Abstract
Objective
Accumulating evidence indicates an important role of neutrophils in the development of rheumatoid arthritis (RA). Recruitment of neutrophils to the joint space and release of proteolytic enzymes can exacerbate tissue damage and the inflammatory response related to RA. Engagement of β2 integrin and subsequent activation of downstream signaling have been shown to be fundamental for activation of neutrophil effector functions. The aim of this study was to test the hypothesis that Vav and phospholipase Cγ2 (PLCγ2), two molecules involved in integrin signaling, are required for arthritis generation and neutrophil activation in a mouse model of arthritis.
Methods
Arthritis was induced in wild-type (WT), Vavnull, and PLCγ2−/− mice using the K/BxN serum–transfer model. Neutrophil function was assessed by analyses of adhesion, spreading, and degranulation on integrin-dependent substrates. Regulation of integrin signaling was determined by analyzing the phosphorylation of Pyk-2, Src, and ERK.
Results
Vavnull and PLCγ2−/− mice were protected from inflammation and bone erosion in the K/BxN serum–transfer model of arthritis. Mechanistically, Vav and PLCγ2 control neutrophils mediated spreading and degranulation on integrin-dependent substrates. Consequently, the Vav/PLCγ2 axis, acting downstream of the integrin receptor, modulated the activation of Pyk-2, Src, and ERK.
Conclusion
Our findings show that Vav cooperates with PLCγ2 in modulating neutrophil activation downstream of the integrin receptor. This study identifies a Vav/PLCγ2-dependent signaling pathway as a possible therapeutic target for the treatment of inflammation and bone disruption in arthritis.
Rheumatoid arthritis (RA) is a debilitating chronic autoimmune disease characterized by progressive inflammation that affects the synovial membranes of the joints. Accumulating evidence suggests that the innate branch of the immune system is fundamental in the induction of arthritis (1). The K/BxN serum–transfer model of inflammatory arthritis bears clinical and histopathologic similarities to human RA, such as the recruitment of leukocytes to the joint space, pannus formation, and bone and cartilage damage (2). Transfer of serum from K/BxN mice containing autoantibodies directed toward glucose-6-phosphate isomerase into a recipient mouse leads to the development of an inflammatory arthritis that is strongly dependent on neutrophils (3). In particular, neutrophils are recruited to the joint space within 1–2 days from the initial injection of arthritic serum. Within 1 week, synovial inflammation, pannus formation, and erosion of bone and cartilage are observed (4). A crucial mechanism for the induction of inflammation includes the release of neutrophil granules that contain proteolytic enzymes, such as elastase, matrix metalloproteinases, and collagenase, that can exacerbate the tissue damage and amplify the neutrophil response (5).
Regulation of neutrophil activity is largely dependent on integrin-mediated adhesive interactions (6,7). Integrins are transmembrane heterodimeric glycoproteins consisting of an α/β heterodimer that mediates cell–cell and cell–extracellular matrix interactions (8) and transmits intracellular signals, leading to gene expression and cytoskeleton rearrangement (9,10). In the immune system, integrins play essential roles in leukocyte trafficking and function (11). These include immune cell attachment to endothelial and antigen-presenting cells, cytotoxicity, and extravasation into tissues, all of which are essential functions for activation of the inflammatory response (12). In particular, β2 integrins are implicated in the regulation of neutrophil effector functions, such as bacterial clearance, oxidative burst, and degranulation (7,13).
The Vav family of guanine nucleotide exchange factors mediates signaling downstream of integrin receptors by promoting the activation of Rho family GTPases, thereby modulating the reorganization of the cytoskeleton in response to cell adhesion (14–17). Three Vav proteins exist: Vav1 is expressed primarily in hematopoietic cells, whereas Vav2 and Vav3 are more broadly expressed (18). In neutrophils, Vav1 and Vav3 regulate β2 integrin–mediated adhesion and signaling through activation of Pyk-2, Src, and ERK (15). In a recent study, we also showed that Vav proteins control the phosphorylation of phospholipase Cγ2 (PLCγ2) downstream of integrin signaling, thereby modulating the induction of neutrophil oxidative burst by a variety of adhesion-dependent stimuli (19).
PLCγ has been implicated in the activation of integrin receptor signaling in various cell types (20,21). Two isoforms of PLCγ are known: PLCγ1, which is widely expressed, and PLCγ2, which is more specifically expressed in the hematopoietic lineage (22). While PLCγ1 participates in T cell receptor signals, where it modulates calcium fluxes, PLCγ2 has been linked to integrin function in platelets, where its phosphorylation downstream of the αIIbβ3 integrin is necessary for platelet spreading (23). We have also observed that PLCγ2 mediates αvβ3 integrin signaling in osteoclasts, which is required for efficient bone resorption (24).
In the present study, we show that Vav1/2/3 triple-knockout (Vavnull) mice and PLCγ2−/− mice are protected from inflammatory arthritis in the K/BxN serum–transfer model. Using neutrophils from Vavnull and PLCγ2−/− mice, we demonstrated that the Vav/PLCγ2 pathway controls integrin-dependent neutrophil functions, such as spreading and degranulation. We also show that Vav and PLCγ2 are required for β2 integrin–mediated phosphorylation of Pyk-2, Src, and ERK. Our data suggest that targeting the Vav/PLCγ2 signaling axis might be of clinical relevance for amelioration of arthritis.
MATERIALS AND METHODS
Mice
The PLCγ2−/− and Vavnull mice used in these studies have been described previously (25,26). All experiments were approved by the Animal Care and Use Committee of the Washington University School of Medicine.
Serum induction of arthritis and scoring of arthritis
Serum from K/BxN mice (200 µl) was injected intraperitoneally into recipient mice on day 0, day 2, and day 7. Paw swelling was determined in terms of the fold induction, which was calculated as follows:
Mice were killed on day 7 or on day 14, and the front paws and hind paws were collected for histologic analysis and RNA extraction.
Real-time polymerase chain reaction (PCR) analysis
Paws from arthritic mice were frozen in liquid nitrogen and then pulverized with a dismembrator (B Braun Biotech International, Melsungen, Germany). Total RNA was extracted, and real-time PCR was performed using the following primers: for GAPDH, GACGGACACATTGGGGGTAG (reverse) and CTTCACCACCATGGAGAAGGC (forward); for interleukin-1 (IL-1), TCAAAAGGTGGCATTTCACAGT (reverse) and GCTTCCTTGTGCAAGTGTCTGA (forward); for IL-6 TGCAAGTGCATCATCGTTGTT (reverse) and TTCTCTGGGAAATCGTGGAAA (forward); for tumor necrosis factor α (TNFα), TTGAGATCCATGCCGTTG (reverse) and CTGTAGCCCACGTCGTAGC (forward); and for Gr-1, TCTGCTTGATGACATGCCAACT (reverse) and CACGTGGATTACGGCTTTCA (forward).
Relative quantification of transcription was calculated as the power of the difference between amplification of the target gene and amplification of GAPDH (i.e., 2 −[Ct target gene − Ct GAPDH], where Ct represents threshold cycle).
