Abstract
The combination of PET and MR in one system is currently emerging and opens up new domains in the functional examinations of living systems. This paper reports on relevant influences of a PET insert on MR imaging. The basic conditions of main magnetic field and RF field homogeneity were measured as well as image quality and signal-to-noise ratio when applying the usual MR sequence types including echo-planar techniques. Moreover, the influence of the PET insert on the RF noise level and on RF interferences was measured by comparing results achieved with and without the PET insert. The temporal stability of EPI imaging with and without the PET insert was assessed. Small but significant decreases in the signal-to-noise ratio were revealed when the PET insert was present, whereas B0 and B1 homogeneity as well as RF noise level were not adversely affected. A higher signal intensity drift was found for EPI imaging studies; however, this can be compensated by post processing. In summary, this study shows that PET inserts can be designed for and used within an MR system practically, without substantially affecting the MR image quality.
Keywords: simultaneous PET/MR, mutual interference, EPI stability, functional imaging
Introduction
The combination of Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI) has been a subject of intensive research over the last years. The strengths of PET lie in its ability to trace tiny amounts, in the picomolar range, of radioactive biomarkers, which allows in vivo imaging of a multitude of functional processes on the molecular level. A general downside of PET is that it lacks the ability to resolve detailed anatomical structures. This is in sharp contrast to MRI, which typically has a sensitivity in the millimolar range, making it roughly nine orders of magnitude less sensitive than PET. However, MRI excels in morphological imaging and offers a variety of functional and biochemical methods, such as spectroscopy, diffusion, perfusion and blood oxygen level dependent (BOLD) contrast.
In contrast to PET/CT systems, PET/MR machines are usually designed to allow simultaneous PET and MR data acquisitions (1-3). A fundamental problem when aiming to combine PET with MR is the effect of the magnetic field on the photomultiplier tubes of conventional PET systems (4). One approach to solve this challenge is the use of light fibers, which are associated with a loss of light intensity (5-7). Split magnet systems, which have the PET detector built between two magnet halves (8), or field cycling systems that switch off the main magnetic field during measurement (9,10) are also under development. Another approach is to substitute the photomultiplier tubes by avalanche photodiodes (APDs) that are solid-state light detectors. This can either be done by using small light fibers that allow for the placement of PET electronics outside the PET/MR field of view (FOV) (2,11) or by connecting the scintillation crystals by a short light guide to the APDs (1,12). The latter approach does not suffer from light loss by optical fibers and allows for an expansion of the volume covered by PET. The systems mentioned above are dedicated to small animal imaging (1,2,5,6,11). Moreover, a first PET/MR system based on APDs for human brain scans has already been developed (3).
With the advent of this new hybrid imaging modality, it is important to consider the question of mutual interference. On the one hand, the MR system could have a negative impact on the PET imaging performance. This question has been addressed quite thoroughly in the literature with the general conclusion that the effect of MR on PET can be minimized if proper shielding of the PET detector and read-out electronics is chosen (1,2,6,11,13). On the other hand, there could be a negative influence of the PET system on the MR, which, based on the flexibility of different MR sequences, is more complex to evaluate. Especially demanding imaging sequences, such as Echo Planar Imaging (EPI), could be more affected than standard spin echo MR imaging sequences. In 1999, Slates at al. conducted a study on the mutual interference of PET and MR systems, but concentrated mainly on MR signal-to-noise ratio (SNR) and image uniformity as well as geometrical distortions. The study concluded that it is possible to collect MR images in the presence of a PET insert without noticeable artifacts or loss of image quality for standard MR sequences (13). However, this article dealt with a PET insert that used long optical fibers to lead the light outside the magnet. In contrast, recently built systems based on APDs have the majority of their PET detection electronics placed inside the main magnetic field.
Whereas earlier studies concentrated mainly on the interference of the MR on PET imaging (1), the aim of the present study was to provide a detailed evaluation of the effects of a PET insert on the performance of a commercial small animal MR system. In particular, the effects of PET on the temporal stability of the MR signal are carried out in detail, which is important for BOLD fMRI studies. Our earlier work also included MR imaging stability, but did not compare the results to the system without the PET insert (1). The mapping of RF noise, the main magnetic field (B0) or the radio frequency (RF) field (B1) in the presence of the PET insert has not been previously performed.
Methods
MR System
All MR measurements were conducted using a 7 T, 300 mm bore ClinScan small animal MR system (Bruker BioSpin MRI, Germany) with a maximum gradient strength of 300 mT/m. A 35 mm inner diameter, quadrature volume coil was used for the transmission and reception of the MRI signal for the compatibility evaluation. The coil was either installed inside the PET insert for the combined PET/MR measurements, or was mounted in a plastic holder that fit inside the bore of the magnet for measurements without the PET insert. The MR RF coil uses shielded BNC connector cables.
