold particles may be a safe platform for a new drug delivery system (28-31).
Pre-clinical studies with CYT-6091, a colloidal gold, rhTNF and thiolayted polyethylene glycol construct, indicate that PEGylation of the gold nanoparticles avoids immediate uptake by the RES, reduces the toxicity of rhTNF, and allows the nanomedicine to sequester in solid tumors. This sequestration in tumors, which is thought to be due to the leakiness of the tumor neovasculature, increased over time, while, in contrast over this same time period, rhTNF levels declined in blood and other vital organs. In TNF-sensitive tumor models, CYT-6091 had a dramatic effect on reducing tumor burden, but in TNF-insensitive tumor models CYT-6091 was much less effective. In those models, combination treatment with CYT-6091 followed by chemotherapy was effective (32).
Here we present the first clinical trial utilizing a nanoparticle to target rhTNF directly to the tumor, via systemic delivery. In this single agent trial in patients with advanced cancer, CYT-6091 was well tolerated and an MTD was not reached. Biologic effects of rhTNF were noted as evidenced by predictable pyrogenic and hematologic changes. Pre-treatment with anti-pyretics was used to manage fever, while the hematologic effects resolved themselves within 24 hours after treatment. In addition, core biopsies, taken 24 hours after treatment, revealed the presence of gold nanoparticles in tumor tissue.
Patients and Methods
Patient selection
Between May 2006 and April 2008, 30 patients with advanced and/or metastatic, histologically documented solid organ cancers, no longer considered responsive to available conventional modalities or treatments were enrolled in this phase I study. Patients were 18 years of age or older, with a clinical performance status of 0 to 1 on the Eastern Cooperative Oncology Group (ECOG) scale and life expectancy of greater than 3 months. Eligible patients had adequate hematological function (platelet count ≥ 100 × 109/L, absolute neutrophil count ≥ 1.5 × 109/L, and hemoglobin ≥ 10 g/dL), adequate renal and hepatic function (serum creatinine ≤ 2.0 mg/dL, alanine transaminase and aspartate transaminase ≤ 1.5-fold the upper limit of normal [≤ 10-fold the upper limit in presence of liver metastases], bilirubin ≤ 2.5 mg/dL) and adequate pulmonary function, as evidenced by FEV1 greater than 30% predicted in patients who have a history of pulmonary disease. Eligible patients were also required to have had no treatment with cytotoxic agents or treatment with biologic agents within 3 weeks prior to treatment or within 6 weeks of prior treatment with nitrosureas and were to have fully recovered from toxicities of any prior treatment with cytotoxic drugs, radiotherapy, surgery, or other anti-cancer modalities. All patients signed an informed consent approved by the Institutional Review Board of the National Cancer Institute.
Treatment
This was a phase I, dose-escalation trial of CYT 6091. The following dose levels were evaluated: 50 μg/m2 (n=3), 100 μg/m2 (n=3), 150 μg/m2 (n=3), 200 μg/m2 (n=3), 250 μg/m2 (n=3), 300 μg/m2 (n=4), 400 μg/m2 (n=3), 500 μg/m2 (n=3), and 600 μg/m2 (n=4). Two doses (day 1 and 15) constituted one course. CYT-6091 was reconstituted in sterile water for injection by the National Institutes of Health Pharmacy Department personnel and delivered to the patient care unit (PCU) or intensive care unit (ICU) in a syringe labeled with the dose, volume, and time of reconstitution. It was then administered through a central catheter as a single IV push injection over 20-30 seconds followed by 15 – 20 mL of 0.9% Sodium Chloride Injection flush. IV hydration was initiated prior to treatment and maintained for 4 hours post injection at a rate of 100 mL/hour.
The patients were monitored for 48 hours after treatment. The first patient of each cohort received his/her first dose of CYT-6091 in the ICU. If acute toxicities were not observed, additional patients could receive the drug administration on the intermediate care unit (or ICU as determined by staffing requirements), with telemetry and pulse oximetry during the first 4 hrs after dosing. Patients remained hospitalized for at least 48 hours, but could be discharged at that time if clinically stable. During and following each dose, vital signs (temperature, pulse, blood pressure, and respiration rate) were obtained at 5 minutes and then every 15 minutes for 60 minutes, then at 1.5, 2, 2.5, 3, 4, and 8 hours, then routinely if stable. After discharge from the hospital, patients were followed as outpatients. After two weeks (+/- 2 days), provided the patients had not experienced DLT and all toxicities had reversed to a grade 2 or less, they received a second dose of CYT-6091 on the PCU. A month following the second dose of CYT-6091, patients returned to clinic for re-staging studies and final evaluation of toxicity. No intra-patient dose escalation or dose reduction was allowed.
If a patient had stable disease or a partial response at the time of evaluation (4 weeks after completing one course of therapy), then the patient was eligible for up to 3 re-treatment courses with the same dose and schedule that had been given safely before.
Dose-limiting toxicities (DLT) were defined as: (1) all grade 4 toxicities with the exception of grade 4 myelosuppression of less than or equal to 5 days duration unless accompanied by a fever greater than or equal to 38.5°C for longer than 24 hours; (2) all grade 3 toxicities with the exception of: (i) grade 3 transaminase elevations occurring within the first week and resolving by day 15, (ii) grade 3 constitutional symptoms that persist for less than 72 hours, (iii) grade 3 myelosuppression of less than 5 days duration, or (iv) grade 3 metabolic/laboratory events that are correctable within 24 hours; or (3) hypotension associated with oliguria or the requirement for pressors to maintain adequate urine output or blood pressure. The MTD was defined as the highest dose that induced DLT in no more than 1 patient among a cohort of 6 patients. If a DLT occurred in 1 of 3 patients accrued to a cohort, then that cohort would have been expanded to 6 patients. If another DLT occurred, then that cohort would have exceeded the MTD.
Assessments
Adverse events were graded according to the NCI-Common Terminology Criteria for Adverse Events (CTCAE version 3.0). Treatment-related toxicities were those toxicities considered by the investigators to be possibly, probably, or definitely related to CYT-6091. Response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST; 33).