Neutrophil preparation
Bone marrow was harvested from long bones, and red blood cells were lysed in hypotonic saline solution (0.2% NaCl). Cells resuspended in Hanks’ balanced salt solution (HBSS; without calcium and magnesium) were overlaid on a discontinuous Percoll gradient (80% and 55% Percoll fractions). Neutrophils were recovered from between the 2 Percoll fractions after centrifugation for 30 minutes at 1,000g and resuspended in HBSS with calcium and magnesium.
Neutrophil migration in vitro and in vivo
In vitro neutrophil migration was assessed in 24-well Transwell plates (3-µm filters) obtained from Costar (Corning Costar, Cambridge, MA). Neutrophils (1 × 105) were seeded into the upper filter and allowed to migrate to the lower well, which contained C5a (Sigma, St. Louis, MO) at 10, 50, or 100 ng/ml or control medium for 1 hour. Phorbol myristate acetate (PMA; 1 µg/well) was added to the lower well for 10 minutes to allow migrated cells to adhere. Cells were fixed, stained with crystal violet, and counted.
To evaluate the migratory capacity of neutrophils in vivo, mice were injected intraperitoneally with 2 ml of 4% thioglycollate in phosphate buffered saline (Sigma). After 4 hours, cells were recovered from peritoneal exudates, and the numbers of neutrophils were determined by counting and by flow cytometric analysis.
Neutrophil adhesion and spreading assay
Neutrophils were plated on coverslips that had been precoated with 1% bovine serum albumin (BSA) or 1 µg/ml of p-RGD (Sigma). PMA (500 ng/ml, Sigma) was added as a positive control. After 15 minutes at 37°C, adherent cells were fixed, stained with crystal violet, and counted. For spreading assays, cells were stained with a fluorescein isothiocyanate–conjugated phalloidin (Molecular Probes, Eugene, OR), and photomicrographs were taken using an Olympus fluorescence microscope with a 20× objective (Olympus, Lake Success, NY).
Neutrophil degranulation assay
Neutrophils (5 × 105/well) were plated on 96-well plates that had been coated with p-RGD (1 µg/ml) or fibrinogen (100 µg/ml; Sigma). In some experiments, cells were also stimulated with C5a (5 µg/ml; Sigma) and TNFα (200 ng/ml; PeproTech, Rocky Hill, NJ). PMA (500 ng/ml) was added to the cells as a positive control. After 2 hours, the media were collected from the wells, centrifuged to separate cells, and subjected to Western blot analysis using a polyclonal antibody against lactoferrin (Sigma).
Western blot analysis
Freshly isolated neutrophils (2 × 106) were plated on 24-well plates that had been coated with p-RGD (1 µg/ml). Cells were lysed in radioimmunoprecipitation assay buffer and subjected to Western blot analysis using polyclonal antibodies against p-p42/p44 (p-ERK) and p-Src-416 (both from Cell Signaling Technology, Beverly, MA), p-Pyk-2-402 (BioSource International, Camarillo, CA), and β-actin (Sigma).
Statistical analysis
Student’s 2-tailed t-test was used for all comparisons. P values less than 0.05 were considered significant.
RESULTS
Decreased inflammatory response of PLCγ2−/− mice to serum-induced arthritis
We recently demonstrated that PLCγ2–/– mice have an osteopetrotic phenotype due to defective RANKL-mediated osteoclast differentiation (27). To analyze whether PLCγ2−/− mice are protected from inflammatory-mediated bone loss, we used the K/BxN passive serum–transfer model of arthritis, which is known to induce focal bone erosion in vivo (28). WT and PLCγ2−/− mice were injected on day 0, day 2, and day 7 with arthritogenic serum, and inflammation was evaluated daily by measuring the paw thickness.
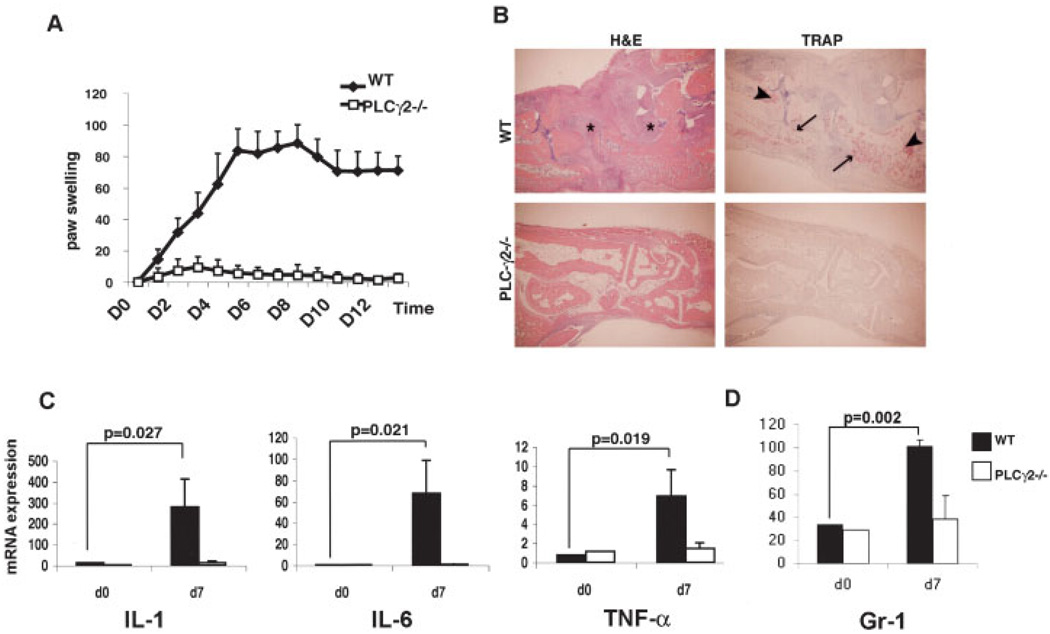
Strikingly, while WT mice exhibited substantial paw and ankle redness and swelling by day 2, reaching maximum thickness on day 5, PLCγ2−/− mice were completely protected from developing the disease (Figure 1A). Serum injection on day 7 prolonged the inflammation in WT mice, but was completely unable to trigger a response in the absence of PLCγ2.
Figure 1.
Protection of PLCγ2−/− mice from inflammatory arthritis. A, Hind paw thickness measured daily in wild-type (WT) and PLCγ2−/− mice injected intraperitoneally with serum from K/BxN mice. Increase in paw swelling was expressed as the fold induction from baseline. Values are the mean and SD of 4 mice per group. Results from 1 of 3 representative experiments are shown. B, Histologic features of hind paws obtained on day 14 from WT and PLCγ2−/− mice treated as in A. Sections were stained with hematoxylin and eosin (H&E) to detect inflammatory infiltrates (*) or with tartrate-resistant acid phosphatase (TRAP) to detect osteoclasts (arrowheads). Arrows indicate bone erosion (original magnification × 10). C, Levels of expression of mRNA for the inflammatory cytokines interleukin-1 (IL-1), IL-6, and tumor necrosis factor α (TNFα) in tissue extracts from paws obtained on day 7 from WT and PLCγ2−/− mice injected with arthritogenic serum. Data were normalized for GAPDH expression. Values are the mean and SD of at least 4 mice per group. D, Levels of expression of mRNA for the neutrophil marker Gr-1 in tissue extracts from paws obtained on day 7 from WT and PLCγ2−/− mice treated as in C. Values are the mean and SD of 4 mice per group.