PET Insert
An MR compatible PET insert (Fig. 1) was developed by our group, as previously described (1,12). The PET insert features ten PET detector modules, which are arranged in a circular shape to form a PET ring with an inner diameter of 60.5 mm and an outer diameter of 118 mm. Each detector module comprises a 12×12 lutetium oxyorthosilicate (LSO) crystal, with a single crystal size of 1.6×1.6×4.5 mm3. The LSO scintillator is coupled via a 3 mm tapered light guide to a 3×3 APD array (Hamamatsu Photonics, Japan). The signal of the APD array is fed to custom-made, charge sensitive preamplifier electronics. The crystal, APD array, preamplifier and electronics of each detector module are shielded by a 20 μm copper coated epoxy material to minimize RF noise. The individual detector cassettes are grounded via separate conductors outside the MR system to avoid eddy current induction. The grounding is utilized via the shielding of the PET signal cables, connecting the PET detector cassettes with the PET acquisition electronics outside the scanner room. Grounding is permanent in this configuration. The ten PET detector modules form a multi-slice PET scanner with 23 axial image planes, each with a thickness of approximately 0.8 mm, and an axial field of view (FOV) of 19 mm and a transverse FOV of about 40 mm. For simultaneous PET/MR operation, the PET ring is inserted inside the gradient coil of the 7 T scanner, and the 35 mm RF coil is placed in the open bore of the PET system. The usable transverse FOV for combined PET/MR imaging is therefore reduced to 35 mm, limited by the RF coil, and to 19 mm in the axial direction, limited by the PET crystal size. For simultaneous PET/MR imaging, the center of the PET FOV is placed in the isocenter of the MR system.
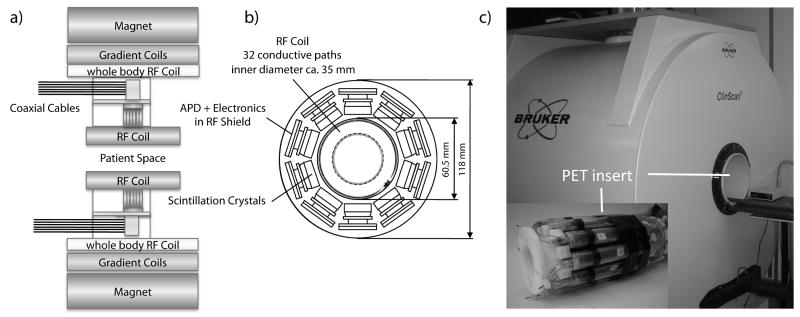
FIG. 1.
a) The schematic structure of the MR system with the built-in PET insert. The PET insert is installed inside the magnet bore. For PET/MR experiments, a transmit/receive RF coil installed inside the PET insert is used. The whole body coil of the MR system is muted for the PET/MR experiments. This design allows an easy transformation from an MR only system to a PET/MR system by just installing the PET insert inside the magnet. b) A CT image of the RF coil used in this study is superimposed on a schematic view of the 10 PET detectors of the PET insert. The coil has 32 conductive paths, which are symmetrically positioned with respect to the PET detectors. Therefore, a symmetric γ-radiation attenuation effect caused by the RF coil can be expected. However, when surface or local RF coils are used, asymmetric γ-radiation attenuation effects have to be expected. In general, the attenuation caused by the RF coil is an important parameter for PET/MR imaging. c) Photograph of the 7 T, small animal MR scanner as well as the PET insert that is installed at the center of the MR field of view.
Some important PET insert performance parameters, as previously described (1), are summarized here. The full width at half maximum (FWHM) energy resolution of the PET insert was 25.8% at 511 keV and changed to 25.1% inside the MRI during PET/MRI data acquisition. The coincidence time resolution was on average 8.0 ns outside of the MRI and changed to 8.2 ns inside the MRI. Single and prompt detection count rates of the PET insert showed, on average, variations of less than 2% between the PET insert operating outside or inside of the MR. These measurements show that even with the RF coil installed, an adequate performance of the PET insert can be achieved. However, it is important to note that the RF coil certainly has an attenuating effect on the detected γ-radiation and therefore needs to be taken into account for quantitative image analysis and reconstruction. Figure 1b shows a CT transversal image of the coil used in this study, which consists of 32 conductive paths that are symmetrically arranged with respect to the 10 PET detectors. A feasible approach for attenuation correction, caused by the RF coil, could be the collection of a CT image of the coil that can be used to generate an attenuation map. This coil attenuation map can then be used in image reconstruction, in combination with attenuation maps generated for the studied object. However, this requires that the coil is repositioned in the same way, relative to the imaging object and to the PET detectors, during every examination. The exact repositioning is less of a problem when symmetrically constructed coils are used, as in the present study. However, this issue is more pronounced for local coils, which show an asymmetric γ-radiation attenuation pattern. Alternatively, the coil attenuation parameters and its relative position to the imaged object can be obtained by means of suitable MR sequences for every PET/MR scan. The study of these important effects, however, was beyond the scope of this paper.