Pharmacokinetics
The pharmacokinetic (PK) profile of CYT-6091 was determined using heparinized whole blood samples collected at pre-treatment and at 5, 15, 30 minutes, and 1, 2, 3, 4, 8, and 24 hours following the completion of the first infusion of CYT-6091. PK analysis was performed for total rhTNF as it is not possible to assay for bound versus free rhTNF, nor is it possible to quantify the amount of gold based upon the sensitivity of available assay techniques. The blood samples were serially diluted in assay diluent and analyzed using a sandwich ELISA (CytImmune Sciences, Inc.) specific for rhTNF. The potency estimates were further analyzed using a pharmacokinetic program (WinNonLin; Pharsight Corporation) to estimate Cmax, Volume of distribution (Vd), area under the curve (AUC) and terminal half-life. The data are reported as the mean ± SD for each cohort.
Tissue Biopsies and Electron Microscopy (EM)
Biopsies for the assay of colloidal gold particles were obtained using either palpation and transcutaneous core biopsy or CT-guided core biopsy. The maximal effective radiation dose of two tissue biopsies, using CT scanning was 0.234 rem. This exposure was within the Radiation Safety Committee Guidelines for Adults (i.e., 5.0 rem). Other biopsies could be obtained during the second cycle of therapy. Where possible, the tumor was to be completely excised, along with a margin of normal skin or adjacent tissue. Only cutaneous lesions or superficial lymph nodes, which could be removed with local anesthesia (with or without sedation), were completely excised. Lesions identified for possible excision during the course of treatment were labeled as non-target lesions. In the case of core biopsies (i.e., liver), a biopsy of both tumor and normal tissue was obtained. EM biopsy tissues were fixed in standard gluteraldehyde fixative and stored at 2 to 8°C. High-power photomicrographs were obtained to examine for the presence of colloidal gold nanoparticles after the tissue had been embedded, sectioned, and stained using standard osmium tetroxide staining techniques. When possible, specimens from both malignant and normal tissue were obtained contemporaneously.
Immunogenicity
The immunogenicity of CTY-6091 was assessed via an assay for anti-rhTNF Immunoglobulin G (IgG) titers from blood samples obtained at protocol-specified time-points. For this immunologic assay, a 10 mL red-top vacutainer tube was used to collect a blood sample prior to the first and second dose of CYT-6091, and at week 6, the time of tumor re-staging evaluation. The samples were stored on ice (or in a 4°C refrigerator) and processed within 1 hr after collection, as follows. Blood samples were centrifuged for 10 min at room temperature and 2400× g. The resultant supernatant serum was separated and stored at -80°C for assay of anti-rhTNF antibodies (IgG). A direct enzyme-linked immunosorbent assay (ELISA) was used to determine the IgG titers to rhTNF. For this assay, rhTNF antigen, human IgG or human serum albumin was coated onto the wells of an ELISA plate and incubated overnight. The following day, the plates were blocked with 1% polyethylene glycol (PEG) for 2 hours and washed again. Subject samples, collected at pre, 2 and 6 weeks post treatment, were diluted 1:5, 1:50 and 1:250 in assay diluent (2% Albumin, 0.1% Tween diluted in Tris Buffered Saline) and added to designated wells of rhTNF-coated ELISA plates with mouse anti-rhTNF or rabbit-anti-rhTNF (R&D Systems, Inc., or CytImmune Sciences, Inc, respectively) diluted in assay diluent being used as positive controls. The plates were then incubated overnight. The following day the plates were washed and alkaline phosphatase-conjugated, species-specific goat antibodies, diluted in assay diluent, were added to the appropriate wells. The plates were incubated for an additional hour, washed once again followed by the addition of substrate. The plates were read at a wavelength of 405 nm once the optical density (OD) for the mouse positive control reached 1.4 (at which point the rabbit positive control had an OD of about 2.5). A positive antibody response was defined as a 20-fold difference in signal between the pre-treatment sample and the post-treatment samples for each patient at a 1:250 dilution.
Statistical Analysis
To evaluate whether there was any consistent effect on blood pressure, changes in systolic and diastolic blood pressure (BP) measurements were evaluated in treated subjects by time-point following each infusion, evaluating first changes in the population of treated subjects as a whole and then in dose cohorts divided into thirds as follows: Low Dose (50, 100 and 150 μg/m2), Mid Dose (200, 250, and 300 μg/m2), and High Dose (400, 500, 600 μg/m2). Changes for each time-point were compared to baseline by a T-test.
Results
Patient Characteristics
Thirty patients were enrolled in the protocol and 29 were treated. One patient with an ampulla of Vater adenocarcinoma was enrolled; however this patient suffered a stroke prior to receiving any treatment. Patients with a variety of histologies were treated; however, most had ocular melanoma (n=9) or adenocarcinoma of the colon (n=8). The rest of the cohort was comprised of 3 patients with adenocarcinoma of the pancreas, 2 patients with ductal carcinoma of the breast, and one patient of each of the following histologies: melanoma, adenocarcinoma of the lung, adenocarcinoma of the rectum, leiomyosarcoma, adrenocortical carcinoma, soft tissue sarcoma, and one of an unknown primary histology. Most patients had at least one prior systemic regimen before enrolling on this study. The median time on treatment was 43 days. Most patients received 2 doses of CYT-6091 (one course); one patient received 2 courses and one patient received 4 courses. Two patients received a single cycle of CYT-6091 and were taken off study prior to the second dose due to disease progression. Of the 29 patients treated, 26 were taken off study due to disease progression, 2 patients refused further treatment, and one patient died of his disease.
