Abstract
The study examined the relationship between risk-taking behavior during selection of monetary rewards and activations in the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC) and medial prefrontal cortex (mPFC), brain regions that are associated with decision-making. Thirty-three adolescents with no personal or family history of any psychiatric illness were administered the Wheel of Fortune (WOF) task using a functional magnetic resonance imaging protocol. The WOF is a computerized two-choice, probabilistic monetary reward task. Selection of a reward, particularly a low-probability/high-magnitude reward choice, induced greater activations in dorsal ACC, ventrolateral OFC and mPFC than the control condition. Although similar findings have been reported by earlier studies, the results from this study were not impacted by reaction times and expected values and persisted even after controlling for sociodemographic factors. Post-hoc analysis revealed greater activation of ACC and mPFC in response to selection of rewards of larger magnitude than those of smaller magnitude when the probability of reward was maintained constant. Adolescents with greater frequency of high-risk behavior (defined as low-probability/high magnitude reward choice) had lower activation of ACC, OFC and mPFC than those who engaged in this behavior less frequently. These findings suggest individual differences in prefrontal cortical function with regards to decision-making process in adolescents.
Keywords: Neurobiology, choice, selection, adolescents, decision-making, rewards
1. Introduction
Adolescence is a developmental period characterized by impulsive decision-making (Byrnes 2002; Chambers et al . 2003; Spear 2000a) and risky behavior that give rise to an increased incidence of unintentional injuries and physical violence, gambling, alcohol and drug abuse (Chambers and Potenza 2003; Steinberg et al. 2008), unintended pregnancy and sexual indiscretion (Steinberg et al. 2004). These risk-taking behaviors have been associated with a high level of morbidity and mortality during adolescence (Eaton et al. 2006) and may be explained by relative immaturity of brain structures during the adolescent years (Gogtay et al. 2004; Spear 2000b). This is especially true for the prefrontal cortex (PFC) that is linked with higher-order cognitive functions and emotional regulation (Casey et al. 2000; Giedd et al. 2004; Luna et al. 2004). For example, the PFC regions of the anterior cingulate cortex (ACC) and orbital prefrontal cortex (OFC) along with medial prefrontal cortex (mPFC) have been involved in cognitive processes of conflict monitoring and response-inhibition that are pivotal for decision-making in adults (Bechara 2001; Kannerley and Wallis 2009; Knutson et al. 2001; O'Doherty et al. 2001; O'Doherty et al. 2002; Rolls 2000). Increased vulnerability to risk-taking during adolescence may be a reflection of relatively higher tendency to seek rewards and still maturing capacities for self-control (Steinberg et al. 2010). Thus, neuromaturational differences in PFC during adolescence are thought to contribute to frequent underestimation of risks (Benthin et al. 1993) and increased risk-taking behaviors (Ernst et al. 2006; Spear 2000a; Steinberg et al. 2004; Steinberg et al. 2008).
Ernst et al. (2009) proposed a triadic model that provides an understanding of the neural underpinnings of the patterns of motivated behavior, which plays a significant role in decision-making. This model comprises of three brain regions with striatum mediating approach, amygdala representing avoidance and PFC providing behavioral regulation. Earlier, Casey et al. (2008) had proposed that heightened responsiveness to rewards and incentives while impulse control is still relatively immature is a biologically plausible explanation of the neural mechanisms underlying high-risk behavior during adolescence. Findings from recent neuroimaging studies supported this view by suggesting differential development of the prefrontal executive versus limbic reward systems during adolescence (Galvan et al., 2006). This developmental disparity could explain predisposition towards risky decision-making, increasing the risk for poor outcomes. However, it is worth mentioning that risky decision-making could be an adaptive process, which may be important for the adolescents to learn from and mature based on their early life experiences. Nevertheless these observations underscore the need to investigate the neurobiology of reward-related decision-making during adolescence when high-risk behavior may be relatively easier to intervene than in adults when the behavior becomes more entrenched.
Decision-making involves choice selection (forming preferences based on available options and executing the selected choice), anticipation of the outcome based on the selection, and evaluation of the outcome (Bjork et al. 2004; Ernst and Paulus, 2005). Although several studies have assessed the brain activation patterns in reward-related behavior in pediatric populations (Bjork et al. 2004; Bjork et al. 2010; Eshel et al. 2007; Galvan et al. 2006; May et al. 2004; van Leijenhorst et al. 2006), there are limited data on the activation patterns in PFC sub-regions specifically during the selection process that leads to outcomes (Ernst and Paulus 2005). In addition, none of these studies differentiated between risk- and reward-induced prefrontal activations (Smith et al. 2009), which underscores the need for such investigation in adolescents.
The study by Eshel et al. (2007) used a monetary decision-making task (the Wheel of Fortune) that allowed the analysis of choice selection (separately from anticipation of outcome or response to reward) to examine differences in PFC activations between adolescents and adults while making decisions to obtain rewards of varying magnitude and probability. High-risk behavior (defined as selection of an option with relatively lower probability of winning a reward of high-magnitude and a higher probability of winning nothing) was associated with greater activations in OFC/ventrolateral PFC (VLPFC) and ACC compared to low-risk behavior in both adolescents and adults (although adults showed greater activation than adolescents). In regards to reward magnitude, only one study has examined this relationship in children, adolescents and adults (Galvan et al. 2006). Although this study reported a significant relationship between reward magnitude and prefrontal activation in children and adults, none was found in adolescents (Galvan et al. 2006). However, this study was comprised of small sample sizes within different age groups (thereby resulting in inadequate power to detect more modest relationships) and the decision-making paradigm did not allow separate analysis of choice selection from other phases of decision-making.
Although comparison of different age groups in prior studies has been informative in understanding the common developmental patterns of decision-making, it is important to mention that even within the adolescent period there were individual differences in frequency of risky behavior and how fast youngsters develop (Gullo and Dawe 2008; Steinberg 2005). Thus, some adolescents may be especially prone to engage in risky behaviors due to developmental changes in concert with variability in a given individual's predisposition to engage in risky behavior, rather than to simple changes in impulsivity (Galvan et al. 2007). Accounting for these individual differences may not only be helpful to reveal patterns that are present only for a subgroup of a certain age group (for example, adolescents who are more prone to risk-taking), but also to have a better understanding of the brain-behavior relationships (Galvan et al. 2007). For instance, Eshel et al. (2007) observed that both adolescents and adults who more frequently engaged in risk-taking behavior on the laboratory task were less likely to have PFC activation during reward selection than those who manifested the risk-taking behavior less frequently. Although the various options on the laboratory task were characterized as a function of risk, they also differed on expected values (Eshel et al. 2007). Therefore, it was not clear whether the above-described brain-behavior relationship was related to risky choices or to reward values. The disentanglement of risk- from reward-related activations will be pivotal to identify the neurobiological underpinnings of high-risk behavior in adolescents.