If the PET insert was removed from the MR magnet at any time, the RF coil as well as the phantom also had to be removed from their original position. Great care was taken to position the phantom and the RF coil in the same place for comparative measurements with and without the PET insert. Nevertheless, due to the technical constraints of the system, the repositioning accuracy was not optimal. Therefore, slight deviations on the order of about 1 mm can be seen in the measurements.
Signal-to-Noise Ratio and MR Image quality
To assess the influence of the PET insert on SNR and the quality of recorded MR images, four different MR sequences were applied. The sequences and their parameters are listed in Table 1. Two spin-echo based sequences are commonly employed for morphological small animal imaging, whereas the 2D gradient-echo sequence is used for fast imaging studies, e.g., in perfusion bolus tracking. The MPRAGE (Magnetization Prepared RApid Gradient Echo) sequence provides high T1 contrast at short measuring time. A cylindrical phantom with a volume of 50 ml and an inner diameter of 27 mm, filled with an aqueous solution of 1g/l CuSO4 and 4.31 g/l NaCl (T1 = 420 ms, T2 = 366 ms), was placed inside the 35 mm RF coil, both with the PET insert installed in the MR and without the PET insert. For each sequence, 18 transverse slices (each 1.0 or 1.1 mm thick), placed symmetrically around the center of the PET/MR FOV, were recorded. Each measurement was repeated five times with and without the PET insert, yielding a total of 90 images per sequence. Images were converted from the DICOM format to the ASI Pro format and analyzed using the ASI Pro software (Concorde Microsystems, USA). A circular region of interest (ROI) with an area of 3.5 cm2 was placed in the center of the signal-producing region of each phantom slice and copied from slice to slice. The size of the ROI was chosen in such a way as to avoid Gibbs ringing and influences from susceptibility artifacts from the fluid-plastic-air interface at the shell of the phantom. The mean pixel intensity from this ROI was used as a value for the MR signal S. Another circular ROI of approx. 0.27 cm2 was placed outside the phantom; the standard deviation of the signal in this ROI was used to assess the image noise σ. Care was taken to avoid any influence of Gibbs ringing and other artifacts on this ROI. The signal to noise ratio was then calculated as:
Table 1.
MR sequences and its parameters used for SNR and image homogeneity measurement. A fast spinecho (FSE), a spinecho (SE), a 3D magnetization-prepared rapid gradient echo sequence (MPRAGE) and a spoiled gradient-echo (GRE) sequence were applied.
| Sequence | TR [ms] | TE/TI [ms] | Voxel Size [mm3] | Matrix Size | α [°] | Bandwith [Hz/Pixel] |
|---|---|---|---|---|---|---|
| 2D FSE | 2770 | 42 | 0.14×0.14×1.0 | 256×256 | 90/180 | 130 |
| 2D SE | 500 | 10 | 0.14×0.14×1.0 | 256×256 | 90/180 | 130 |
| 3D MPRAGE | 2000 | 2.07/800 | 0.14×0.14×1.0 | 256×256 | 20 | 380 |
| 2D GRE | 18 | 7 | 0.55×0.55×1.1 | 256×256 | 20 | 1000 |
| [1] |
The factor 0.655 was introduced because magnitude images have been used to assess the noise in the images according to Henkelman et al. (14).
For the calculation of the image homogeneity as a marker for image quality in a homogeneous phantom, the same ROI from the measurements described above was used. Inside this ROI, the minimum Smin and maximum Smax signal intensities were determined. The area of the ROI is 61% of the total phantom cross-sectional area, smaller than the American Association of Physicists in Medicine recommendations (15) of 75% for the evaluation of image homogeneity. This smaller ROI was chosen to avoid artifacts at the border of the phantom and to obviate image spikes that could have adversely influenced homogeneity values. Moreover, the AAPM recommendations are mainly intended for human MR systems; therefore, it is difficult to transfer them without modifications to small animal MR scanners. Image uniformity was then calculated using Eq. [2]:
| [2] |
A t-test was performed for each sequence type for testing whether the SNR or the homogeneity was significantly different between the configuration without the PET insert and with the PET insert installed in the MR. Data are presented as mean ± one standard deviation (SD).