Histologic assessment of bone erosion and cellular infiltration in the knee joints and ankles confirmed the clinical findings, with WT mice displaying massive cellular infiltration and evidence of cartilage and bone erosion (Figure 1B), whereas PLCγ2−/− mice did not show such changes. Tartrate-resistant acid phosphatase staining revealed pronounced recruitment of osteoclasts in WT bone tissues, in contrast to PLCγ2−/− mice, which showed virtually no osteoclasts in the ankle (Figure 1B) and knee joints (results not shown).
We next quantified by real-time PCR the levels of messenger RNA (mRNA) for cytokines known to be present in the inflamed joints of patients with RA, which are also implicated in the progression of the disease in mice. To this end, we measured levels of expression of mRNA for IL-1, IL-6, and TNFα in snap-frozen, pulverized paws from WT and PLCγ2−/− mice 7 days after the first injection of arthritic serum. Reflecting the histologic findings, levels of IL-1, IL-6, and TNFα in the paws of PLCγ2−/− mice were comparable to baseline (day 0) control levels. In contrast, there was a significant increase in all of these cytokines in arthritic tissues from WT mice compared with baseline (Figure 1C).
The K/BxN serum–transfer model of arthritis is strongly dependent on neutrophils (3). Thus, we determined by real-time PCR the expression levels of Gr-1, a surface protein marker specific for neutrophils, in the paws of animals injected with arthritic serum. Confirming the clinical findings, we did not observe an appreciable increase in Gr-1 expression in the paws of PLCγ2−/− mice above the basal levels (Figure 1D).
Requirement of Vav proteins for the inflammatory response in the serum-induced arthritis model
Vav proteins are essential modulators of PLCγ in T cells and B cells downstream of their antigen receptors (29), and they control PLCγ2 phosphorylation following integrin-mediated adhesion in neutrophils (19). Thus, we hypothesized that mice deficient in Vav proteins (Vavnull mice) would be protected from inflammatory arthritis, as were the PLCγ2−/− mice.
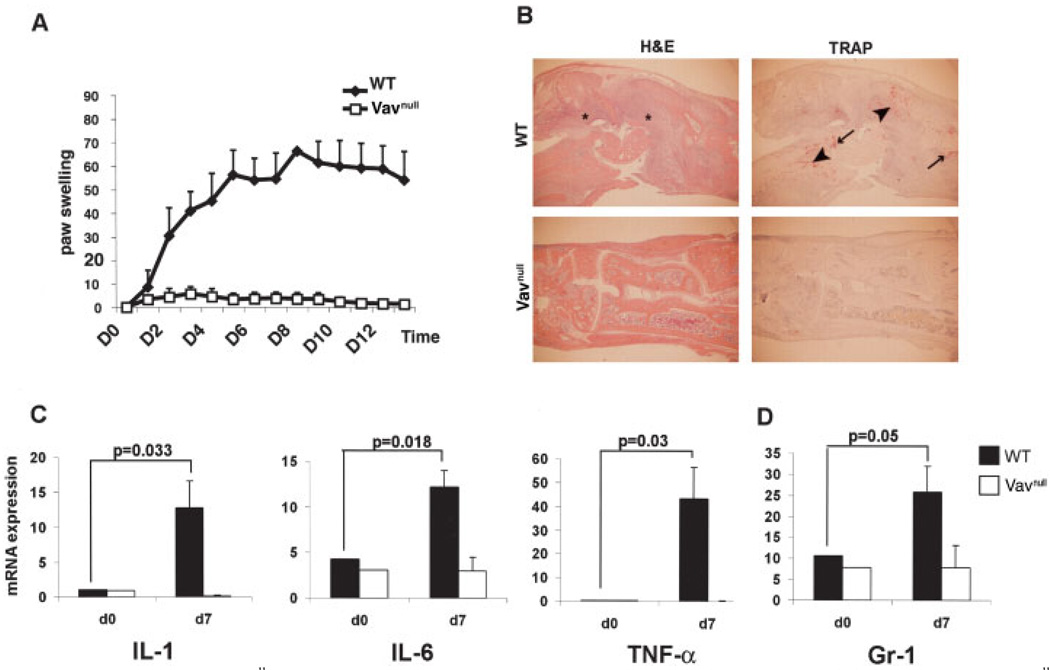
Similar to the findings in the PLCγ2−/− mice, the front and hind paws of Vavnull mice failed to develop any signs of inflammation following injection of K/BxN serum (Figure 2A). Histologic sections of the hind paws confirmed the lack of cellular infiltration, osteoclast recruitment, and cartilage and bone erosion in Vavnull mice compared with WT controls (Figure 2B). Furthermore, Vavnull animals displayed decreased levels of expression of mRNA for inflammatory cytokines following injection of arthritic serum (Figure 2C), similar to the findings in mice lacking PLCγ2. We also confirmed the histologic absence of a neutrophil response by quantitating the levels of mRNA for Gr-1 on day 0 and day 7. While Gr-1 levels were increased 3-fold in WT mice from day 0 to day 7, there was no change in Gr-1 levels in Vavnull mice (Figure 2D), resembling the phenotype of the PLCγ2−/− animals.
Figure 2.
Protection of Vavnull mice from inflammatory arthritis. A, Hind paw thickness measured daily in WT and Vavnull mice injected intraperitoneally with serum from K/BxN mice. Increase in paw swelling was expressed as the fold induction from baseline. Values are the mean and SD of 4 mice per group. Results from 1 of 3 representative experiments are shown. B, Histologic features of hind paws obtained on day 14 from WT and Vavnull mice treated as in A. Sections were stained with hematoxylin and eosin (H&E) to detect inflammatory infiltrates (*) or with tartrate-resistant acid phosphatase (TRAP) to detect osteoclasts (arrowheads). Arrows indicate bone erosion (original magnification × 10). C, Levels of expression of mRNA for the inflammatory cytokines interleukin-1 (IL-1), IL-6, and tumor necrosis factor α (TNFα) in tissue extracts from paws obtained on day 7 from WT and Vavnull mice injected with arthritogenic serum. Data were normalized for GAPDH expression. Values are the mean and SD of at least 4 mice per group. D, Levels of expression of mRNA for the neutrophil marker Gr-1 in tissue extracts from paws obtained on day 7 from WT and Vavnull mice treated as in C. Values are the mean and SD of 4 mice per group.
Importantly, the lack of neutrophil recruitment in the arthritic joints of Vavnull and PLCγ2−/− mice was not due to absent or diminished numbers of circulating neutrophils, since we did not observe alterations in the neutrophil counts in blood and bone marrow from these animals as compared with WT mice (results not shown). Collectively, these data represent the first demonstration that Vav proteins and PLCγ2 modulate the development of the inflammatory response and the associated bone destruction in the K/BxN model of RA.