The present study was undertaken to examine the reward-related behavior in a large sample of adolescents during the choice selection of decision-making. Choice selection is the first step in the decision-making process (Ernst and Paulus 2005) and involves a behavioral component in contrast to the more cognitively-oriented anticipation and feedback phases, thereby providing an opportunity to examine the associations between manifest behavior and neural responses. The task (i.e., wheel of fortune) selected for this study included varying probabilities and magnitudes of rewards (for details, see materials and methods section). Despite including different probabilities and rewards, the expected value was maintained constant in order to assess risk-taking behavior (which in this study was defined as the selection of low-probability/high magnitude rewards) without the confounding impact of reward-magnitude. Developmental (age-related) as well as individual differences in the neuronal substrates underlying reward selection was examined. Additionally, the influence of other sociodemographic characteristics on behavioral performance and neuronal activation patterns was also examined.
The following hypotheses were postulated: (1) compared to the control condition, risk-reward selections will be associated with greater activation in the prefrontal regions representing response-inhibition and reward values (OFC) and monitoring conflict and error (ACC and mPFC); (2) high-risk behavior (low-probability/high-reward choices) will be associated with greater activation in these prefrontal regions compared with low-risk behavior (high-probability/low-reward choices), representing greater conflict while making a low-probability choice than a high probability choice; (3) older adolescents will have greater prefrontal activations during reward selections than younger adolescents; and (4) adolescents with higher risk-taking behavior (higher frequency of low probability/high-reward choices) will have reduced activation in these brain regions than those with lower risk-taking behavior, suggesting that impulsive adolescents will have less conflict in making low-probability choice than high-probability choice.
We also formulated an exploratory hypothesis that adolescents from high SES will exhibit greater prefrontal activation during reward selections compared to those from low SES.
2. Materials and methods
2.1. Participants
The participants included 33 adolescents (age range 12-20) recruited from local schools and churches, through advertisements in local newspapers and by word-of-mouth. Prior to any research procedure, all adolescents signed a written assent form and parents signed an informed consent document, approved by the local Institutional Review Board. All participants were medically healthy and free from alcohol or illicit drug use, as determined by physical examination, full chemistry panel, thyroid function tests, electrocardiogram, and urine drug screens. The participants had to be free from any psychiatric disorder in their lifetime, and all first-degree relatives also had to be free from a psychiatric disorder. Females with suspected pregnancy and subjects with metallic devices or objects (e.g., pacemakers, artificial heart valves, implanted infusion pumps, cochlear implants, etc.), or those having symptoms of claustrophobia or color blindness were excluded from the study.
2.2. Diagnostic evaluation
Assessment of psychiatric disorders was done using the Schedule for Affective Disorders and Schizophrenia for School-Age Children - the Present and Lifetime Version (K-SADS-PL; (Kaufman et al. 1997). The K-SADS-PL was administered separately to the adolescent and the parent, and both were re-interviewed to resolve any discrepancies. Summary scores were tabulated based on the information obtained from both informants. The Family History-Research Diagnostic Criteria (FHRDC) was used for the evaluation of psychiatric disorders in family members (Andreasen et al. 1977). The FHRDC is sensitive for obtaining information from knowledgeable relatives (Thompson et al. 1982). Socioeconomic status (SES) was assessed with the Hollingshead Four Factor Index of Social Status (Hollingshead 1975), and Intelligence Quotient (IQ) was estimated from vocabulary and block design scores using Wechsler Intelligence Scale for Children (WISC IV) (Wechsler 2003) for ages < 16 years and Wechsler Adult Intelligence Scale (WISC III) (Wechsler 1997) was employed for ages ≥ 16 years. The pubertal stage was assessed with the Tanner's scale (Marshall and Tanner 1969; Marshall and Tanner 1970).
2.3. Behavioral task
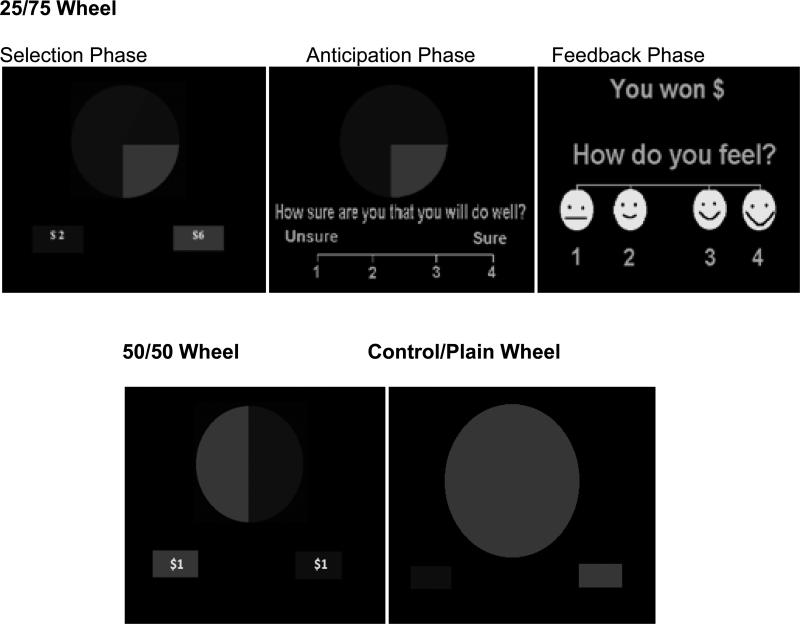
The Wheel of Fortune (WOF) is a computerized two-choice decision-making task (Ernst et al. 2004) involving probabilistic monetary rewards with varying levels of risk (see Figure 1). This task has been used in other studies of adolescents and adults (Dichter et al. 2009; Ernst et al. 2004; Eshel et al. 2007; Smith et al. 2009; Smoski et al. 2009) and allows an analysis of choice selection separately from other phases of decision-making. The task has been validated as a measure of real life risky behavior in adolescents in a recent study by Rao et al. (in press). Subjects completed four runs of 7.8 minutes each. Each run was comprised of 39 trials/stimuli including twenty-four 25/75 wheels, eight 50/50 wheels and seven control wheels. Each wheel type/trial lasted 12 s and included a choice-selection phase, during which subjects selected one of the options of the wheel (4 s); an anticipation phase, during which subjects rated how confident they were in the selection they made (4 s); and the feedback phase, during which the outcome was displayed and subjects rated how they felt about it (4 s). However, in this report, data are presented only for the selection phase. No jitter was used in this study.
Figure 1.
Wheel of Fortune (WOF) task showing 25/75 (risk/reward), 50/50 (equal-risk), and control/plain wheels.