B0 Mapping
The phantom described in the previous section was placed at the center of the PET/MR FOV, and automatic shimming was performed. To measure local magnetic field variations ΔB0(r), a phase measurement method was used (16). Two phase images were obtained in coronal orientation with a gradient echo sequence (TR = 500 ms, TE1 = 10 ms and TE2 = 20 ms, FOV = 100 × 100 mm2, Matrix size = 128 × 128, slice thickness = 2.0 mm, bandwidth = 260 Hz/pixel, flip angle = 25°). Subsequently, a phase unwrapping algorithm with an SNR threshold of 10 was applied to generate continuous phase maps Φ(r, TE1) and Φ(r, TE2) from the obtained images (17). Local magnetic field variations were calculated from these images employing Matlab (The MathWorks, USA), according to Eq. [3], where γ represents the gyromagnetic ratio for 1H protons:
| [3] |
The B0 maps were color coded and displayed by contour maps, allowing a qualitative comparison of the obtained B0 maps calculated for the configurations with and without the PET insert installed in the MR scanner.
B1 Mapping
To measure whether the flip angle distribution generated by the excitation field is altered due to the presence of the PET insert, the same phantom was used, as described in the SNR and image homogeneity section. For RF field mapping, a spin echo sequence (TR = 300 ms, TE1 = 17 ms, TE2 = 17 ms, FOV = 100 × 100 mm2, Matrix = 128 × 128, slice thickness = 2.0 mm, bandwidth = 260 Hz/pixel) was employed. After a slice selective excitation (90°), two refocusing pulses (180°, time difference 17 ms) were employed that generated a spin echo and a stimulated echo signal. Further echoes were spoiled by gradients. The signals of the spin echo (SSE) and of the stimulated echo (SSTE) were collected. The flip angle distribution α inside the object can be calculated according to Eq. [4]:
| [4] |
where T1 is the relaxation time in the phantom (T1 = 420 ms) and t is the time difference between the two echoes (t = 17 ms). The exponential term accounts in this case to an approximately 4% difference, compared to a calculation neglecting the T1 effects.
Slices in coronal, sagittal and transverse orientation were obtained in the center of the FOV, either without the PET insert or with the PET insert installed. Data were displayed in color-coded contour maps.
RF Noise measurements
To evaluate possible RF interferences, especially the RF of the PET electronics affecting the MR system, a special “noise sequence,” provided by the manufacturer of the MR system, was applied. This sequence repeatedly receives data from the RF channel of the system without applying any RF by the MR transmitter or gradient pulses. By varying the carrier frequency, a total of 50 images were collected. Each image had a matrix size of 256 × 256 and a bandwidth of 39 Hz/pixel, resulting in a total receiver bandwidth of 10 kHz. Because the system frequency is at 300.4 MHz, a frequency range between 300.15 MHz and 300.65 MHz was monitored for RF noise contributions from the PET. The RF noise sequence was performed without the PET insert built into the MR system, with the PET insert installed but powered off, with the PET insert installed and powered on, with the PET insert installed, powered on, and collecting data from the phantom mentioned above but in addition filled with an activity of 14 MBq [18F]fluoride, and, finally, with an earlier design of the PET detector that was not fully shielded for comparison. The Fast Fourier Transform (FFT) scaling factor was adapted to visualize even small effects in the RF noise pattern (FFT = 100 fold for the measurements with and without the PET insert, and FFT = 1 for the measurement with the not fully shielded detector). All 50 images of the sequence were imported into Matlab for calculation of a sum over all pixels in a single column, yielding an RF noise spectrum.
Quality of Echo Planar Imaging
The procedures outlined in the function Biomedical Informatics Research Network (fBIRN) protocol (18) were used to evaluate the feasibility of functional MRI (fMRI) data acquisition when the PET insert was installed in the MRI. The size of the phantom and some of the MR sequence parameters, as defined in the original fBIRN protocol for human MR scanners, were adjusted to accommodate them to our high-field, small animal scanner. A cylindrical phantom with a volume of 15 ml and an inner diameter of 15 mm, filled with the same solution used for the SNR measurements, was placed inside the 35 mm RF coil. The phantom was chosen according to the size and coil load of a mouse brain. A gradient-recalled EPI sequence was used (transverse orientation, TR = 2000 ms, TE= 30 ms, flip angle = 90°, FOV = 25 × 25 mm2, matrix = 64 × 64, slice thickness = 2.0 mm, 5 slices, bandwidth = 2298 Hz/pixel). A total of 200 volume scans (one volume contains all slices measured per scan) were acquired per experimental run (acquisition time 6 min 44 s), including two initial volumes that were discarded to allow for T1 equilibration. Image analysis was performed on the center slice (i.e., #3). However, the other slices were controlled by visual inspection to ensure that they provided similar results and did not exhibit image artifacts (data not shown). The data in a central ROI (20 × 20 voxels) inside the phantom were analyzed using a custom-written Matlab script to determine the following parameters:
- Mean signal intensity in the ROI, averaged across all 198 volumes.