Failure of Vav/PLCγ2 axis to control neutrophil migration
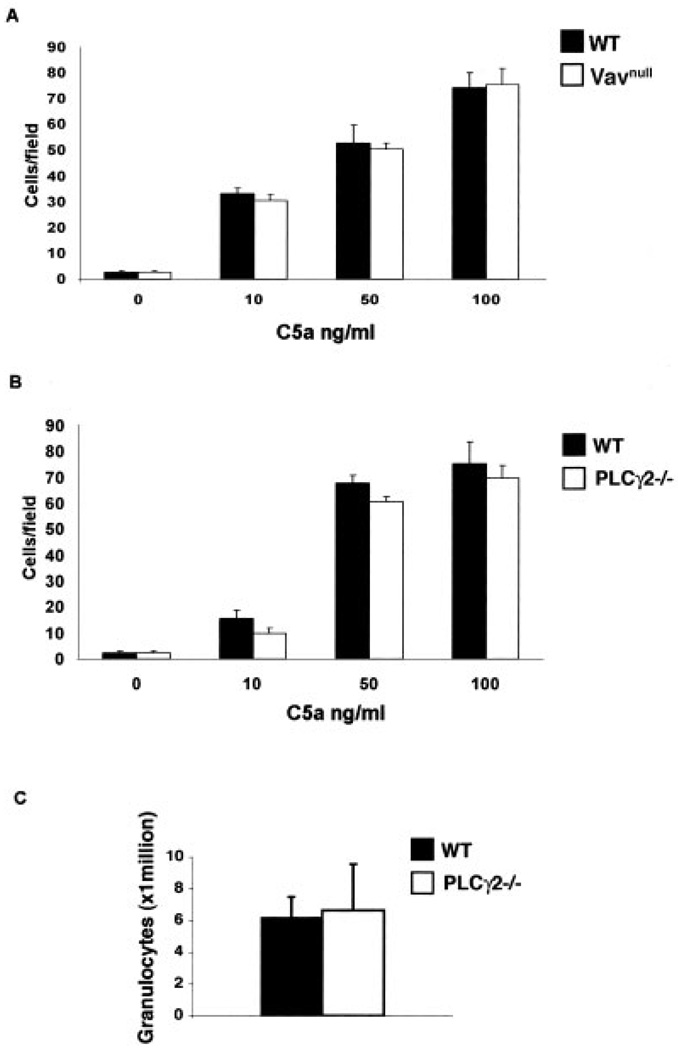
One explanation for the lack of inflammation in the joints of Vavnull and PLCγ2−/− mice in response to arthritogenic serum would be that neutrophils have motility defects. To test this hypothesis, we first examined the chemotactic response of neutrophils in vitro. Purified bone marrow–derived neutrophils isolated from WT, Vavnull, and PLCγ2−/− mice were allowed to migrate toward different concentrations of C5a, a chemotactic stimulus required for the induction of arthritis in the serum-transfer model (30). Interestingly, both Vavnull and PLCγ2−/− neutrophils migrated across perforated Transwells toward increasing concentrations of C5a with efficiencies similar to those of WT neutrophils (Figures 3A and B), indicating that the Vav/PLCγ2 axis is not required for neutrophil migration, at least not in vitro.
Figure 3.
Failure of Vav and PLCγ2 to control neutrophil chemotaxis. A and B, In vitro Transwell migration of neutrophils from wild-type (WT), Vavnull, and PLCγ2−/− mice in response to the indicated concentrations of C5a. Values are the mean and SD. Results are representative of 3 independent experiments. C, In vivo migration of neutrophils from WT and PLCγ2−/− mice to the peritoneum following a single intraperitoneal injection of 4% thioglycollate. Values are the mean and SD number of peritoneal exudate cells. Results are representative of 3 independent experiments.
In vivo, neutrophils must travel through the blood vessels and respond to numerous stimuli to reach sites of primary inflammation, adding greater complexity to the migration process. Therefore, we determined the migratory capacity of neutrophils in vivo using a peritonitis model. Peritonitis was induced by injecting thioglycollate intraperitoneally, and 4 hours later, the cells recruited to the peritoneum were counted. As we previously reported (31), we did not observe any motility defect in neutrophils lacking Vav (results not shown). Furthermore, and consistent with the in vitro findings, PLCγ2−/− neutrophils migrated into the peritoneal cavity in numbers similar to those of WT neutrophils (Figure 3C), suggesting that the lack of an inflammatory response in Vavnull and PLCγ2−/− animals is likely not to be dependent upon a decreased capacity of neutrophils to reach the site of inflammation.
Requirement of Vav and PLCγ2 for cell spreading in response to integrin stimuli
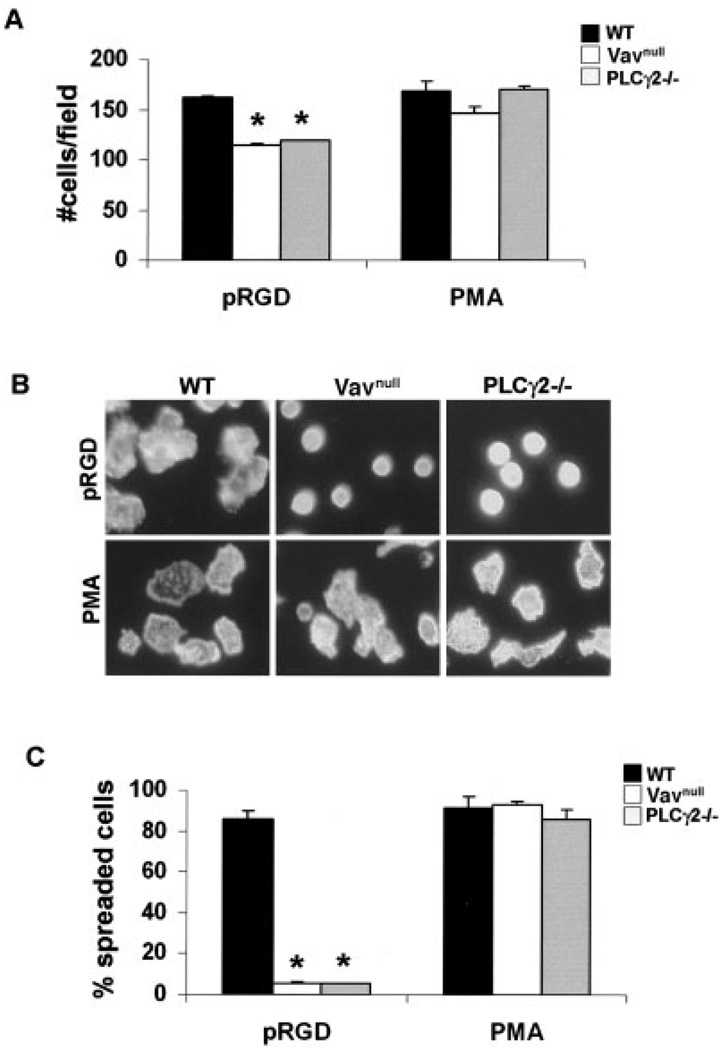
Neutrophils express the integrin receptors αLβ2 (or, lymphocyte function–associated antigen 1) and αMβ2 (or, Mac-1), both of which are known to contribute to neutrophil adhesion and to modulate neutrophil effector functions, such as oxidative burst, degranulation, and phagocytosis (32,33). To examine the role of the Vav/PLCγ2 axis in integrin-dependent neutrophil functions, neutrophils were analyzed for their ability to adhere to and spread on pRGD, a synthetic peptide with high affinity for the integrin extracellular domain, which bypasses the need for the integrin to be activated by other intracellular signals (34). As a positive control, we stimulated the cells with PMA, which acts directly on downstream effectors, bypassing the requirement of the integrin, and as a negative control, we stimulated the cells with BSA. Following adhesion for 15 minutes, adherent cells were stained with phalloidin to visualize the actin cytoskeleton or with 4′,6-diamidino-2-phenylindole to visualize the nuclei for counting.