In each trial, monetary and plain wheels were presented. With regard to the monetary wheel, participants were asked to select one of the slices by its color (blue or magenta) using a button press. For example, when the magenta slice was on the left and subjects opted for this color, they pressed the left button. If the computer randomly selected the same color as the subject, the subject won the designated amount of money (receipt of reward); if the computer randomly selected the other color, the subject won nothing (omission of reward). The monetary wheel conditions comprised of (1) a risk/reward (25/75) condition, where subjects had to choose between a 25% chance of winning either of the two rewards of high magnitude and a 75% chance of winning nothing versus a 75% chance of winning either of the two rewards of low magnitude or a 25% chance of winning nothing ( i.e. $6.00 vs. $2.00 or $3.00 vs. $1.00); (2) an equal-risk/reward (50/50) condition, where subjects had a 50/50 chance of winning $1.00 or nothing (i.e. $1.00 vs. $1.00). The plain wheel was monochromatic (all blue, or all magenta) with no monetary value, which represented the control condition to account for the sensory-motor attributes of the monetary conditions, while lacking the decision-making process. The participants were instructed to press the button whose color corresponded to the color of the wheel (left or right button). Order of presentation for all conditions was fully randomized. Subjects were instructed to try to win as much money as possible and their compensation was up to $50 for completing the WOF fMRI task in 2 out of 4 randomly selected runs. In addition, a fixed amount of $50 was paid to each subject for participating in the scans. The WOF task was administered using E-Prime software (Schneider et al. 2002).
2.4. Imaging parameters and processing
Functional magnetic resonance imaging (fMRI) technique was used to measure regional blood oxygen level dependent (BOLD) signal during performance of the task. A General Electric 1.5 Tesla scanner was employed and gradient echo planar images (EPI) were acquired in 26, 5 mm sagittal slices per brain volume. The following imaging parameters were used: EPI gradient echo pulse sequence, TR = 2000 ms; TE = 20 ms; Flip = 90°; slice spacing = 0; field of view = 200 mm; matrix = 64 mm × 64 mm. Head movement was restricted by the use of a strap on the forehead in addition to the foam padding provided with the head coil. The raw fMRI data acquired from each subject were slice-time corrected and converted to ANALYZE image format using AFNI software (Cox 1996). The first 6 scans (or images acquired during first 12 seconds) were deleted to remove potential artifacts related to signal stabilization. At the individual subject level (i.e., time series), event-related response amplitudes were estimated employing the General Linear Model (GLM) in Statistical Parametric Mapping 2 (SPM2; Friston et al. 1995) for each of the two active (25/75 and 50/50) and the control (monochromatic wheel) conditions.
The waveform used to model each type of event-related response in the GLM was a rectangular pulse of the duration of the event (4 seconds) convolved with the synthetic hemodynamic response. Contrast images were generated for each subject using pair-wise comparisons of the event-related BOLD changes across event types. The seventh scan in each run was compared with the EPI template provided in SPM2 and translation and rotation parameter correction numbers were plugged-in at image display until that image volume was closely oriented with the template, and this orientation was applied to all the images within the run. Each run was separately realigned using INRIAlign (Freire et al. 2002). Each participant's motion parameter corrections were examined to ensure there was no excessive motion. The data from two subjects (from a total sample of 33 subjects) were excluded from analysis because of excessive head motion (more than 3 mm in any one or more of x, y or z translational parameters). A mean image was generated in this step from the realigned functional volumes. This mean image volume was then used to determine the parameters for spatial normalization into Montreal Neurological Institute (MNI) standardized space employed in SPM2. The normalization parameters determined for the mean functional scan volume were then applied to the corresponding reoriented and realigned functional image volumes for each participant.
All the normalized images were then smoothed with a 10×10×10 mm Gaussian kernel. Finally, the smoothed images were filtered using a Butterworth low pass filter with a cutoff frequency of 0.15 to remove any high frequency noise. An EXCEL Visual Basic for Applications (VBA) macro was run on the E-Prime task data to obtain scan onset vectors that were used to model the design matrix (Schneider et al. 2002). Six motion regression parameters generated during the realignment step were added as regressors in the design to regress out motion artifacts. The fMRI design was specified in scans with vectors obtained from the above step. The mean image generated in the realignment step is the mean of all functional scan volumes within the run, which were imported into the design matrix with a standard SPM2 high-pass filter of 128 seconds and no global scaling.
2.5 Statistical analysis
At the individual level, 3 sets of analyses were conducted with events during the selection phase: (1) risk/reward (25/75 probability wheel) vs. control (plain wheel) condition; (2) (25/75 probability wheel vs. 50/50 probability wheel; and (3) high-risk (25% probability) vs. low-risk (75% probability) reward selection events within the 25/75 probability wheel. In this context, the risk/reward (combination of high- and low-risk selections) condition provided the assessment of reward-related behavior compared to the control condition represented by a plain wheel containing no reward and requiring no decision-making. The 50/50 probability (equal-risk) wheel included a reward but it provided less cognitively challenging choices with each selection resulting in a similar outcome (50% probability of winning $1 or $0) compared to more cognitively challenging options of risk/reward involving the selection of rewards associated with different probabilities. The high-risk versus low-risk reward selection events provided a decision-based contrast of selecting a low probability high magnitude (high-risk) or a high probability low magnitude (low-risk) option.
In addition to the whole brain analysis, activation patterns were looked in selected a priori prefrontal regions consistently linked to incentive and cognitive-conflict processing (Arana et al. 2003; Bush et al. 2002; Ernst et al. 2002; Ernst et al. 2004; Paulus et al. 2001; Rushworth et al. 2005; Walton et al. 2004); right and left dorsal ACC (Brodmann's area BA 24 and 32), right and left OFC (BA 11 and 47), and right and left mPFC (BA 10 and 25) from the standard Wake Forest University (WFU) Pickatlas toolbox with statistical threshold set at p ≤ and K equivalent of 5 voxels. The data from three out of 31 participants who chose to respond to less than 10% of trials in the 25% (high-risk) probability in the high-risk versus low-risk contrast were excluded from the ROI-based analyses for statistical reasons. None of responses to the 75% probability (low-risk) were less than 10%. Spearman Rank Test was used to examine the correlation between percent BOLD signal change for the specified contrasts and sociodemographic characteristics or high-risk behavior (frequency of low-probability/high-reward selections, i.e., 25% choices).