Adverse Events and Response Assessment
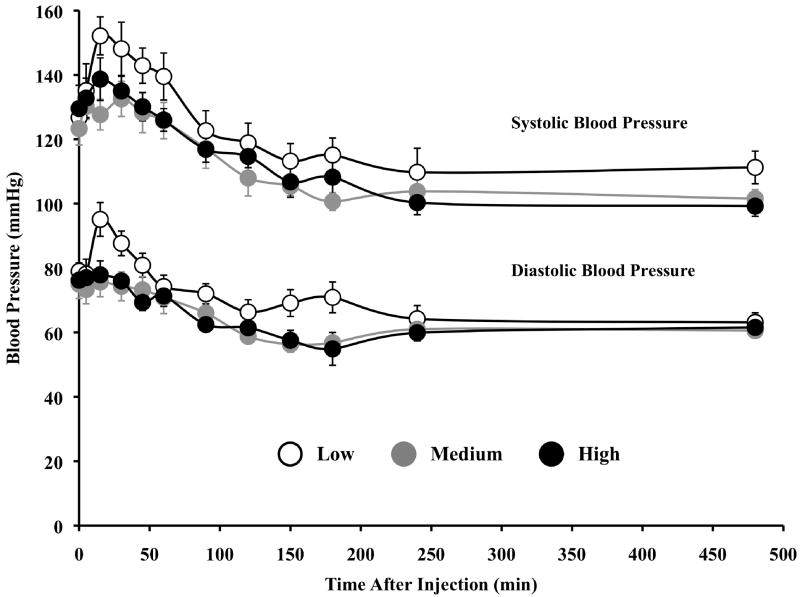
The treatment was well tolerated and there were no DLTs. The first 2 patients treated on the study did not receive any prophylactic anti-pyretics or H2 blockade, in order to accurately evaluate the toxicity profile. However, a predicted, yet controllable fever occurred in these patients and all patients subsequently treated received prophylactic anti-pyretics and H2 blockers. Table 1 lists all grade 3 and 4 adverse events reported by dose level, regardless of attribution. The most frequent grade 3 and 4 adverse events were lymphopenia (26/29 patients), hypoalbuminemia (5/29), hypokalemia (5/29), hypophosphatemia (5/29), hyperbilirubinemia (5/29), and increased AST (5/29). In addition, a predictable (34) redistribution in circulating lymphocytes and neutrophils that was dose dependents was seen (Figure 1). All events resolved per protocol requirements and were not considered DLTs. The main DLTs of intravenous recombinant rhTNF in previous trials were hypotension and hepatoxicity. Administration of doses up to 600 μg/m2 of CYT6091, exceeding our target dosage of 1 mg rhTNF per treatment, did not result in hypotension (Figure 2) or in hepatoxicity. All clinical responses, including blood pressure and hematological measures, at all doses following the second dose of CYT-6091 were indistinguishable from those following the first treatment.
Table 1.
Grade 3-4 Adverse Events experienced by dose level. AEs numbers represent the highest AE grade per person for all injections received, regardless of attribution.
| Adverse Event (CTCAE v3.0) | 50 μg/m2 (n=3) | 100 μg/m2 (n=3) | 150 μg/m2 (n=3) | 200 μg/m2 (n=3) | 250 μg/m2 (n=3) | 300 μg/m2 (n=4) | 400 μg/m2 (n=3) | 500 μg/m2 (n=3) | 600 μg/m2 (n=4) | Total (n=29) |
|---|---|---|---|---|---|---|---|---|---|---|
| Blood/Bone Marrow | ||||||||||
| Lymphopenia | 2 | 3 | 1 | 3 | 3 | 4 | 3 | 3 | 4 | 26 |
| Leukocytes (total WBC) | 1 | 1 | 2 | |||||||
| Hemoglobin | 1 | 1 | 2 | |||||||
| Neutrophils/granulocytes | 1 | 1 | ||||||||
| Metabolic/Laboratory | ||||||||||
| Hypoalbuminemia | 1 | 1 | 1 | 2 | 5 | |||||
| Hypokalemia | 1 | 1 | 1 | 2 | 5 | |||||
| Hypercalcemia | 1 | 1 | 2 | |||||||
| AST/SGOT | 1 | 1 | 1 | 2 | 5 | |||||
| ALT/ SGPT | 1 | 1 | ||||||||
| Alkaline phosphatase | 1 | 1 | ||||||||
| Hypophosphatemia | 2 | 2 | 1 | 5 | ||||||
| Hyperbilirubinemia | 1 | 4 | 5 | |||||||
| Adverse Events | ||||||||||
| Fatigue | 1 | 1 | 1 | 3 | ||||||
| Pain, chest wall | 1 | 1 | ||||||||
| Pain, liver | 1 | 1 | ||||||||
| Pain, abdomen | 2 | 1 | 1 | 4 | ||||||
| Pain, bone | 1 | 1 | ||||||||
| Pain, head | 1 | 1 | ||||||||
| Pain, NOS | 1 | 1 | ||||||||
| Nausea | 1 | 1 | 2 | |||||||
| Vomiting | 1 | 1 | 2 | |||||||
| Diarrhea | 1 | 1 | ||||||||
| Infection, pneumonia | 1 | 1 | ||||||||
| Infection, urinary | 1 | 1 | 2 | |||||||
| Anorexia | 1 | 1 | ||||||||
| Thrombosis/embolism | 1 | 1 |
Figure 1.
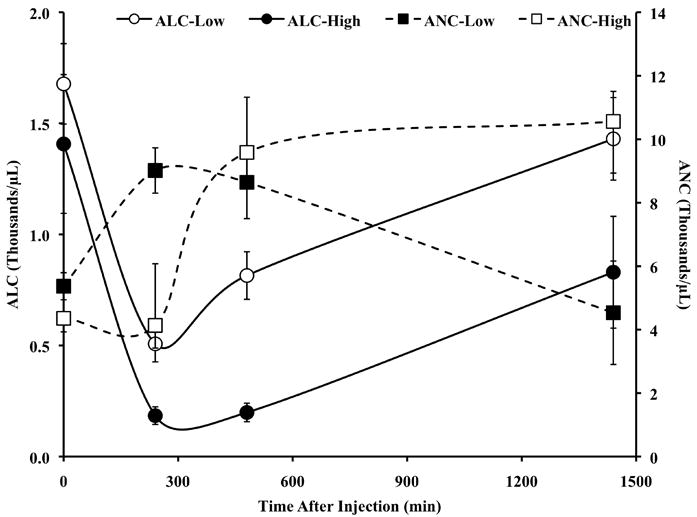
CYT-6091 induces a predictable redistribution of lymphocytes and neutrophils. All patients from all the dosing groups of CYT-6091 were sorted into three dose cohorts: HIGH (400, 500, 600 μg/m2), MEDIUM (200, 250, 300 μg/m2), and LOW (50, 100, 150 μg/m2). Data are presented as the mean±S.E.M for the counts from two of the three dosing cohorts.
Figure 2.
Effect of CYT-6091 on blood pressure (BP). Patients were grouped into three cohorts as described in Figure 1. Data are presented as the mean±S.E.M. Mean systolic BP did not drop below 80 mmHg, a threshold for hypotension requiring medical intervention.