Neutrophils from WT mice rapidly attached to and spread on pRGD and PMA (Figure 4A), but they remained round and poorly adherent to BSA (results not shown). In contrast, neutrophils from Vavnull and PLCγ2−/− mice adhered to pRGD, although to a lesser extent than WT cells (Figure 4A), but maintained a round morphology, indicating a defect in cell spreading (Figures 4B and C). The reduced capacity of Vav and PLCγ2−/− neutrophils to attach to or spread on the integrin substrate was not due to impaired integrin expression, since they expressed levels of β2 integrin that were similar to those of WT cells (data not shown). Importantly, the ability of both Vavnull and PLCγ2−/− cells to spread was rescued by adding PMA (Figures 4B and C) or FMLP, a microbially derived ligand that signals through G protein–coupled receptor (GPCR) (results not shown). These data suggest that Vav and PLCγ2 deficiency does not affect the intrinsic ability of neutrophils to rearrange their cytoskeleton, but is involved in controlling downstream integrin signaling events.
Figure 4.
Requirement of Vav and PLCγ2 for cell spreading in response to integrin stimuli. A, Adhesion of neutrophils from wild-type (WT), Vavnull, and PLCγ2−/− mice to pRGD or in response to phorbol myristate acetate (PMA; positive control). Values are the mean and SD. * = P < 0.05 versus WT cells. B, Phalloidin immunostaining of cells treated as in A to detect actin organization (original magnification × 20). C, Capacity of neutrophils to spread on pRGD or in response to PMA. Spreading was defined as cells having lamellipodia, as indicated by phalloidin staining. Values are the mean and SD percentage of cells that had spread versus the total cell number. * = P < 0.05 versus WT cells.
Failure of Vavnull and PLCγ2−/− neutrophils to undergo adhesion-mediated degranulation
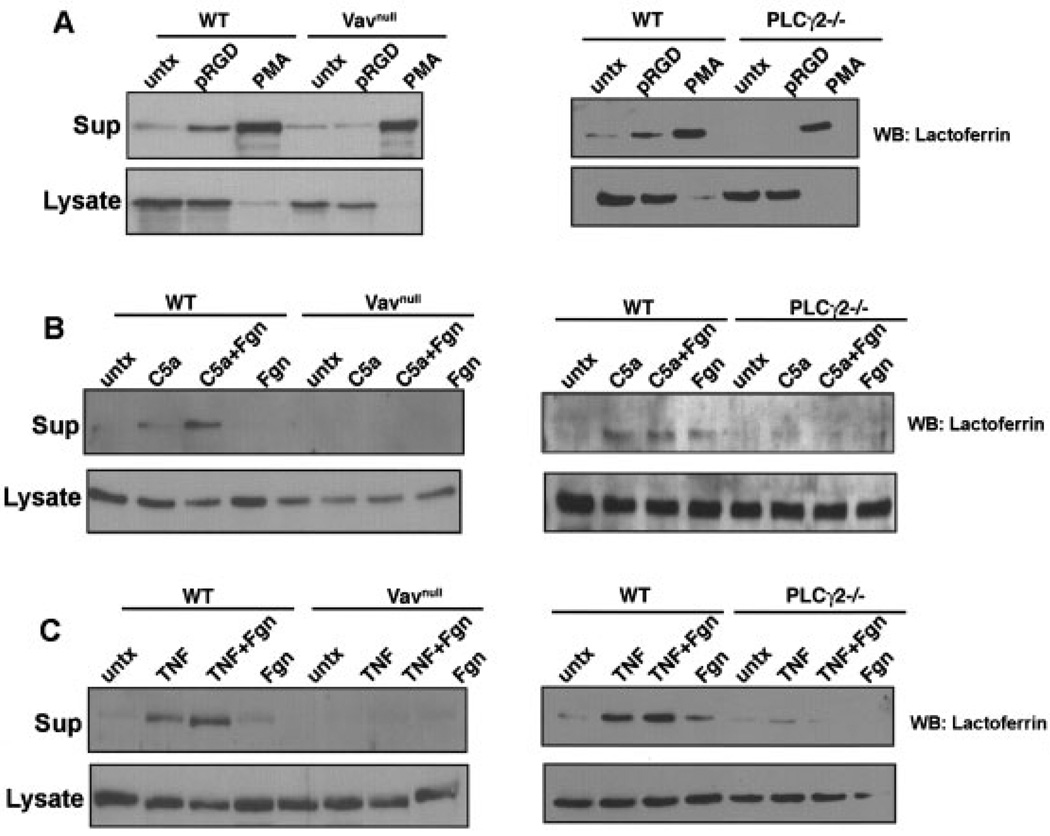
Increasing evidence indicates that integrin-mediated adhesion promotes degranulation, a critical effector mechanism used by neutrophils when they are exposed to mediators of inflammation (5,35). Thus, we monitored neutrophil degranulation following adhesion to integrin-dependent substrates by examining the release of lactoferrin, a known component of neutrophil secretory granules. WT, Vavnull, and PLCγ2−/− neutrophils were plated on pRGD for 2 hours, the supernatant was subsequently recovered and resolved by sodium dodecyl sulfate– polyacrylamide gel electrophoresis for detection of lactoferrin release by Western blotting. As a positive control, PMA was added to some wells, while cells plated onto BSA represented the negative control.
WT neutrophils degranulated within 2 hours of adhesion to pRGD and PMA (Figure 5A). In contrast, Vavnull and PLCγ2−/− neutrophils failed to degranulate in response to the integrin ligand, although they responded to PMA stimulation at levels similar to those of WT cells (Figure 5A). To more accurately reproduce neutrophil activation in the arthritic joints, we plated the cells on the integrin ligand fibrinogen in the presence or absence of mediators of inflammation, such as TNFα and C5a, which dramatically augment integrin signaling and have been shown to mediate inflammatory arthritis.
Figure 5.
Failure of neutrophils from Vavnull and PLCγ2−/− mice to undergo adhesion-mediated degranulation. Degranulation of neutrophils from wild-type (WT), Vavnull, and PLCγ2−/− mice in response to A, pRGD and phorbol myristate acetate (PMA), B, C5a and fibrinogen (Fgn), and C, tumor necrosis factor α (TNFα) and fibrinogen was determined by Western blotting (WB), according to the release of lactoferrin into the supernatant (Sup). The lactoferrin content in the cell lysate was also determined. Results are representative of 5 independent experiments. Untx = untreated.