- Temporal drift of the mean signal intensity across EPI volumes, as determined by fitting a second order polynomial (Fig. 4b) to the time series and determining the difference between the minimum and maximum of the fitted curve. The result is expressed as a percentage relative to the overall mean signal intensity.
- Fast temporal fluctuations of the mean signal (root mean square stability, or RMS). After removing the slow temporal drift from the mean signal, the SD of the detrended signal is expressed as a percentage relative to the overall mean signal intensity. As BOLD signal recorded in fMRI experiments manifests itself as a systematic experiment-related signal fluctuation around the slowly drifting baseline, the RMS stability can be seen as index of the signal-to-noise ratio between the observed brain activations and the physical noise of the MR scanner.
- Signal-to-fluctuation-noise ratio (SFNR): The ratio between the mean intensity and the SD of the detrended signal (determined voxelwise, and then averaged across all voxels in the ROI) is a further index to characterize the impact of scanner noise on the SNR in fMRI experiments. Visualizing the individual SFNR values of all voxels as two-dimensional image allows for an efficient inspection of the distribution of the noise across the imaged slice.
- The rdc (correlation radius) is based on a Weisskoff analysis(19) and characterizes the amount of intervoxel correlation. Averaging the time series of neighboring voxels reduces noise with the square root of the number of voxels, given that its origin is purely thermodynamic (i.e., completely uncorrelated). When scanner instabilities occur that affect a large number of voxels simultaneously (e.g., spikes), averaging will be less effective, and noise will decrease more slowly with an increasing number of voxels. The parameter rdc characterizes the number of voxels (expressed as the radius of a circular ROI) at which the noise reduction by averaging is first less than optimal. A poorly performing scanner will have a relatively low rdc of 1 or 2 voxels (indicating a strong intervoxel correlation), whereas better human MR scanners can reach rdc values of 5 or higher (20).
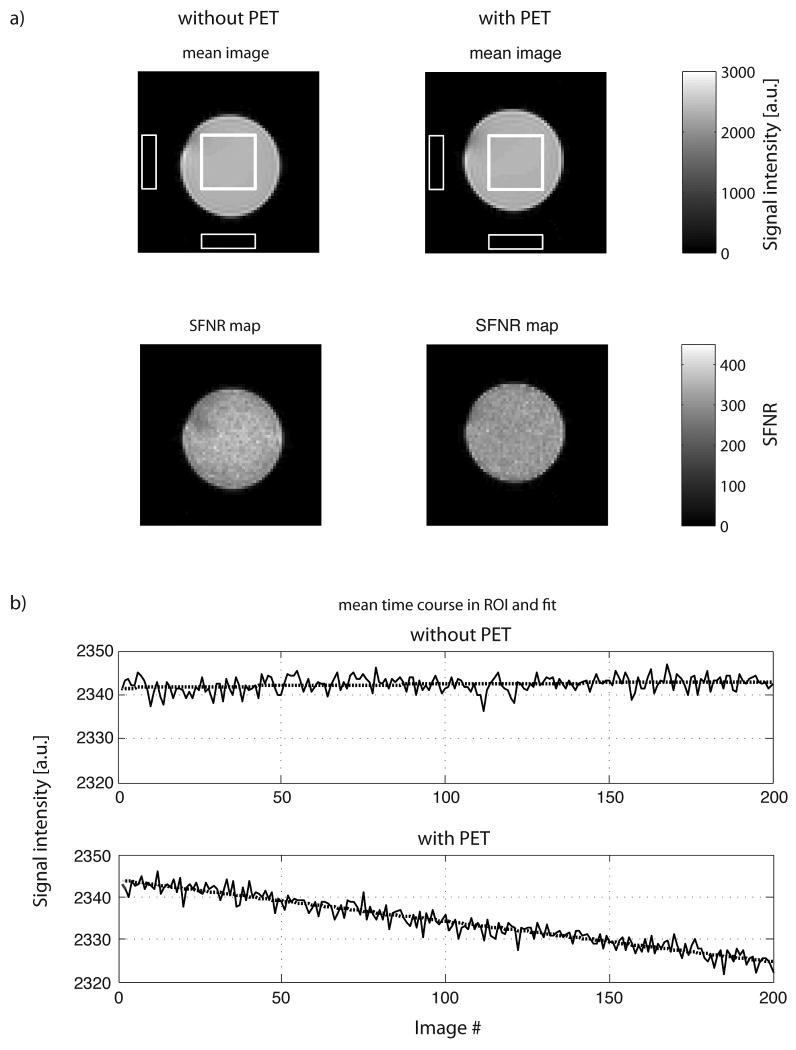
FIG. 4.
a) The mean images (top row) and SFNR maps (bottom row) for the configuration without and with the PET insert (equally scaled for both system configurations) show no differences in EPI image quality. The white squares in the center of the phantom and background are used for the calculation of the fBIRN measures reported in Table 3. b) Time series of the average signal intensity in the central ROI and 2nd order trend (dotted line) for two typical EPI experiments without (top) and with the PET insert installed (bottom). The drift in these measurements was 0.06% and 0.823% over a time range of 6 min and 44 s, respectively, without and with the PET insert in place.