Of the 29 patients treated 62% (18/29) experienced at least one episode of hypotension following treatment (Figure 2). There were statistically significant decreases in the mean systolic BP after both treatment infusions that initially occurred sporadically but then became consistent about one to two hours post-dosing (p<0.001). Analysis by dose cohort (Low Dose, Medium Dose and High Dose) suggested that the changes were not strongly dose-related in that while the High Dose cohort showed the greatest mean decrease at 3, 4 and 8 hours (p<0.0001, p=0.0009, and p<0.0001, respectively), this was not a consistent observation across all time points.
This analysis also revealed, however, that even at the times when the largest decreases occurred, the resulting mean systolic BP values were still within the normal range (90 to <130mm Hg)) and there were no subjects who received pressors in response to hypotensive episodes. Indeed, using the FDA toxicity grading scale for normal volunteers (FDA, 2007) only 2 subjects achieved a grade 3 hypotension (systolic BP <80mm Hg) and in both cases, these were single excursions, not requiring medical intervention.
Of the 27/29 subjects evaluated for disease response, one patient had a partial response by RECIST criteria, while 4 patients had stable disease. This former patient, who had melanoma and was treated with 100 μg/m2, continued to respond through three cycles (7 months in duration). The four subjects, one each in the 100, 250, 300 and 600 μg/m2 cohorts, respectively, all achieved stable disease in the first cycle. However, of these four subjects only the one in the 250 μg/m2 cohort underwent a second cycle, at the end of which disease progression was observed. All of the remaining 22 evaluated subjects, i.e., 76% of those receiving treatment, experienced disease progression.
Pharmacokinetics
Blood samples were taken at 5, 15, 30, 60, 120, 180, 240, 480, and 1440 minutes for pharmacokinetic analysis and evaluated for rhTNF concentrations using the rhTNF ELISA detailed above. As indicated in Table 2, the concentration-time profiles showed gradually decreasing concentrations of rhTNF through 4 hours (480 min) after the start of the infusion for all cohorts and through 24 hours (1440 minutes) for the 3 highest dose cohorts, i.e., doses below 400 μg/m2 produced no detectable levels of rhTNF at the last time point, i.e., 24 hours post-dosing. These data indicate that the plasma concentrations at all time-points show a monotonic relationship with the dose administered.
Table 2.
Mean ± SEM Whole Blood Concentrations of rhTNF (ng/ml) Following Infusion with Various Dose Levels of CYT-6091
| Time (min) | Dose Administered (μg/m2) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | 300 | 400 | 500 | 600 | |
|
5 |
7.7 ± 0.1 | 17.2 ± 2.6 | 25.2 ± 1.3 | 47.6 ± 5.8 | 58.2 ± 16.4 | 62.6 ± 12.1 | 85.1 ± 11.9 | 106.7 ± 9.7 | 154.7 ± 34.7 |
|
15 |
5.2 ± 0.7 | 10.1 ± 1.5 | 16.5 ± 1.0 | 34.2 ± 5.6 | 43.8 ± 9.7 | 48.1 ± 13.6 | 74.9 ± 13.4 | 73.8 ± 15.8 | 147.5 ± 22.8 |
|
30 |
4.0 ± 0.6 | 6.8 ± 1.0 | 11.4 ± 1.9 | 25.9 ± 6.9 | 24.4 ± 4.8 | 33.3 ± 9.8 | 58.4 ± 12.2 | 60.0 ± 16.2 | 109.2 ± 16.1 |
|
60 |
2.5 ± 0.4 | 3.5 ± 0.3 | 6.5 ± 1.2 | 15.5 ± 4.7 | 21.7 ± 2.8 | 21.3 ± 6.7 | 46.5 ± 4.6 | 43.3 ± 13.3 | 90.2 ± 12.7 |
|
120 |
1.3 ± 0.3 | 1.7 ± 0.1 | 3.2 ± 0.7 | 8.4 ± 2.9 | 10.9 ± 1.5 | 10.9 ± 4.0 | 21.7 ± 4.5 | 24.6 ± 8.3 | 47.2 ± 3.4 |
|
180 |
0.9 ± 0.2 | 1.1 ± 0.1 | 1.8 ± 0.4 | 4.6 ± 1.3 | 4.7 ± 0.6 | 4.3 ± 1.4 | 11.8 ± 3.8 | 12.2 ± 3.8 | 24.0 ± 1.8 |
|
240 |
0.6 ± 0.1 | 0.7 ± 0.1 | 1.3 ± 0.3 | 3.1 ± 0.9 | 2.6 ± 0.4 | 2.4 ± 0.8 | 6.1 ± 1.6 | 8.9 ± 3.0 | 12.3 ± 1.1 |
|
480 |
0.2 ± 0.1 | 0.2 ± 0.0 | 0.4 ± 0.2 | 1.0 ± 0.2 | 0.8 ± 0.0 | 0.8 ± 0.4 | 1.6 ± 0.5 | 2.6 ± 0.6 | 2.6 ± 0.0 |
| 1440 | BDL | BDL | BDL | BDL | BDL | BDL | 0.3 ± 0.1 | 1.2 ± 0.1 | 0.6 ± 0.0 |
| BDL= Below the limit of detection. | |||||||||
| Analysis (Mean ± S.D.) of the Pharmacokinetic Parameters of CYT-6091 in Man | |||||||||
| Dose of CYT-6091 (μg/m2) | Total Dose (μg) | Cmax (ng/mL) | Terminal Half Life (min) | AUC (min*ng/mL) | Volume of Distribution (mL) | Clearance (mL/min) | |||
|
50 |
90 ± 5 | 7.7 ± 0.2 | 120.7 ± 19.6 | 611 ± 192 | 18686 ± 2988 | 155 ± 40 | |||
|
100 |
202 ± 33 | 17.2 ± 4.6 | 131.3 ± 21.0 | 977 ± 64 | 24064 ± 8308 | 209 ± 48 | |||
|
150 |
270 ± 49 | 25.2 ± 2.3 | 127.0 ± 34.4 | 1575 ± 412 | 18641 ± 2845 | 177 ± 67 | |||
|
200 |
314 ± 7 | 47.7 ± 9.9 | 146.3 ± 22.2 | 3789 ± 1465 | 12063 ± 5174 | 90 ± 28 | |||
|
250 |
410 ± 62 | 58.2 ± 16.4 | 112.7 ± 27.2 | 3888 ± 890 | 11355 ± 3536 | 107 ± 23 | |||
|
300 |
563 ± 45 | 62.6 ± 21.0 | 113.0 ± 46.2 | 4403 ± 2223 | 13241 ± 4637 | 159 ± 96 | |||
|
400 |
785 ± 148 | 85.1 ± 20.7 | 266.0 ± 79.0 | 9297 ± 3845 | 16265 ± 9021 | 97 ± 51 | |||
|
500 |
823 ± 153 | 106.7 ± 16.7 | 371.1 ± 133 | 11206 ± 4359 | 31248 ± 27137 | 84 ± 53 | |||
| 600 | 1208 ± 214 | 154.7 ± 34.7 | 160.5 ± 52.0 | 17730 ± 2928 | 10865 ± 5666 | 71 ± 24 | |||
As seen in Table 2, the half-life (t½) data across all cohorts show no apparent relationship to dose and are all of the same order of magnitude, indicating that the removal of CYT-6091 follows a first-order reaction. AUC and Cmax values, however, increase with increasing dose in an approximately monotonic relationship to dose.