In post-hoc analyses, ROI based analysis of the ventral striatum was examined since this region is strongly implicated in reward-related behaviors. Additionally, brain activation patterns with respect to magnitude of the reward were examined. Despite the proportional variations in the probability of winning and magnitude of reward in the 25/75 probability wheel, the expected value (EV) for each of the two options of the 2575 wheel were kept constant in the high-risk vs. low-risk contrast [i.e., 25% chance of winning $3 (EV = 3*0.25 = 0.75) or 75% chance of winning $1 (EV = 1*0.75 = 0.75), respectively; and 25% chance of winning $6 (6*0.25 = 1.50) or 75% chance of winning $2 (2*0.75 = 1.50), respectively].
3. Results
3.1. Sample characteristics
Demographic characteristics of the sample are provided in Table 1. All participants were between 12-20 years. Majority of the participants were in Tanner Stage IV (18.5%) or V (66.7%) of pubertal development (Marshall and Tanner 1969; Marshall and Tanner 1970). One study volunteer had an estimated IQ score of 62 but this subject had no evidence of mental retardation based on clinical evaluation, academic achievement scores and psychosocial functioning. Exclusion of this participant from the analysis did not alter the results.
Table 1.
Demographic characteristics of the sample
| Age (years) | 15.8 ± 2.2; (range = 12-20) |
| Gender | Female = 11 (35.5%), Male = 20 (64.5%) |
| Ethnicity | Caucasian = 18 (58.1%), Non-Caucasian = 13 (41.9%) |
| Tanners's Stage | Stage II = 7.4%; Stage III = 7.4%; Stage IV = 18.5%; Stage V = 66.7% |
| Hollingshead SES score1 | 50.9 ± 8.4 (range = 36-66) |
| Full-Scale IQ score | 107 ± 19.4 (range = 62-141)* |
Data are presented as means and standard deviations along with ranges, or as raw numbers and proportions
Although one participant had an estimated IQ score <70 based on the Wechsler Intelligence Scale for Children, this participant had no evidence of mental retardation based on clinical evaluation, academic achievement scores and psychosocial function. Hence, this participant was included in the study. Analysis of data excluding this participant did not change the results.
3.2. Behavioral performance
Information on behavioral performance on the WOF task is provided in Table 2. Age, gender, race and IQ did not influence the behavioral performance. However, adolescents with higher SES had a higher frequency of making high-risk selections than those with lower SES (r = 0.42, p = .03). There were no significant differences in reaction times in the selection of high- versus low-risk options (t = 0.48, df = 60, NS). Similarly, percent selections between the two high-risk options ($6 vs. $3) and the two low-risk options ($1 vs. $2) were not statistically different (t = 1.2, df = 31, NS; and t = -1.2, df = 31, NS, respectively). The mean amount of cumulative earnings was $32±8.7 based on randomly selected 2 out of 4 runs. In addition, each subject was paid $50 for completing the scan procedures.
Table 2.
Behavioral Performance on the Wheel of Fortune Task
| Percent high-risk (25% probability) selections | 35 ± 22.9 (1-87) |
| Reaction time for high-risk (25% probability) selections | 1804 ± 303 (1233-2488)) |
| Cumulative earnings (average of 4 runs) | 32 ± 8.7 (17-55) |
| Total amount paid to each subject | $50 for participation plus cumulative earnings over 4 runs |
| Reaction time for low-risk (75% probability) selections | 1667 ± 297 (1163-2340) |
| Reaction time for $6 selections | 1731 ± 349 (1015-2958) |
| Reaction time for $2 selections | 1635 ± 330 (1138-2275) |
| Reaction time for $3 selections | 1725 ± 327 (1171-2613) |
| Reaction time for $1 selections | 1613 ± 263 (1183-2174) |
Data are presented as means and standard deviations along with ranges
3.3. Brain activation patterns
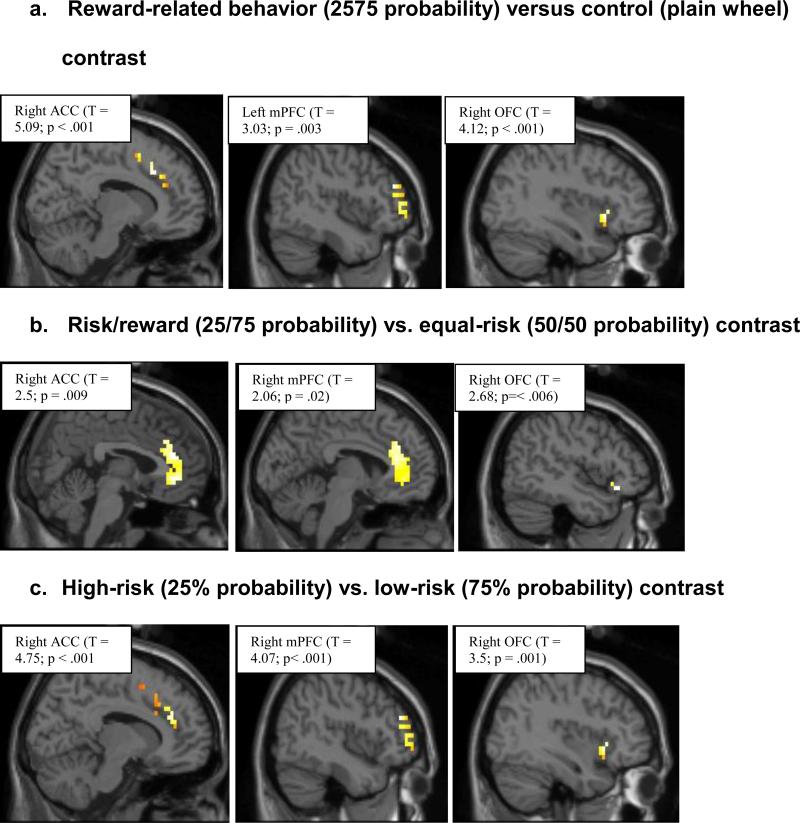
3.3.1 Risk/reward (25/75 probability) vs. control (plain wheel) contrast
The ACC showed significant activation bilaterally (see Table 3 and Figure 2). Specifically, the dorsal ACC (BA 24) showed greater activations on the right side than on the left side. With regards to OFC, adolescents recruited only the right-sided OFC (BA11) in this contrast. These activations were primarily in the ventral and lateral portions of the OFC (see Table 3). While this contrast produced significant activation of only the left mPFC (see Table 3). There were no detectable activations in the reverse contrast.
Table 3.