Consistent with previous reports (35), WT neutrophils degranulated in response to stimulation with C5a or TNFα, and this response was augmented when the cells were plated on fibrinogen (Figures 5B and C). In contrast, Vavnull and PLCγ2−/− neutrophils were not responsive to C5a or TNFα, even in the presence of fibrinogen (Figures 5B and C). These data indicate that the Vav/PLCγ2 axis is involved in the regulation of neutrophil degranulation, suggesting that this might be an important mechanism responsible for the protection of PLCγ2−/− and Vavnull mice from tissue damage in the K/BxN serum–transfer model of arthritis.
Decreased β2 integrin–dependent signals in Vavnull and PLCγ2−/− neutrophils
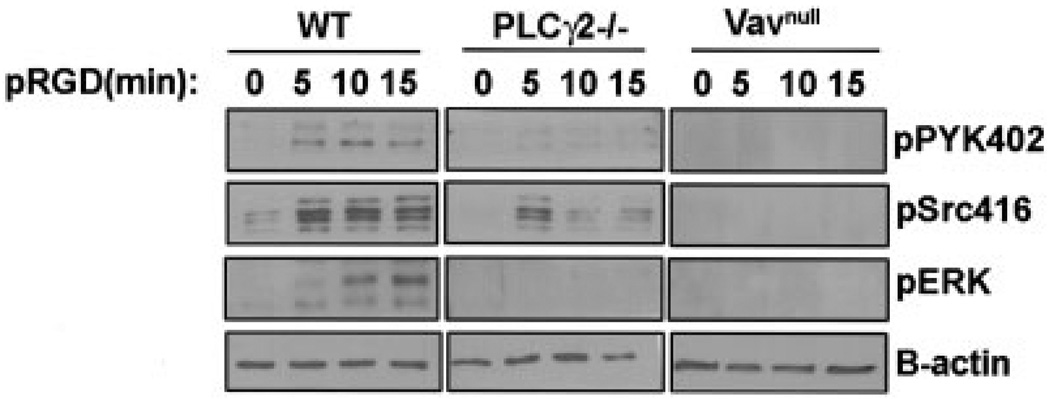
Pyk-2, Src, and ERK are known mediators of αMβ2 integrin signaling in neutrophils (15,36,37). To examine whether Vav and PLCγ2 modulate the activation of these signaling pathways downstream of integrin ligation, WT, Vavnull, and PLCγ2−/− neutrophils were plated on pRGD for different times, and adhesion-induced tyrosine phosphorylation of Pyk-2, Src, and ERK was examined by Western blotting. Previous reports indicated that neutrophils lacking Vav1 and Vav3 isoforms displayed defective integrin signaling (15). Similarly, we observed that Vavnull neutrophils, as well as PLCγ2−/− neutrophils, failed to promote Pyk-2, Src, and ERK activation in response to integrin engagement (Figure 6).
Figure 6.
Requirement of Vav and PLCγ2 for Pyk-2, Src, and ERK activation of neutrophils. Activation of the phosphorylated forms of Pyk-2, Src, and ERK in neutrophils from wild-type (WT), Vavnull, and PLCγ2−/− mice was determined by Western blotting of cells plated on pRGD for 5, 10, or 15 minutes. β-actin served as a loading control. Results are representative of 3 independent experiments.
DISCUSSION
The interaction between the immune system and the skeletal system is increasingly recognized as a significant cause of pathologic bone loss in RA (38). The inflammatory response associated with the human arthritic disease generates TNFα, macrophage colony-stimulating factor, IL-1, and RANKL, cytokines that fuel osteoclastogenesis and arthritic bone destruction. TNF blockade is widely used in the prevention of inflammatory bone loss in RA; however, not all patients are responsive to anti-TNF therapy (39). RANKL-neutralizing antibodies are likely to become the treatment of choice for blocking RANKL in RA, but a major limitation of RANKL antagonism is that it does not treat synovitis (40). Thus, the importance of identifying common signaling molecules affecting the osteo-immune system and determining their impact on pathologic bone loss is clear and may lead to novel therapies that would help to ameliorate the inflammatory condition and limit focal bone erosion.
Our findings reveal that PLCγ2−/− and Vavnull mice are protected from inflammatory bone loss induced by arthritogenic serum from K/BxN mice and that the 2 molecules modulate neutrophil activation, namely, degranulation. Our data are the first indication of the important contribution of PLCγ2 and Vav proteins in the modulation of the neutrophil-mediated inflammatory response associated with RA. As a consequence of the impaired neutrophilic response, the release of proosteoclastogenic cytokines is dampened, and therefore, these mice are also protected from inflammation-induced bone loss.
Vav proteins are known regulators of PLCγ activation in several hematopoietic cells (29). Both Vav and PLCγ2 play an important role in bone homeostasis. We have previously shown that Vav-deficient mice display defective recruitment of osteoclasts in vivo in response to RANKL (41). More recently, we have also documented the importance of PLCγ2 in regulating osteoclast differentiation and activation (27). Targeted deletion of PLCγ2 in mice leads to an osteopetrotic phenotype, with blockade of osteoclastogenesis, but with intact osteoblast function (27). In the present study, we demonstrated that Vavnull and PLCγ2−/− mice do not develop inflammation, and thus focal bone erosion associated with RA, in the serum-transfer model of arthritis. This finding suggests a previously unknown role for the Vav/PLCγ2 axis in the regulation of innate immune responses and positions these molecules as prime candidates that control both the immune system and the bone system.
The K/BxN serum–transfer model is an established model of neutrophil-dependent RA (3). In the complex picture of induction of inflammation in RA, neutrophils are known to play a biphasic role. In the initiation phase, immune complexes and fixed complement fragments can recruit and activate neutrophils in situ through Fc receptors and integrins. Their activation leads to degranulation and the release of inflammatory and chemotactic cytokines that recruit more neutrophils, thereby amplifying and sustaining the inflammatory response (42). Thus, we hypothesized that the resistance to inflammatory arthritis in Vavnull and PLCγ2−/− mice could be due to impaired function of neutrophils.
Given that we did not observe cellular infiltration into the joint space in either the Vavnull mice or the PLCγ2−/− mice after serum transfer, we examined their neutrophil migratory capacity. As previously reported (15), we confirmed that Vav-deficient neutrophils have a normal ability to migrate in response to chemotactic gradients. When we tested the chemotactic response of PLCγ2−/− neutrophils after stimulation with C5a complement in vitro and in a chemical peritonitis model in vivo, we also observed that PLCγ2 was not required for neutrophil migration, thus indicating that the Vav/PLCγ2 axis is not required for neutrophil extravasation. Indeed, our data indicate that both Vav and PLCγ2 are required for neutrophil effector functions. Specifically, we demonstrated that Vav or PLCγ2 deficiency completely abrogates neutrophil degranulation following attachment to integrin ligands and/or following C5a and TNFα stimulation. Thus, our data support a model in which inflammation is a self-perpetuating cycle, where a primary phase of initiation is followed by subsequent amplification.