Electron Microscopy of Biopsied Samples
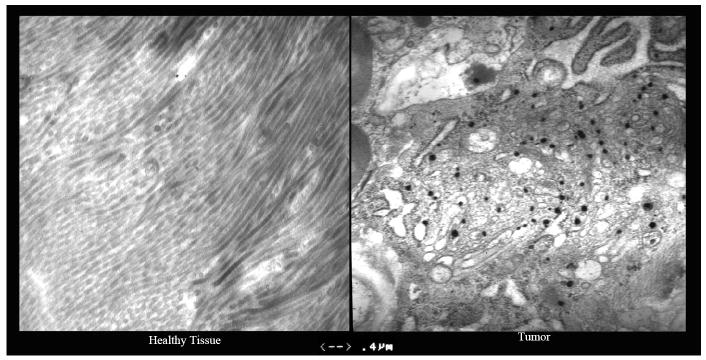
Detection of the gold particles in tissue biopsy samples via transmission electron microscopy was used as initial proof of concept of the tumor targeting ability of CYT-6091 (Table 3). Biopsies (punch, core or excisional) were obtained from twenty subjects 24 hours post administration of CYT-6091. Tissue samples were obtained from both tumor and adjacent healthy tissue. The samples were fixed in Formaldehyde/Glutaraldehyde, 2.5% each in 0.1M Sodium Cacodylate Buffer, pH 7.4. Post fixation tissues were analyzed by TEM using standard osmium tetroxide staining techniques. Figure 3 presents a side-by-side comparison of normal and neoplastic breast tissue taken from a subject with invasive ductal carcinoma of the breast 24 hours following the first infusion of CYT-6091. The black particles seen in the tumor tissue are gold particles, indicating that the nanoparticles trafficked to the tumor, but not to healthy breast tissue. In other patients where healthy and cancerous liver tissue was taken, gold particles were also detected in healthy liver tissue, an anticipated clearance site. However, the data shown in Figure 3 was the clearest example of differential tissue distribution. Given the limitations of the analysis and our inability to fully quantify the number of gold particles as a function of tissue mass, targeting at this point may only be inferred.
Table 3.
Detection of Gold Nanoparticles in Normal and Neoplastic Tissues from Subjects Treated with Various Doses of CYT-6091. (ND=not determined)
| Dose (μg/m2) | Patient ID | Diagnosis | Site of Biopsy | Normal Tissue | Tumor Tissue | ||
|---|---|---|---|---|---|---|---|
| Particles | Fields | Particles | Fields | ||||
| 50 | 0601-01 | Cutaneous Melanoma | Chest Wall | 10 | 10 | 91 | 6 |
| 0601-02 | Colon Adenocarcinoma | Liver | 2 | 6 | 13 | 11 | |
| 0601-03 | Ocular Melanoma | Liver | 0 | 4 | 5 | 11 | |
| 100 | 0601-04 | Colon Adenocarcinoma | Liver | 0 | 5 | 13 | 10 |
| 0601-05 | Colon Adenocarcinoma | ND | - | - | - | - | |
| 0601-06 | Ocular Melanoma | Scalp | 1 | 7 | 0 | 7 | |
| 150 | 0601-07 | Lung Adenocarcinoma | Liver | 0 | 6 | 33 | 5 |
| 0601-08 | Pancreatic Adenocarcinoma | Liver | 0 | 6 | 135 | 12 | |
| 0601-09 | Pancreatic Adenocarcinoma | Liver | 2 | 5 | 0 | 5 | |
| 200 | 0601-10 | Invasive Ductal Carcinoma | Breast | 0 | 5 | 167 | 5 |
| 0601-11 | Leiomyosarcoma | Muscle | 3 | 4 | 31 | 10 | |
| 0601-12 | Ocular Melanoma | Liver | 7 | 6 | 8 | 10 | |
| 250 | 0601-13 | Ocular Melanoma | Muscle | 19 | 7 | 0 | 4 |
| 0601-15 | Pancreatic Adenocarcinoma | Liver | 1 | 5 | 4 | 7 | |
| 0601-16 | Ocular Melanoma | Liver | 0 | 5 | 0 | 5 | |
| 300 | 0601-17 | Colon Adenocarcinoma | ND | - | - | - | - |
| 0601-18 | Ocular Melanoma | Liver | 8 | 10 | 16 | 12 | |
| 0601-19 | Ocular Melanoma | Liver | 15 | 6 | 5 | 5 | |
| 0601-20 | Ocular Melanoma | Liver | 23 | 6 | 0 | 6 | |
| 400 | 0601-21 | Desmoplastic Small Round Cell | Omentum | 0 | 5 | 0 | 6 |
| 0601-22 | Rectal Adenocarcinoma | Liver | 27 | 7 | 10 | 5 | |
| 0601-23 | Colorectal Adenocarcinoma | Liver | 78 | 6 | 0 | 5 | |
| 500 | 0601-24 | Ocular Melanoma | Skin | 0 | 8 | 1 | 9 |
| 0601-25 | Invasive Ductal Carcinoma | Liver | 148 | 9 | 0 | 7 | |
| 0601-26 | Colorectal Adenocarcinoma | ND | - | - | - | - | |
| 600 | 0601-27 | Desmoplastic Small Round Cell | ND | - | - | - | - |
| 0601-28 | Colorectal Adenocarcinoma | ND | - | - | - | - | |
| 0601-29 | Colorectal Adenocarcinoma | ND | - | - | - | - | |
| 0601-30 | Adenocarcinoma | ND | - | - | - | - | |
Figure 3.