The focus of the measurements not only revealed the effects of the PET insert on the stability within a single experimental run, but also on the long-term stability of the system across several hours. Therefore, the fBIRN measurements were repeated several times. Initially, nine EPI runs were performed without the PET insert, resulting in 9 × 200 = 1800 volumes in total (three successive runs were acquired in 20 min 12 s and were followed by a break of 40 min; this was repeated three times). Subsequently, the PET insert was installed, and 30 additional EPI runs were performed using the same time scheme as above, yielding 6000 acquired volumes. The PET insert was kept powered on during the entire time period over approximately 9 hours, and the cooling of the PET insert was refilled with a mixture of water and ice when necessary. The fBIRN results are reported as mean values ± SD across runs for each of the two system configurations.
Results
Signal-to-Noise Ratio and MR Image quality
The results of the SNR and image homogeneity measurements, over all 18 slices and 5 measurements (n = 90) and for different sequences, are presented in Table 2.
Table 2.
Results of the SNR and image homogeneity evaluation (in mean ± SD across runs). The SNR is statistically significant lower in the configuration with PET insert. The image homogeneity is only significantly impaired for the GRE sequence, which has already an inherent low SNR value.
| Sequence | SNR | Image Homogeneity [%] | ||||
|---|---|---|---|---|---|---|
| without PET | with PET | statistical significance | without PET | with PET | statistical significance | |
| FSE | 85.1 ± 2.3 | 73.7 ± 5.3 | Yes (p<0.01) | 92.9 ± 0.5 | 92.9 ± 0.6 | No (p>0.05) |
| SE | 49.9 ± 2.0 | 45.6 ± 2.3 | Yes (p<0.05) | 90.1 ± 0.6 | 89.5 ± 1.0 | No (p>0.05) |
| MPRAGE | 49.7 ± 6.5 | 44.6 ± 6.0 | Yes (p<0.05) | 89.1 ± 1.3 | 87.8 ± 2.5 | No (p>0.05) |
| GRE | 24.4 ± 1.0 | 23.1 ± 1.1 | Yes (p<0.05) | 82.3 ± 1.5 | 79.3 ± 3.0 | Yes (p<0.01) |
The values reflect a small but statistically significant decrease of the SNR when the PET insert was present: -13.4% (p<0.01) for the FSE, -8.6% (p<0.01) for the SE, -10.3% (p<0.05) for the MPRAGE and -5.2% (p<0.05) for the GRE sequence. The deviations of the image homogeneity were in general smaller: 0% (p>0.05) for the FSE, -0.6% (p>0.05) for the SE, -1.5% (p>0.05) for the MPRAGE and -3.8% (p<0.01) for the GRE sequence. The differences between the pure noise levels without and with the PET insert were small; therefore, the slight decrease in the SNR is mainly caused by modified RF excitation and/or signal recording.