Specific to the K/BxN model of arthritis, the initiation phase is likely related to activation of in situ neutrophils that adhere to immune complexes and then degranulate. The released proteases contribute to the destruction of surrounding vessels and tissue, and the inflammatory cytokines can promote the massive recruitment of neutrophils (and other cells), thereby amplifying the whole process. Thus, despite the intrinsic capacity of Vavnull and PLCγ2−/− neutrophils to reach the joint space, the impaired capacity of the local neutrophils to degranulate inhibits the development and amplification of the inflammatory response.
Similar to our observations, other studies have shown the role of granule release for massive neutrophil recruitment in models of neutrophil-dependent inflammation (43). Mice lacking the lysosomal cysteine protease dipeptidylpeptidase 1, an enzyme that controls the catalytic activity of granule proteases, have normal in vitro neutrophil chemotaxis and in vivo neutrophil accumulation during sterile peritonitis, but are protected against acute arthritis induced by the transfer of monoclonal antibodies against type II collagen (44). These findings, in conjunction with the findings of our study, suggest that preventing the release of neutrophil granules could restrain the inflammatory response associated with RA.
Engagement of β2 integrins is crucial in modulating neutrophil effector functions, such as degranulation (45), by synergizing with numerous mediators of inflammation, including C5a and TNFα. It has been established that Vav and PLCγ2 become phosphorylated upon integrin engagement in several cell types, and specifically in neutrophils, Vav is required for PLCγ2 activation (19,21,23). Therefore, we hypothesized that Vav and PLCγ2 could modulate neutrophil degranulation by affecting β2 integrin–mediated functions. Our data indicate that both molecules control the spreading of neutrophils on integrin substrates. Vavnull and PLCγ2−/− neutrophils can adhere to pRGD, albeit to a lesser extent than WT neutrophils, but the cytoskeletal changes following the initial interaction with the extracellular matrix are impaired. Gakidis et al (15) showed decreased activation of Pyk-2, Src, and ERK after integrin engagement in neutrophils lacking Vav1 and Vav3. Consistent with this finding, we noted aberrant activation of integrin signaling in Vavnull and PLCγ2−/− neutrophils.
Interestingly, despite defective integrin-mediated spreading, Vav-deficient or PLCγ2-deficient neutrophils were still capable of responding to chemotactic stimuli and migrating. Our observations are consistent with those from other studies of neutrophils lacking the Src family kinase members Hck and Fgr, which exhibit a reduced capacity to sustain prolonged adhesion to β2 integrin ligands in vitro, without impaired migration (36). Furthermore, another molecule in the same signaling pathway as Vav and PLCγ2, namely, SLP76, has been shown to be required for neutrophil spreading but not migration (46). The mechanisms underlying divergent effects on integrin-mediated adhesion and migration are not clear, but it is possible that these processes are regulated by different pathways. Many neutrophil chemoattractants are GPCR agonists, which act in a signaling pathway that is independent of Vav and PLCγ2. In fact, it has been demonstrated that, despite defective integrin-mediated signaling, Vavnull mice show normal GPCR signaling. Consistent with this finding, Vavnull and PLCγ2−/− neutrophils can migrate toward C5a, and cell spreading is intact when these cells are stimulated with GPCR ligands such as FMLP (data not shown).
The importance of neutrophils in the development of RA in humans is becoming increasingly recognized. Accumulating evidence suggests that neutrophils play an essential role in the inductive phase of the joint-specific inflammation that occurs in the early stages of RA (3). Recent clinical trials using granulocyte/monocyte apheresis in patients with RA have shown improvements in clinical parameters, consistent with the importance of neutrophils in the pathogenesis of the disease (47). Using a genetic approach, we provide new data indicating that the Vav/PLCγ2 pathway regulates integrin-dependent signals in neutrophils that are required for induction of the inflammatory response in the serum-transfer model of arthritis. We cannot exclude the possibility that Vav and PLCγ2 modulate inflammation mediated by other native immune cells, such as macrophages and mast cells. However, the K/BxN serum–transfer model of arthritis is known to be primarily dependent on neutrophils, since neutrophil depletion prevents the induction of disease, despite the presence of functional mast cells and macrophages (48).
Our findings demonstrate that targeting the Vav/PLCγ2 pathway may be an effective way to modulate the inflammatory response elicited during arthritis. While β2 integrin and Vav are broadly expressed, PLCγ2 may itself represent a viable therapeutic target because of its more confined role in B cells, neutrophils, and osteoclasts, all of which are important players in the initiation and development of arthritis.
ACKNOWLEDGMENTS
We gratefully acknowledge Yousef Abu-Amer (Department of Orthopedics, Washington University) for the K/BxN arthritogenic serum. We thank Tonia Thompson for research administration and Karon Hertlein for secretarial support (Department of Orthopedics, Washington University).
Dr. Graham’s work was supported by NIH grant T32CA-009547. Dr. Swat’s work was supported by NIH grants AI-06107703 and AI-06302402. Dr. Faccio’s work was supported by the Arthritis Foundation and by NIH grant R01-AR-52921.
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Faccio had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Cremasco, Faccio.
Acquisition of data. Cremasco, Graham.
Analysis and interpretation of data. Cremasco, Graham, Novack, Swat, Faccio.
Manuscript preparation. Cremasco, Faccio.
Statistical analysis. Cremasco.