Region of interest (ROI) activation patterns based on probability of risk/reward on the Wheel of Fortune task.
| L-ACC | R-ACC | |||||||
|---|---|---|---|---|---|---|---|---|
| Equivk | p voxel | T | x, y, z | Equivk | p voxel | T | x, y, z | |
| 2575 vs. control contrast | 102 | 0.001 | 3.31 | –12, 9, 48 | 69 | 0.000 | 5.09 | 15, 27, 32 |
| 2575 vs. 5050 contrast | 65 | .008 | 2.53 | -3, 33, 16 | 57 | .009 | 2.50 | 3, 39, 20 |
| 25 vs. 75 contrast | 110 | 0.000 | 3.84 | -3, 36, 28 | 88 | 0.000 | 4.75 | 12, 36, 24 |
| L-OFC | R-OFC | |||||||
|---|---|---|---|---|---|---|---|---|
| Equivk | p voxel | T | x, y, z | Equivk | p voxel | T | x, y, z | |
| 2575 vs. control contrast | 28 | 0.000 | 4.12 | 24, 45, -16 | ||||
| 2575 vs. 5050 contrast | 22 | 0.006 | 2.68 | 48, 27, -12 | ||||
| 25 vs. 75 contrast | 19 | 0.005 | 2.74 | -42, 15, -8 | 29 | 0.001 | 3.50 | 39, 18, –4 |
| L-mPFC | R-mPFC | |||||||
|---|---|---|---|---|---|---|---|---|
| Equivk | p voxel | T | x, y, z | Equivk | p voxel | T | x, y, z | |
| 2575 vs. control contrast | 11 | 0.003 | 3.03 | –36, 48, 16 | ||||
| 2575 vs. 5050 contrast | 10 | 0.02 | 2.06 | 45, 54, -4 | ||||
| 25 vs. 75 contrast | 37 | 0.002 | 3.24 | -24, 45, 28 | 64 | 0.000 | 4.07 | 45 42 24 |
Region of interest activation in the contrasts [2575 (risk/reward) versus control (plain wheel)], [2575 (risk/reward) versus 50/50 (equal-risk)] and [25 (high-risk) versus 75 (low- risk). The regions of interest include the left and right anterior cingulate cortices (L-ACC, R-ACC); left and right orbitofrontal cortices (L-OFC, R-OFC), and left and right medial prefrontal cortices (L-mPFC; R-mPFC). Results show significant activations (p < 0.05 small volume corrected p-values centered on peak voxels found to be significantly activated by risk/reward selections relative to control condition and high-risk selections relative to low-risk selections. Equivk is the cluster size of voxels, and x, y, z (mm) are the coordinates of the peak voxel based on the brain template provided by the Montreal Neurological Institute (MNI). No ROI showed significantly more activation during control (plain wheel), low-risk (75% probability), or equal-risk (50/50 probability) selections than during risk/reward or high- risk selections (25% probability).
Figure 2.
Peak voxels in anterior cingulate (ACC), orbitofrontal (OFC), and medial prefrontal cortex (mPFC) after minimum volume correction.
3.3.2 Risk/reward (25/75 probability) vs. equal-risk (50/50 probability) contrast
Bilateral ACC activations also were observed in this contrast (see Table 3 and Figure 2). However, the right-sided activation included more ventral areas of ACC than the left side. The reverse (50/50 vs. 25/75 probability) contrast produced activation of the more caudal areas of right ACC. This contrast also produced activation of only the right ventrolateral OFC (see Table 3). The reverse contrast produced activation of more medial and dorsal areas of the right OFC. While no detectable activations were observed in the mPFC in this contrast or the reverse contrast (see Table 3).
3.3.3 High-risk (25% probability) vs. low-risk (75% probability) contrast
This contrast yielded significant activations in the dorsal ACC (BA 24) bilaterally but right-sided activations were stronger than on the left side (see Table 3 and Figure 2). OFC was also activated bilaterally in this contrast. But this activation was limited to more posterior part of the ventrolateral OFC, especially on the left side (see Table 3). This contrast also induced mPFC activation bilaterally but greater activation was observed on the right side than the left side (see Table 3). There was no detectable activation in the reverse contrast.
3.3.4. ROI -Striatum
Both ventral and dorsal portions of the striatum were significantly activated in the high-risk (25%) versus low-risk (75%) contrast. The reverse contrast did not show any activation in this region.
3.5.5. Effect of reward magnitude
Within high-risk or low-risk selection choices, rewards of large magnitude ($6 vs. $3, or $ 2 vs. $1) showed greater activation of the left ACC (t = 2.66; p = .007), right ACC (t = 2.04; p = .02), left mPFC (t = 2.55; p = .008), and right mPFC (t = 2.47; p = .01). Rewards of greater magnitude also induced significant activation of the left ventral striatum (t = 2.66; p= .006) than rewards of smaller magnitude. The reverse contrasts did not show any activation patterns in these regions.
3.3.6. Relationship between sociodemographic factors and percent change BOLD signal
There were no significant relationships between activations in any of the pre-specified ROIs and age, gender, ethnicity, pubertal scores or IQ. Age also was dichotomized into younger (≤15 years) and older (>15 years) groups, and these two groups did not differ significantly on prefrontal activation patterns. Higher SES was associated with lower activation in the right mPFC in the risk/reward (25/75) vs. control contrast (r = -0.55; p = 0.005).
3.3.7 Correlations between functional ROIs and high-risk behavior
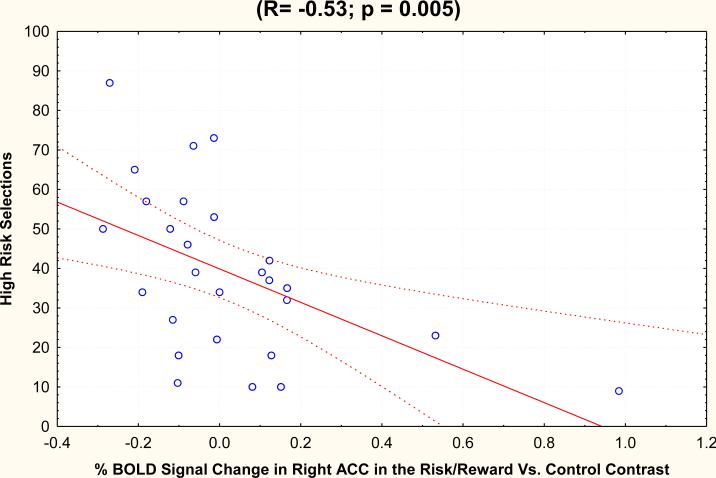
In the risk/reward (25/75 wheel) versus control (plain wheel) condition, frequency of high-risk (25% probability) choice was negatively correlated with percent BOLD signal change in the right ACC (r = -0.53, p = 0.005) (see Figure 3), left ACC (r = -0.60, p = 0.0008), right OFC (r = -0.58, p = 0.001) and left mPFC (r = -0.40; p = 0.04). The effect of high-risk behavior on neuronal activation persisted after controlling for sociodemographic characteristics (see Table 4). Frequency of high-risk behavior did not correlate with activation in the striatal region.
Fig 3.
Scatterplot of high risk selections and % BOLD signal change in risk/reward versus control contrast in Right ACC
Table 4.