B0 Mapping
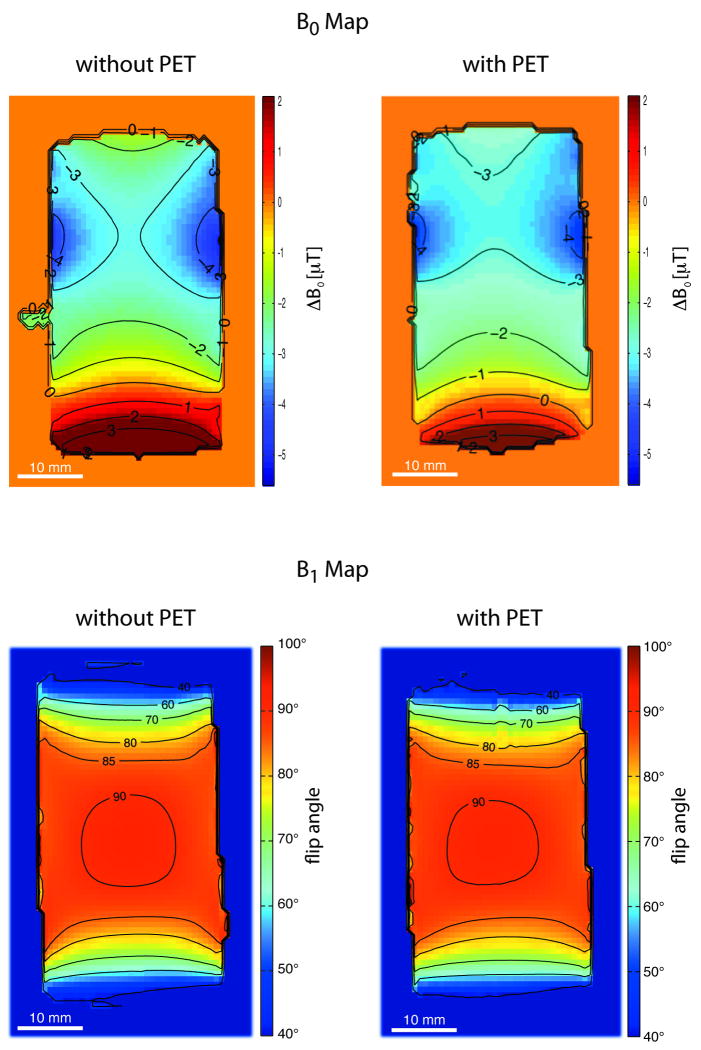
Figure 2a shows the distribution of the static magnetic field in a coronal slice obtained without and with the PET insert installed. A relatively homogenous region can be seen in the frontal part of the phantom. Here, field inhomogeneities are in both cases on the order of a maximum of 4 μT. At the bottom of the images, a non-uniform phantom region is identified, where ΔB0 ranges from approximately -1 μT to +4 μT. This marked change is seen in both B0 field maps obtained without and with the PET insert. Differences between the two configurations were found to be smaller than 3.5 μT. At a field strength of 7 T, a field non-uniformity of 3.5 μT transfers to 0.5 ppm. The main magnetic field of the scanner was specified by the manufacturer, with an inhomogeneity of less than 1 ppm in a spherical volume with a diameter of 11 cm.
FIG. 2.
a) B0 field mapping. The main static magnetic field homogeneity is shown by a coronal slice obtained with a phantom placed at the center of the PET/MR FOV. In both cases, without (left) and with (right) the PET insert installed into the MR, a relative homogenous region is found in the frontal part of the phantom (top of the images). The back of the phantom (bottom of the images) features a change in the main magnetic field from -1 μT to +2 μT. Susceptibility effects induced by the fluid, plastics and especially by phantom cap interfaces cause these changes. b) B1 field homogeneity. Flip angle distribution in a coronal slice obtained without (left) and with (right) the PET insert installed. No fundamental alteration of the B1 field was observed when the measurements for the two configurations were compared. Small differences in the B1 field between the two configurations might partly be caused by tolerances in slice positioning.
B1 Mapping
A typical result of the RF field mapping inside the phantom is displayed in Figure 2b for a coronal slice. Transverse as well as sagittal slices showed similar results. For the configuration without the PET insert, a homogenous region (flip angle 90° to 85°) of approximately 27 mm in the axial and 27 mm in the transverse orientation was found in the center of the PET/MR FOV. In the axial direction, the flip