REFERENCES
- 1.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FMA, Boackle SA, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 2.Walsh NC, Crotti TN, Goldring SR, Gravallese EM. Rheumatic diseases: the effects of inflammation on bone. Immunol Rev. 2005;208:228–251. doi: 10.1111/j.0105-2896.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 3.Wipke BT, Wang Z, Nagengast W, Reichert DE, Allen PM. Staging the initiation of autoantibody-induced arthritis: a critical role for immune complexes. J Immunol. 2004;172:7694–7702. doi: 10.4049/jimmunol.172.12.7694. [DOI] [PubMed] [Google Scholar]
- 4.Nandakumar K, Holmdahl R. Antibody-induced arthritis: disease mechanisms and genes involved at the effector phase of arthritis [review] Arthritis Res Ther. 2006;8:223. doi: 10.1186/ar2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burg ND, Pillinger MH. The neutrophil: function and regulation in innate and humoral immunity. Clin Immunol. 2001;99:7–17. doi: 10.1006/clim.2001.5007. [DOI] [PubMed] [Google Scholar]
- 6.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 7.Berton G, Lowell CA. Integrin signalling in neutrophils and macrophages. Cell Signal. 1999;11:621–635. doi: 10.1016/s0898-6568(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 8.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 9.Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruoslahti E. Integrin signaling and matrix assembly. Tumour Biol. 1996;17:117–124. doi: 10.1159/000217975. [DOI] [PubMed] [Google Scholar]
- 11.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 12.Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 13.Lowell CA, Soriano P. Knockouts of Src-family kinases: stiff bones, wimpy T cells, and bad memories. Genes Dev. 1996;10:1845–1857. doi: 10.1101/gad.10.15.1845. [DOI] [PubMed] [Google Scholar]
- 14.Swat W, Fujikawa K. The Vav family: at the crossroads of signaling pathways. Immunol Res. 2005;32:259–265. doi: 10.1385/IR:32:1-3:259. [DOI] [PubMed] [Google Scholar]
- 15.Gakidis MAM, Cullere X, Olson T, Wilsbacher JL, Zhang B, Moores SL, et al. Vav GEFs are required for β2 integrin-dependent functions of neutrophils. J. Cell Biol. 2004;166:273–282. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham DB, Cella M, Giurisato E, Fujikawa K, Miletic AV, Kloeppel T, et al. Vav1 controls DAP10-mediated natural cytotoxicity by regulating actin and microtubule dynamics. J Immunol. 2006;177:2349–2355. doi: 10.4049/jimmunol.177.4.2349. [DOI] [PubMed] [Google Scholar]
- 17.Turner M, Billadeau DD. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat Rev Immunol. 2002;2:476–486. doi: 10.1038/nri840. [DOI] [PubMed] [Google Scholar]
- 18.Bustelo XR. Vav proteins, adaptors and cell signaling. Oncogene. 2001;20:6372–6381. doi: 10.1038/sj.onc.1204780. [DOI] [PubMed] [Google Scholar]
- 19.Graham DB, Robertson CM, Bautista J, Mascarenhas F, Diacovo MJ, Montgrain V, et al. Neutrophil-mediated oxidative burst and host defense are controlled by a Vav-PLCγ2 signaling axis in mice. J Clin Invest. 2007;117:3445–3452. doi: 10.1172/JCI32729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones NP, Peak J, Brader S, Eccles SA, Katan M. PLCγ1 is essential for early events in integrin signalling required for cell motility. J Cell Sci. 2005;118:2695–2706. doi: 10.1242/jcs.02374. [DOI] [PubMed] [Google Scholar]
- 21.Jones NP, Katan M. Role of phospholipase Cγ1 in cell spreading requires association with a β-Pix/GIT1-containing complex, leading to activation of Cdc42 and Rac1. Mol Cell Bio. 2007;27:5790–5805. doi: 10.1128/MCB.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilde JI, Watson SP. Regulation of phospholipase Cγ isoforms in haematopoietic cells: why one, not the other? Cell Signal. 2001;13:691–701. doi: 10.1016/s0898-6568(01)00191-7. [DOI] [PubMed] [Google Scholar]
- 23.Wonerow P, Pearce AC, Vaux DJ, Watson SP. A critical role for phospholipase Cγ2 in αIIbβ3-mediated platelet spreading. J Biol Chem. 2003;278:37520–37529. doi: 10.1074/jbc.M305077200. [DOI] [PubMed] [Google Scholar]
- 24.Epple H, Cremasco V, Zhang K, Mao D, Longmore GD, Faccio R. Phospholipase Cγ2 modulates integrin signaling in the osteoclast by affecting the localization and activation of Src kinase. Mol Cell Biol. 2008;28:3610–3622. doi: 10.1128/MCB.00259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Feng J, Wen R, Marine JC, Sangster MY, Parganas E, et al. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors. Immunity. 2000;13:25–35. doi: 10.1016/s1074-7613(00)00005-4. [DOI] [PubMed] [Google Scholar]
- 26.Fujikawa K, Miletic AV, Alt FW, Faccio R, Brown T, Hoog J, et al. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J Exp Med. 2003;198:1595–1608. doi: 10.1084/jem.20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. PLCγ2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Invest. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 29.Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katschke KJ, Jr, Helmy KY, Steffek M, Xi H, Yin J, Lee WP, et al. A novel inhibitor of the alternative pathway of complement reverses inflammation and bone destruction in experimental arthritis. J Exp Med. 2007;204:1319–1325. doi: 10.1084/jem.20070432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miletic AV, Graham DB, Montgrain V, Fujikawa K, Kloeppel T, Brim K, et al. Vav proteins control MyD88-dependent oxidative burst. Blood. 2007;109:3360–3368. doi: 10.1182/blood-2006-07-033662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrew DP, Spellberg JP, Takimoto H, Schmits R, Mak TW, Zukowski MM. Transendothelial migration and trafficking of leukocytes in LFA-1-deficient mice. Eur J Immunol. 1998;28:1959–1969. doi: 10.1002/(SICI)1521-4141(199806)28:06<1959::AID-IMMU1959>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 34.Newbrough SA, Mocsai A, Clemens RA, Wu JN, Silverman MA, Singer AL, et al. SLP-76 regulates Fcγ receptor and integrin signaling in neutrophils. Immunity. 2003;19:761–769. doi: 10.1016/s1074-7613(03)00305-4. [DOI] [PubMed] [Google Scholar]
- 35.Mocsai A, Ligeti E, Lowell CA, Berton G. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. J Immunol. 1999;162:1120–1126. [PubMed] [Google Scholar]
- 36.Giagulli C, Ottoboni L, Caveggion E, Rossi B, Lowell C, Constantin G, et al. The Src family kinases Hck and Fgr are dispensable for inside-out, chemoattractant-induced signaling regulating β2 integrin affinity and valency in neutrophils, but are required for β2 integrin-mediated outside-in signaling involved in sustained adhesion. J Immunol. 2006;177:604–611. doi: 10.4049/jimmunol.177.1.604. [DOI] [PubMed] [Google Scholar]
- 37.Yan SR, Novak MJ. Diverse effects of neutrophil integrin occupation on respiratory burst activation. Cell Immunol. 1999;195:119–126. doi: 10.1006/cimm.1999.1524. [DOI] [PubMed] [Google Scholar]
- 38.Ochi S, Shinohara M, Sato K, Gober HJ, Koga T, Kodama T, et al. Pathological role of osteoclast costimulation in arthritis-induced bone loss. Proc Natl Acad Sci U S A. 2007;104:11394–11399. doi: 10.1073/pnas.0701971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 40.Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 41.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, et al. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–290. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 42.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirahashi J, Mekala D, Van Ziffle J, Xiao L, Saffaripour S, Wagner DD, et al. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity. 2006;25:271–283. doi: 10.1016/j.immuni.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109:363–371. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowell CA, Berton G. Integrin signal transduction in myeloid leukocytes. J Leukoc Biol. 1999;65:313–320. doi: 10.1002/jlb.65.3.313. [DOI] [PubMed] [Google Scholar]
- 46.Clemens RA, Lenox LE, Kambayashi T, Bezman N, Maltzman JS, Nichols KE, et al. Loss of SLP-76 expression within myeloid cells confers resistance to neutrophil-mediated tissue damage while maintaining effective bacterial killing. J Immunol. 2007;178:4606–4614. doi: 10.4049/jimmunol.178.7.4606. [DOI] [PubMed] [Google Scholar]
- 47.Sanmarti R, Marsal S, Valverde J, Casado E, Lafuente R, Kashiwagi N, et al. Adsorptive granulocyte/monocyte apheresis for the treatment of refractory rheumatoid arthritis: an open pilot multicentre trial. Rheumatology (Oxford) 2005;44:1140–1144. doi: 10.1093/rheumatology/keh701. [DOI] [PubMed] [Google Scholar]
- 48.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167:1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]