Hierarchical linear regression coefficients depicting the contribution of sociodemographic variables and high-risk behavior (proportion of 25% probability selection) to activation of right anterior cingulate cortex.
| Unstandardized Coefficients |
Standardized Coefficients |
||||
|---|---|---|---|---|---|
| Model |
B |
Std. Error |
Beta |
t |
Sig. |
| 1 | . | . | NS | ||
| age | .011 | .034 | .100 | .326 | NS |
| Gender (male/female) | -.120 | .159 | -.198 | -.753 | NS |
| Ethnicity (Caucasian/non-Caucasian) | -.067 | .092 | -.229 | -.726 | NS |
| IQ | -.004 | .005 | -.237 | -.886 | NS |
| SES |
.003 |
.010 |
.097 |
.348 |
NS |
| 2 | |||||
| age | -.002 | .027 | -.016 | -.065 | NS |
| gender | -.011 | .130 | -.018 | -.086 | NS |
| ethnicity | -.045 | .073 | -.155 | -.618 | NS |
| IQ | -.005 | .004 | -.284 | -1.338 | NS |
| SES | .010 | .008 | .299 | 1.308 | NS |
| High-risk selections | -.011 | .003 | -.702 | -3.259 | .005 |
IQ = intelligent quotient; SES = socioeconomic status
3.3.8 Effect of decision-making outcome on the subsequent selection
The influence of attaining a reward (winning a reward vs. not winning) on the subsequent choice of reward (25% probability vs. 75% probability choice) was assessed, and there was no significant association.
4. Discussion
The study examined neural substrates underlying the selection of monetary rewards in healthy adolescent volunteers. As predicted, choice of a reward was associated with greater activations in the prefrontal regions of dorsal ACC, ventrolateral OFC and mPFC, which have been associated with conflictual decision-making. The PFC activations (especially involving ACC) were most robust in response to the high- vs. low-risk choices followed by the combined high-and low-risk (risk/reward behavior) versus control condition, and lastly in comparison with equal-risk (50/50) options. This graded prefrontal activation across the three contrasts employed in this study supports the notion that high-risk selections posed greater cognitive challenge compared to purely reward-related behavior.
Despite greater PFC activation with high- than low-risk choice, an inverse relationship was observed between high-risk behavior and the magnitude of neuronal activation. These contradictory findings can be explained in the following manner. The adolescents, as a group, selected the low-risk options more frequently than the high-risk options. However, it appears that when adolescents made the high-risk selections, they were more conflicted because the probability of gaining a reward was less likely. The group contrast differences showing greater activation in response to high-risk choices might be driven by the subgroup of adolescents who selected the high-risk option less frequently. In contrast, the negative correlation between the proportion of high-risk choice and magnitude of PFC activation indicates that those adolescents who made the high-risk choice less frequently were more conflicted when they made this choice (thereby inducing greater PFC activation), whereas those who made the high-risk choice more frequently might have given greater consideration to the magnitude of reward than the probability of attaining this reward. These results are consistent with findings from other studies in adolescents (Bjork et al. 2007; Bjork et al. 2008; Eshel et al. 2007, van Leijenhorst et al. 2006) and adults (Bjork et al. 2007; Carmichael and Price 1996; Eshel et al. 2007; Schoenbaum and Roesch 2005; Taylor et al. 2006). From a neurobiological standpoint, the negative correlation between high-risk behavior and PFC activations may reflect a propensity for impulsive decision-making, potentially mediated by a relative lack of OFC-mediated impulse control/response inhibition and ACC-mediated conflict monitoring/resolution.
The involvement of ACC and OFC as key neural substrates during decision-making processes, such as choice selection, has been reported in multiple neuropsychological, lesion and functional neuroimaging studies (Arana et al. 2003; Bush et al. 2002; Elliott et al. 1999; Ernst et al. 2002; Ernst et al. 2004; Paulus et al. 2001; Rogers et al. 1999; Rushworth et al. 2005; Walton et al. 2004). Previous work has shown engagement of both regions in studies of reward-related processes (Cohen et al. 2005; Rogers et al. 2004) and the selection of options (Ernst et al. 2004; Hadland et al. 2003; Williams et al. 2004). However, dorsal ACC is thought to provide pivotal cognitive input during behaviors involving conflict resolution and decision-making (Kerns et al. 2004; Krawczyk 2002), whereas ventral ACC has been shown to mediate the emotional salience of stimuli (Hampton and O'Doherty 2007). The OFC is generally involved in response-inhibition and cognitive flexibility (Casey et al. 2001; Cools et al. 2002; Nagahama et al. 2001; O'Doherty et al. 2003), supporting its role in controlling action in a conflictual context including its ability to code for reward values (O'Doherty et al. 2003; Schoenbaum and Roesch 2005).
The activation of dorsal ACC in response to high-risk choices suggests that adolescents in this study may have used their cognitive ability to resolve conflicts to take action rather than the emotional salience of the stimulus. The preference for low-risk option in this study may also reflect an aversive reaction to the conflictual decision-making resulting in a cognitively less challenging and affectively safe selection with the lower probability of a painful outcome. This view is supported by involvement of ACC during conscious processing of pain (Zaki et al. 2010). Similarly, activation of the ventrolateral OFC indicates some level of impulse control during reward-related behavior as proposed by Eshel et al. (2007). However, unlike findings from the study by Eshel et al. (2007), this study failed to observe the extension of OFC activation into the anterior insula. This could be due to a relatively lesser activation of the insula in adolescents compared to adults, and age-related differences in the function of harm-avoidance circuitry (Ernst et al. 2005). However, involvement of more ventral ACC (pregenual ACC) in response to combined high- and low-risk choices as compared to equal-risk selections may reflect greater emotional salience of high- versus equal-risk stimuli (Hampton and O'Doherty 2007). Nevertheless greater activation of these regions during the high-risk as compared to low-risk choices suggests that the participants were more conflicted when they contemplated the choice of high reward due to the low probability of attaining this reward. Taken together, these results suggest that probabilistic monetary rewards with varying levels of risk and magnitude activate distinct sub-regions within ACC and OFC in adolescents as well as adults.