Electron micrographs from a patient with inoperable ductal carcinoma of the breast. Magnification is 20,000 X, and the line at the bottom is 0.4 μm. Black dots in the tumor micrograph are nanoparticles of gold.
Discussion
Striking a favorable balance between efficacy and toxicity is a significant challenge for the development of new cancer therapies. The ideal agent is one that can be administered systemically yet exert its effects exclusively within the local tumor tissue. A variety of strategies have been attempted to achieve this goal. Nanomedicine, utilizing tumor-targeted particles carrying an anti-tumor agent, is one such strategy. In this study we have evaluated the safety, pharmacokinetics, immunogenicity and tumor targeting capability of a novel nanomedicine, CYT-6091. CYT-6091 is a covalently linked pegylated colloidal gold nanoparticle carrying recombinant human tumor necrosis factor (rhTNF). Based on our pre-clinical studies, we found that CYT-6091 selectively trafficked to tumor tissue, delivered a therapeutic dose of rhTNF, caused massive vascular leak, and was safer and more effective that rhTNF (27, 35). Our results in this phase I clinical trial mirrored much of our pre-clinical findings.
Our dose escalation scheme was based on the dose of rhTNF as determined by the amount of rhTNF measured in the CYT-6091 final drug product. We were able to administer CYT-6091 in doses up to 600 μg/m2 of rhTNF without encountering DLTs or immunogenicity. This dose exceeds the MTD of systemically administered, native rhTNF to patients and is within the range of effective rhTNF doses predicted by pre-clinical studies (1-4). In addition, we were able to demonstrate, by EM, accumulation of CYT-6091 in tumor tissue. Finally, although efficacy was not the primary endpoint of this study, we saw a partial response by RECIST criteria for a patient with metastatic ocular melanoma, and stable disease with four others.
The pharmacokinetic (PK) analysis indicated that the half-life of rhTNF was approximately 5-fold longer for CYT-6091 than previous clinical trials with native rhTNF (~130 min vs. ~28 min, respectively). The rhTNF half-life observed in the present clinical study is similar to that seen in a rat push-pull cannula model where both rhTNF and gold were measured (36). In this model, the half-life for rhTNF and gold were quite similar (182 min and 217 min, respectively), suggesting that the CYT-6091 nanoconstruct remains intact in the circulation. Further analysis of the PK data revealed that the AUC for the highest dose of CYT-6091 resulted in an exposure to rhTNF that is 4-fold higher than that seen with highest native rhTNF, but without any DLT (2-4).
Previous studies have suggested that the DLT of systemically administered rhTNF is primarily hypotension resulting from a direct effect of rhTNF on the endothelial cells that comprise normal, healthy blood vessels (37-38). By having rhTNF bound to the colloid gold nanoparticles, we hypothesize that the biodistribution of rhTNF is restricted, improving the safety of higher doses of rhTNF, and most likely eliminating this toxic effect on endothelial cells. Further, the immunogenicity data indicate that no anti-TNF antibodies were detected 2 or 6 weeks after treatment, suggesting that the improved safety was not due to the neutralization of the CYT-6091 rhTNF by TNF-absorbing antibodies. And, of note is that systemically administered CYT-6091 induced fever and a re-trafficking of lymphocytes, but did not induce hypotension, indicating that the CYT-6091 construct is able to separate rhTNF’s clinically manageable responses from its DLT response.
Electron micrographs of the patient biopsies, taken 24-hours after treatment, indicate that the gold nanoparticles traffic to tumors. Such trafficking to the tumor is anticipated as the neovasculature of tumors has been shown to have fenestrations of 200-400 nm in size (20-24). At 27 nm, we hypothesize that CYT-6091 would be able to readily exit the circulation through the well-described enhanced permeability and retention (EPR) effect in tumor neovasculature. In addition, our pre-clinical studies indicate that rhTNF serves as an ideal tumor-targeting ligand, since gold particles with rhTNF on the surface were seen in the tumor within 30 minutes after treatment, while, in contrast, the same amount of gold nanoparticles without rhTNF bound to the surface took approximately 24-hours to be detected in the tumor (27). In addition, the targeting of CYT-6091 to tumors in pre-clinical mouse tumor xenograft models is independent of whether the tumor cells do or do not have TNF receptors (Paciotti, unpublished observations). Consequently, CYT-6091 may target solid tumors by both a passive mechanism (the EPR effect), as well as an active one (rhTNF binding to the tumor neovasculature).
It should be noted that many of these patients also had biopsies of normal liver, and gold nanoparticles were also seen in healthy liver tissue (Table 3). In contrast, when other tissues were biopsied, such as healthy skin or breast tissue, no gold particles were detected. These data are consistent with other pre-clinical studies, showing that one-day post treatment most of the gold administered as CYT-6091 was found in the liver, decreasing by 50% 120 days after treatment (39-41). It is possible that the PEGylation of the gold nanoparticles may keep the particles monodispersed, allowing for better elimination from the liver, which would suggest improved long-term safety of CYT-6091 compared to that seen with chrysotherapy.
Analysis of the EM data indicates that the number of nanoparticles accumulating in tumor tissue was independent of dose. Even at the lowest dose, nanoparticles of gold were detected in tumor biopsies, and at the higher doses of CYT-6091 no proportional increase in nanoparticle accumulation was observed. Of note, two patients, one with ductal carcinoma of the breast and the other with pancreatic cancer, who did not have resections of their primary tumors, appeared to have the greatest number of particles in the biopsies of these primary tumors. This observation suggests that an intact tumor neovascualture may allow for better tumor targeting of these gold nanoparticles, further supporting the strategy of treating patients with nanomedicines prior to surgery.
The clearest clinical benefit of rhTNF is in hyperthermic limb perfusion for in-transit melanoma or sarcoma (11-19). In this protocol, rhTNF is infused first, followed 30-60 minutes later with the infusion of chemotherapy. Now as an EMA approved treatment protocol, improvements have been developed since this procedure was described in 1992. Most significantly, the perfusion dose of rhTNF has been lowered from 4 mg to 1 mg, with no change in the percentage of patients responding, suggesting that a threshold may be reached where additional drug is of no further benefit (18-19). The success of this surgical protocol, where a 70-90% complete response rate has been reported, is the ideal protocol design to mimic, but with systemic treatments using CYT-6091. Assuming CYT-6091 traffics preferentially to solid tumors, a priori the goal of this Phase I study was to achieve systemic dosing of CYT-6091 of at least 1 mg of rhTNF per dose. At 600 μg/m2, this objective was met. Now the efficacy of systemically administered CYT-6091 needs to be tested in combination with chemotherapy.