Recruitment of bilateral mPFC during high-risk behavior, as observed in this study, has been documented earlier (Bush et al. 2002). This is not unexpected as mPFC is part of an interconnected network organized around ACC, which encompasses a wide range of neural and neurochemical systems implicated in autonomic and visceral control (Carmichael and Price 1996; Ongur and Price 2000; Ongur et al. 2003). In addition, mPFC has been implicated in the registration of reward versus punishment (Breiter et al. 2001; Elliott et al. 1999), and controls the reward-related behavior by tracking the magnitude of delivered reward (Breiter et al. 2001; Knutson et al. 2003). The later finding is supported by the observation of bilateral activation of mPFC in response to rewards of higher magnitude than lower magnitude in this study. Of note, higher SES was associated with lower activation in the right mPFC during the reward-related behavior. This could reflect greater mPFC-related “default” activity and less cognitive effort by adolescents from the lower SES compared to those from higher SES, despite more frequent high-risk selections. Based on new EEG data from UC Berkeley (accepted for publication in the Journal of Cognitive Neuroscience), children with low SES were found to have lower prefrontal response to unexpected novel stimuli than those from high SES. http://www.sciencedaily.com/releases/2008/12/081203092429.htm
With regard to reward magnitude, only one study in youngsters (Galvan et al. 2006) and three studies in adults (Galvan et al. 2006; Rogers et al. 2004; Smith et al. 2009) assessed the impact of the magnitude of reward on PFC activations. Galvan et al. (2006) reported greater OFC activation with rewards of large magnitude in children and adults but not adolescents, while Rogers et al. (2004) and Smith et al. (2009) observed the activations of ACC and mPFC in response to rewards of larger magnitude than smaller magnitude. The adolescent sample size in the Galvan et al. (2006) investigation was modest (n = 13), limiting the power to detect more modest effects. Overall, the findings from this study are consistent with results from these earlier studies and rewards of greater than smaller magnitude induced more significant activation of bilateral ACC, left OFC, and bilateral mPFC. It was interesting to observe a similar ventral extension of ACC activation in response to rewards of larger than smaller magnitude as observed with risky versus non-risky behavior in this study. These findings suggest that risky choices and rewards of larger magnitude may have a higher emotional salience than non-risky choices and rewards of smaller magnitude, respectively.
Post-hoc analysis revealed greater activation of the left ventral striatum in response to rewards of larger magnitude, when the probability of reward was maintained constant. This finding is consistent with results from an earlier study, which showed a direct correlation between reward magnitude and activation in the ventral striatum (Knutson et al. 2001). In contrast to this, both ventral and dorsal portions of the striatum were activated while making a choice that involved higher -magnitude reward than lower magnitude reward, suggesting that both ventral and dorsal portions of the striatum are involved in the decision-making process involving monetary rewards (Belleine et al. 2007; Ernst et al. 2005; Galvan et al. 2007). It has been suggested that the dorsal striatum is particularly involved during the selection and initiation process through the integration of sensorimotor, cognitive and emotional/motivational information (Balleine et al. 2007; O'Doherty et al. 2004), whereas the ventral striatum might be better able to critically ascertain the reward magnitude, particularly under uncertain conditions (O'Doherty et al. 2004; Knutson et al. 2001; Pagoni et al. 2002). Consistent with a previous investigation, activation in the striatum was associated with the choice of a high-magnitude reward regardless of age but not specifically with high-risk/impulsive behavior (Galvan et al. 2007).
In contrast to our hypothesis, chronological age did not have a significant influence on brain activations associated with the selection of monetary rewards. These results suggest that, within the adolescent population, individual differences appear to have a greater influence than chronological age per se. Previous investigations reported developmental differences among various age groups (Eshel et al., 2007; Galvan et al., 2006). Despite the developmental differences across age groups, there were significant individual differences within specific age groups and these individual differences contributed greater variance to the brain-behavior relationship in both adolescents and adults (Eshel et al. 2007; Galvan et al. 2007). Also, no relationship was observed between pubertal stage and PFC activations. However, this might be due to the limited variance in pubertal status within this sample, with majority of the adolescents in later pubertal developmental stage. Future investigations should recruit youth across different pubertal states and also include gonadal hormonal measures in order to better understand the association between pubertal status and the neurobiology of decision-making. Finally, adolescents from higher SES selected a high-risk reward more frequently than those from lower SES, suggesting that their economic advantage offers the choice to take greater monetary risks.
The findings from this study should be interpreted in the context of study limitations. The sample comprised of adolescent volunteers with stringent eligibility criteria, and the results might not be applicable to adolescents in the community. Also, the temporal differentiation of BOLD responses associated with the selection phase from the anticipation phase is limited, especially due to the lack of jitter in the WOF task used in this study. However, this is an issue that is not specific to this study because anticipation is probably present even before the selection since it contributes to the selection itself. Although activation of the ventral striatum observed in this study could be attributed to the anticipation phase of decision-making as reported earlier in healthy adolescents and adults (Bjork et al. 2004; Knutson et al. 2001), the striatum, particularly the dorsal portion, is also involved in the selection phase (Balleine et al. 2007). Additionally, the selections could be impacted by the feedback phase as well. However, the regions involved in feedback (i.e., ventral striatum and mPFC) are different from the regions involved in selection (lateral PFC, OFC) (Ernst et al. 2004). Thus, it is unlikely that there might be a systematic bleeding of feedback into selection in the regions examined. Finally, the task was not designed for even distribution of chance trials, which explains the significant inter-subject difference in the selection of 25% versus 75% probabilities within the 2575 wheel. This explains how the three subjects ended up choosing less than 10% of 25% (high-risk) option and were not analyzed in this study due to statistical reasons.
Despite these limitations, this study reports differential pattern of activation in response to varying levels of risk and reward probability without the confounding effects of reward magnitude and reaction times in a relatively large sample of adolescent subjects. The large sample size also provided the opportunity to control for important sociodemographic variables to examine the relationship between high-risk behavior and the magnitude of PFC activations. The unique contribution of high-risk behavior to PFC activations in ACC, OFC, and mPFC suggests that individual differences in risk-taking behavior might be important in recruiting the neural circuits associated with decision-making during adolescent development than chronological age or any other sociodemographic factors.
Conclusion
The present results demonstrate engagement of the PFC monitoring regions (i.e., ACC, mPFC and OFC) during risk/reward behavior, as observed in prior studies. The inverse relationship between high-risk behavior and PFC activations suggests individual differences in recruiting neural circuits during the decision-making process that might have implications for real-world risk-taking behaviors. In a larger sample of adolescents of adolescents that completed the WOF task outside the scanner (also including the sample reported here), risky selections were associated with real-life risk-taking behavior and substance-related problems (Rao et al., in press). It is of interest that the sample reported in the current manuscript is a subset of the participants reported in the Rao et al., paper (in press), indicating that there might be an association between behavioral measures and neuronal function. Of note, the current findings are initial results from an ongoing longitudinal study to monitor the adolescents’ behavior over time to assess whether differential activation of the PFC regions will predict future high-risk behavior and the development of psychopathology.
Acknowledgments
This work was supported, in part, by grants from the National Institutes of Health (DA14037, DA15131, DA17804, DA17805, MH62464 and MH68391), and the Sarah M. and Charles E. Seay Endowed Chair in Child Psychiatry at UT Southwestern Medical Center. The authors also express gratitude to the Dallas and Fort Worth Independent School Districts for their assistance in providing resources and infrastructure for the recruitment of study participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest
The primary author has received funding from Eli Lilly for other research. The other authors have no conflicts of interest.
References
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. Journal of Neuroscience. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosada O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Neurobiology of decision-making: risk and reward. Semin Clin Neuropsychiatry. 2001;6:205–216. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- Benthin A, Slovic P, Severson H. A psychometric study of adolescent risk perception. J Adolescence. 1993;16:153–168. doi: 10.1006/jado.1993.1014. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW. Anticipating instrumentally obtained and passively-received rewards: a factorial fMRI investigation. Behav Brain Res. 2007;12:165–70. doi: 10.1016/j.bbr.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: Similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–19. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;65(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JP. The development of decision-making. J Adolesc Health. 2002;31:208–215. doi: 10.1016/s1054-139x(02)00503-7. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;22:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey NJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, Thomas KM, Noll DC. Sensitivity of prefrontal cortex to changes in target probability: A functional MRI study. Human Brain Mapping. 2001;13:26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN. Neurodevelopment, impulsivity, and adolescent gambling. J Gambl Stud. 2003;19:53–84. doi: 10.1023/a:1021275130071. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA, Pascual-Leone A, Press DZ, Robertson EM. Off-line learning of motor skill memory: a double dissociation of goal and movement. Proc. Natl Acad. Sci. U.S.A. 2005;102:18237–18241. doi: 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The Effects of Psychotherapy on Neural Responses to Rewards in Major Depression. Biol Psychiatry. 2009;66:886–897. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Ross J, Hawkins J, Harris WA, et al. Youth risk behavior surveillance--United States, 2005. MMWR Surveill Summ. 2006;55:1108. [PubMed] [Google Scholar]
- Elliott R, Rees G, Dolan RJ. Ventromedial prefrontal cortex mediates guessing. Neuropsychologia. 1999;37:403–411. doi: 10.1016/s0028-3932(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, et al. Decision-making in a risk-taking task: A PET study. Neuropsychopharmacol. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Eshel N, Zarahn E, et al. Choice selection and reward anticipation: An fMRI study. Neuropsychologia. 2004;42:15851597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of Decision Making: A Selective Review from a Neurocognitive and Clinical Perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith C, Frackowiak RSJ. Statistical Parametric Maps in Functional Imaging: A General Linear Approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Galvan TA, Hare CE, Parra J, Penn H, Voss S, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan TA, Hare CE, Voss S, Glover G, Casey BJ. Risk-taking and the adolescent brain: Who is at risk? Dev Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;25:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullo MJ, Dawe S. Impulsivity and adolescent substance use: rashly dismissed as “all-bad”? Neurosci Biobehav Rev. 2008;32:1507–1518. doi: 10.1016/j.neubiorev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Hadland KH, Rushworth MF, Gaffan D, Passingham RE. The anterior cingulate and reward-guided selection of actions. Journal of Neurophysiology. 2003;89:11611164. doi: 10.1152/jn.00634.2002. [DOI] [PubMed] [Google Scholar]
- Hampton AN, O'Doherty JP. Decoding the neural substrates of reward-related decision making with functional MRI. Proc Natl Acad Sci U S A. 2007;104:1377–1382. doi: 10.1073/pnas.0606297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-aged Children-Present and Lifetime version K-SADS-PL: initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Wallis JD. Evaluating choices by single neurons in the frontal lobe: outcome value encoded across multiple decision variables. Eur J Neurosci. 2009;29:2061–2073. doi: 10.1111/j.1460-9568.2009.06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci Biobehav Rev. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. Review. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, et al. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cerebral Cortex. 2001;11:85–92. doi: 10.1093/cercor/11.1.85. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–26. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Sci. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in human ventral striatum locked to errors of reward prediction. Nat Neurosci. 2002;5:97–98. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Zauscher B, McDowell JE, Frank L, Brown GG, et al. Prefrontal, parietal, and temporal cortex networks underlie decision making in the presence of uncertainty. Neuroimage. 2001;13:91–100. doi: 10.1006/nimg.2000.0667. [DOI] [PubMed] [Google Scholar]
- Rao U, Sidhartha T, Harker KR, Chen L-A, Bidesi AS, Ernst M. Relationship between adolescent risk preferences on a laboratory task and behavioral measures of risk-taking. J Adolesc Health. 2010 doi: 10.1016/j.jadohealth.2010.06.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW. Choosing between small, likely rewards and large, unlikely rewards activate inferior and orbital prefrontal cortex. J Neurosci. 1999;19:9028–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Précis of The brain and emotion. Behav Brain Sci. 2000;23:177–191. doi: 10.1017/s0140525x00002429. discussion 192-233. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Gough PM, Alexander IH, Kyriazis D, McDonald KR, et al. Attentional selection and action selection in the ventral and orbital prefrontal cortex. Journal of Neurosci. 2005;25:11628–11636. doi: 10.1523/JNEUROSCI.2765-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zoccolotto A. E-Prime User's Guide Pittsburgh. Psychology Software Tools Inc.; 2002. [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BW, Mitchell DG, Hardin MG, Jazbec S, Fridberg D, Blair RJ, Ernst M. Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. Neuroimage. 2009;15:600–609. doi: 10.1016/j.neuroimage.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, et al. MRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobeh Rev. 2000a;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000b;24:417–463. [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann N Y Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44:1764–78. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, Himle JA, Gehring WJ. Medial frontal cortex activity and loss-related responses to errors. J Neurosci. 2006;26:4063–4070. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WD, Orvaschel H, Prusoff BA, Kidd KK. An evaluation of the family history method for ascertaining psychiatric disorders. Arch Gen Psychiatry. 1982;39:53–58. doi: 10.1001/archpsyc.1982.04290010031006. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44:2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Westenberg PM, Crone EA. A developmental study of risky decisions on the cake gambling task: age and gender analyses of probability estimation and reward evaluation. Dev Neuropsychol. 2008;33:179–96. doi: 10.1080/87565640701884287. [DOI] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nature Neuroscience. 2004;7:1259–1265. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale: Administration and Scoring Manual. 3th ed. the Psychological Corporation; San Antonio TX: 1997. [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children: Administration and Scoring Manual. 4th ed. the Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat. Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN, Hanelin J, Wager TD, Mackey SC. Different circuits for different pain: patterns of functional connectivity reveal distinct networks for processing pain in self and others. Soc Neurosci. 2007;2:276–91. doi: 10.1080/17470910701401973. [DOI] [PMC free article] [PubMed] [Google Scholar]