A phase II clinical trial in patients with solid tumors that administers CYT-6091 systemically first, followed 30-60 minutes later with chemotherapy, replicates the isolated limb perfusion protocol. Future studies are planned to evaluate whether CYT-6091 induces greater vascular leak as determined by dynamic contrast enhanced-magnetic resonance imaging and to evaluate the safety and efficacy of CYT-6091 in combination with approved chemotherapies to determine whether or not such a combination is not only safe, but improves the response rates of these chemotherapies.
Acknowledgments
This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN2612008000001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Alexander HR, Feldman AL. Tumor necrosis factor: Basic principles and clinical application in systemic and regional cancer treatment. In: Steven A, Rosenberg MD, editors. Biologic Therapy of Cancer. 3. Philadelphia: Lippincott; 2000. pp. 174–93. [Google Scholar]
- 2.Kimura K, Taguchi T, Urushizaki I, Ohno R, Abe O, Furue H, et al. Phase I study of recombinant human tumor necrosis factor. Cancer Chemother Pharmacol. 1987;20:223–229. doi: 10.1007/BF00570490. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi T. Phase I study of recombinant human tumor necrosis factor (rHu-TNF:PT-050) Cancer Detection and Prevention. 1988;12:561–572. [PubMed] [Google Scholar]
- 4.Creaven PJ, Plager JE, Dupere S, Huben RP, Takita H, Mittelman A, et al. Phase I clinical trial of recombinant human tumor necrosis factor. Cancer Chemotherapy and Pharmacology. 1987;20:137–144. doi: 10.1007/BF00253968. [DOI] [PubMed] [Google Scholar]
- 5.Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 6.Heim ME, Siegmund R, Illiger HJ, Klee M, Rieche K, Berdel WE, et al. Tumor necrosis factor in advanced colorectal cancer: a phase II study. A trial of the phase I/II study group of the Association for Medical Oncology of the German Cancer Society. Onkologie. 1990;13:444–447. doi: 10.1159/000216817. [DOI] [PubMed] [Google Scholar]
- 7.Kuei JH, Tashkin DP, Figlin RA. Pulmonary toxicity of recombinant human tumor necrosis factor. Chest. 1989;96:334–338. doi: 10.1378/chest.96.2.334. [DOI] [PubMed] [Google Scholar]
- 8.Aulitzky WE, Tilg H, Gastl G, Mull R, Flener R, Vogel W, et al. Recombinant tumour necrosis factor alpha administered subcutaneously or intramuscularly for treatment of advanced malignant disease: a Phase I trial. European Journal of Cancer. 1991;27:462–467. doi: 10.1016/0277-5379(91)90387-s. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead RP, Fleming T, Macdonald JS, Goodman PJ, Neefe J, Braun TJ, et al. A phase II trial of recombinant tumor necrosis factor in patients with metastatic colorectal adenocarcinoma: A Southwest Oncology Group Study. Journal of Biological Response Modifiers. 1990;9:588–591. [PubMed] [Google Scholar]
- 10.Moritz T, Niedule N, Baumann J, May D, Kurschel E, Osuka R, et al. Phase I study of recombinant human tumor necrosis factor a in advanced malignant disease. Cancer Immunology,Immunotherapy. 1989;29:144–50. doi: 10.1007/BF00199290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lienard D, Lejeune F, Ewalenko I. In transit metastases of malignant melanoma treated by high dose r TNF alpha in combination with interferon-gamma and melphalan in isolation perfusion. World J Surg. 1992;16:234. doi: 10.1007/BF02071526. [DOI] [PubMed] [Google Scholar]
- 12.Lejeune FJ. High dose recombinant tumour necrosis factor (rTNFα) administered by isolation perfusion for advanced tumors of the limbs: a model for biochemotherapy of cancer. Eur J Cancer. 1995;3:1009–1016. doi: 10.1016/0959-8049(94)00512-5. [DOI] [PubMed] [Google Scholar]
- 13.Fraker DL, Alexander HR, Andrich M, Rosenberg SA. Treatment of patients with melanoma of the extremity using hyperthermic isolated limb perfusion with melphalan, tumor necrosis factor, and interferon-gamma: Results of a TNF dose escalation study. J Clin Oncol. 1996;14:479–889. doi: 10.1200/JCO.1996.14.2.479. [DOI] [PubMed] [Google Scholar]
- 14.Alexander HR, Bartlett DL, Libutti SK, Fraker DL, Moser T, Rosenberg SA. Isolated hepatic perfusion with tumor necrosis factor and melphalan for unresectable cancers confined to the liver. J Clin Oncol. 1998;16:1479–1489. doi: 10.1200/JCO.1998.16.4.1479. [DOI] [PubMed] [Google Scholar]
- 15.Eggermont AMM, Koops HS, Liénard D, Kroon BBR, van Gee AN, Hoekstra HJ. Isolated limb perfusion with high dose tumor necrosis factor-a in combination with interferon-gamma and melphalan for irresectable extremity soft tissue sarcomas: A multicenter trial. J Clin Oncol. 1996;14:2653–2665. doi: 10.1200/JCO.1996.14.10.2653. [DOI] [PubMed] [Google Scholar]
- 16.Eggermont AMM, Koops HS, Klausner JM, Kroonm BBR, Schlag PM, Liénard D, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. Annals of Surgery. 1996;224:756–765. doi: 10.1097/00000658-199612000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin induced serum factor that causes necrosis of tumors. P N A S. 1975;72:3666. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Filippo F, Garinei R, Anzà M, Cavaliere F, Giannarelli D, Cagol PP, Rossi CR, Santinami M, Deraco M, Botti C, Perri P, Di Filippo S, Piarulli L, Bruno P. Doxorubicin in Isolation Limb Perfusion in the Treatment of Advanced Limb Soft Tissue Sarcoma. J Exp Clin Cancer Res. 2003;22:81–87. [PubMed] [Google Scholar]
- 19.Bonvalot S, Laplanche A, Lejeune F, Stoeckle E, Le Peéchoux, Vanel D, Terrier P, Lumbroso J, Ricard M, Antoni G, Cavalcanti A, Robert C, Lassau C, Blay JA, Le Cesne A. Limb salvage with isolated perfusion for soft tissue sarcoma: could less TNF-a be better? Annals of Oncology. 2005;16:1061–1068. doi: 10.1093/annonc/mdi229. [DOI] [PubMed] [Google Scholar]
- 20.van Horssen R, ten Hagen TML, Eggermont AMM. TNF-α in Cancer Treatment: Molecular Insights, Antitumor Effects, and Clinical Utility. The Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 21.ten Hagen TLM, Eggermont AMM. Solid Tumor Therapy: Manipulation of the Vasculature with TNF. Technology in Cancer Research & Treatment. 2003;2:195–203. doi: 10.1177/153303460300200303. [DOI] [PubMed] [Google Scholar]
- 22.Papisov MI. Theoretical considerations of RES-avoiding liposomes: molecular mechanisms and chemistry of liposome interactions. Ad Drug Delivery Rev. 1998;32:119–138. doi: 10.1016/s0169-409x(97)00135-x. [DOI] [PubMed] [Google Scholar]
- 23.Moghimi SM, Patel HM. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system—the concept of tissue specificity. Ad Drug Delivery Rev. 1998;32:45–60. doi: 10.1016/s0169-409x(97)00131-2. [DOI] [PubMed] [Google Scholar]
- 24.Woodle MC. Controlling liposome blood clearance by surface grafted polymers. Ad Drug Delivery Rev. 1998;32:139–152. doi: 10.1016/s0169-409x(97)00136-1. [DOI] [PubMed] [Google Scholar]
- 25.Nafayasu A, Uchiyama K, Kiwada H. The size of liposomes: a factor, which affects their targeting efficiency to tumors and therapeutic activity of liposomal antitumor drugs. Ad Drug Del Rev. 1999;40:75–87. doi: 10.1016/s0169-409x(99)00041-1. [DOI] [PubMed] [Google Scholar]
- 26.Maruyama K, Takizawa T, Takahashi N, Tagawa T, Nagaike K, Iwatsuru M. Targeting efficiency of PEG-immunoliposome-conjugated antibodies at PEG terminals. Ad Drug Delivery Rev. 1998;24:235–242. [Google Scholar]
- 27.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal Gold: A Novel Nanoparticle Vector for Tumor Directed Drug Delivery. Drug Delivery. 2004;11:169. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 28.Root SW, Andrews GA, Knieseley RM, Tyor MP. The distribution and radiation effects of intravenously administered colloidal Au 198 in man. Cancer. 1954;7:856–866. doi: 10.1002/1097-0142(195409)7:5<856::aid-cncr2820070506>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Rubin P, Levitt SH. The response of disseminated reticulum cell sarcoma to the intravenous injection of colloidal radioactive gold. J Nuclear Med. 1964;5:581–594. [PubMed] [Google Scholar]
- 30.Gottlieb NL, Gray RG. Pharmacokinetics of gold in rheumatoid arthritis. Agents Actions Suppl. 1981;8:529–538. [PubMed] [Google Scholar]
- 31.Graham-Bonnalie FE. Gold for rheumatoid arthritis. Br Med J. 1971;2:277. doi: 10.1136/bmj.2.5756.277-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myer LD, Jones D, Tamarkin L, Paciotti GF. Nanomedicine-Based Enhancement of Chemotherapy. Presented at the Annual Meeting of the AACR (2008).2008. [Google Scholar]
- 33.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 34.Farma JM, Puhlmann M, Soriano PA, Cox D, Paciotti GF, Tamarkin L, Richard AH. Direct evidence for rapid and selective induction of tumor neovascular permeability by tumor necrosis factor and a novel derivative, colloidal gold bound tumor necrosis factor. Int J Cancer. 2007;120:2474–2480. doi: 10.1002/ijc.22270. [DOI] [PubMed] [Google Scholar]
- 35.Ulich TR, del Castillo J, Ni R, Bikhazi N, Calvin L. Mechanisms of Tumor Necrosis Factor Alpha-InducedLymphopenia, Neutropenia, and Biphasic Neutrophilia: A Study of Lymphocyte Recirculation and Hematologic Interactions of TNFa With Endogenous Mediators of Leukocyte Trafficking. J Leuk Biol. 1989;45:155–167. doi: 10.1002/jlb.45.2.155. [DOI] [PubMed] [Google Scholar]
- 36.Stern ST, Hall JB, Yu LL, Wood LJ, Paciotti GF, Tamarkin L, Long SE, McNeil SE. Translational considerations for cancer nanomedicine. Journal of Controlled Release. 2010;146:164–174. doi: 10.1016/j.jconrel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedl J, Puhlmann M, Bartlett DL, Libutti SK, Turner EN, Gnant MF, et al. Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: relationship between the procoagulant and permeability effects of TNF. Blood. 2002;100:1334–1339. [PubMed] [Google Scholar]
- 38.Ruegg C, Yilmaz A, Bieller G, Bamat J, Chaubert P, Lejeune FJ. Evidence for the involvement of endothelial cell integrin aVb3 in the disruption of the tumor vasculature induced by TNF and IFNg. Nature Medicine. 1998;4:408–414. doi: 10.1038/nm0498-408. [DOI] [PubMed] [Google Scholar]
- 39.Renaud G, Hamilton RL, Havel R. Hepatic metabolism of colloidal gold-low-density lipoprotein complexes in the rat: evidence for bulk excretion of lyosomal contents into bile. Hepatology. 1989;9:380–392. doi: 10.1002/hep.1840090307. [DOI] [PubMed] [Google Scholar]
- 40.Hardonk MJ, Harms G, Koudstaal J. Zonal heterogeneity of rat hepatocytes in the vivo uptake of 17 nm colloidal gold granules. Histochemistry. 1985;83:473–477. doi: 10.1007/BF00509211. [DOI] [PubMed] [Google Scholar]
- 41.Goel R, Shah N, Visaria R, Paciotti GF, Bischof JC. Biodistribution of TNF-alpha coated gold nanoparticles in an in vivo model system. Nanomedicine. 2009;4:401–410. doi: 10.2217/nnm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]