angle decreased within 10 mm from 85° to 40°. In the transverse direction, i.e., within the diameter of the phantom, the flip angle decreased from 90° to 80°. A similar flip angle distribution was observed for the configuration with the PET insert installed. Only a slightly smaller region, indicated as being excited by the regular flip angle (90°), was noted in the images with the PET insert, but already the 85° region showed no fundamental differences to the configuration without the PET insert.
RF Noise Measurements
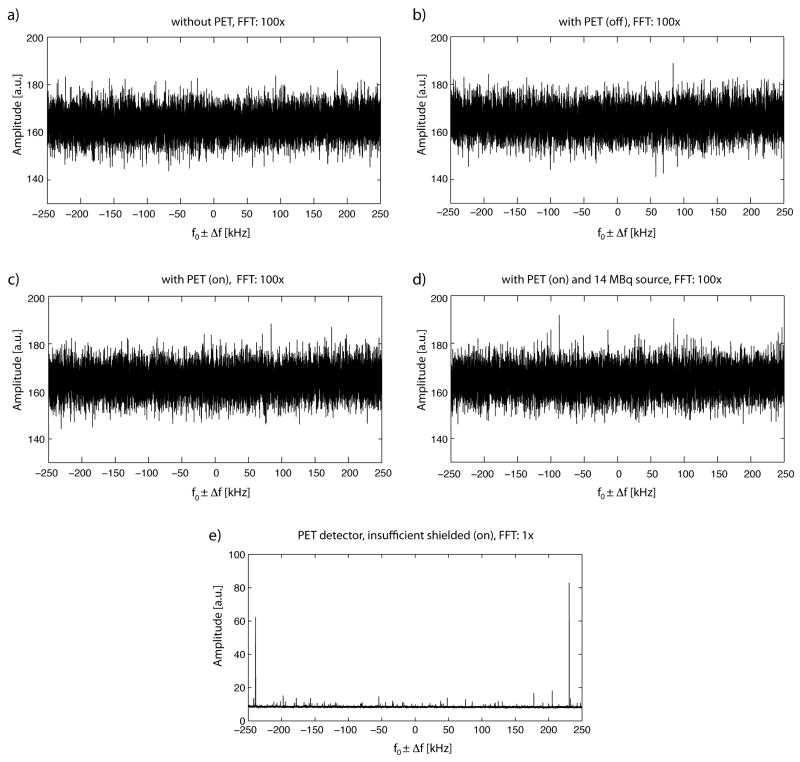
Figure 3 depicts the results of the RF noise measurements. Without the PET insert (Fig. 3a), a noise level at approximately (164 ± 5) a.u. (mean ± SD) was measured, which stayed nearly constant with the installed PET insert (Fig. 3b) at approximately (165 ± 5) a.u. When the PET insert was powered on inside the MR, but without any activity inside the PET FOV (Fig. 3c), the noise level was (164 ± 5) a.u. Finally, when the PET insert was powered on, and a phantom with a radioactivity of 14 MBq was placed in the PET/MR FOV (Fig. 3 d), the noise level was (164 ± 5) a.u. No prominent signal peaks could be detected, regardless of whether the PET insert was powered on or off. For comparison and control of the measurement method, Figure 3d shows that with an insufficiently shielded PET detector, various interferences appear (note also that the FFT scaling factor was reduced to 1 in this case to accommodate the strong signal amplitudes observed).
FIG. 3.
RF noise spectra of the MR system, measured in different configurations. The spectra are displayed at a resonance frequency of f0 = 300.4 MHz ± 250 kHz a) without the PET insert, b) with the PET insert installed, but powered off, c) with the PET insert powered on. d) with the PET insert installed, powered on, and collecting data from a phantom filled with 14 MBq radioactivity. No dominant noise peaks can be observed, even when the PET insert is installed and powered on. e) For control purposes an insufficiently shielded PET detector was used, leading to clearly dominant noise peaks. Note that the FFT scale factor was reduced from 100 to 1 in e).
Quality of Echo Planar Imaging
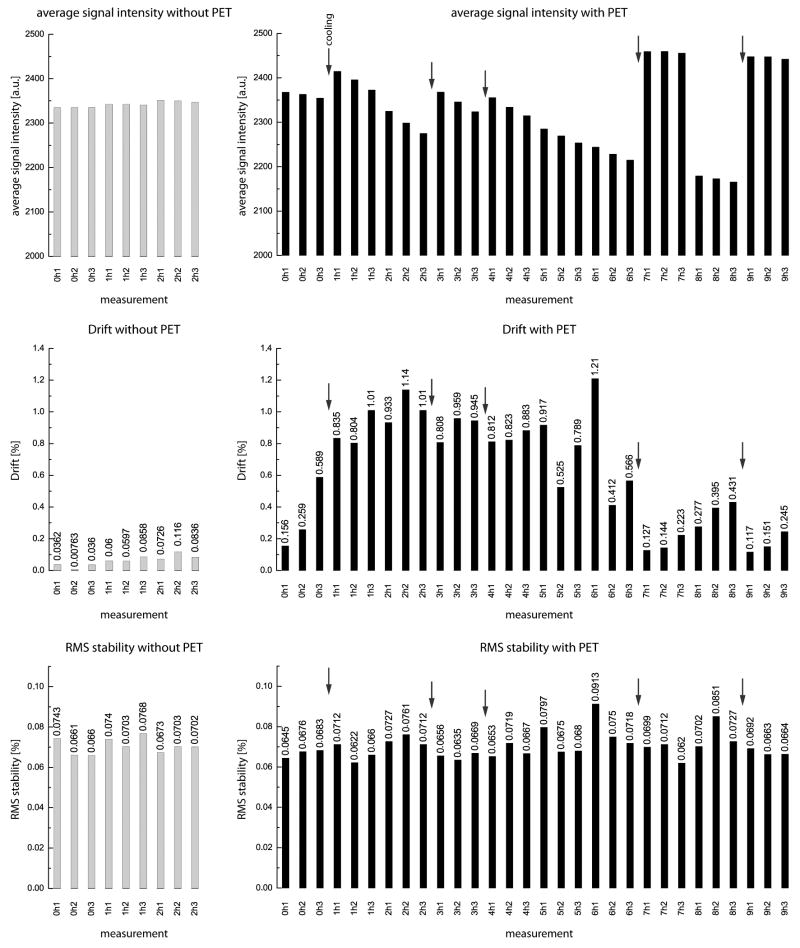
Images of the central EPI slice (#3 of 5) are shown in the top row of Figure 4a for two representative experimental runs without and with the PET insert. As expected from the results of the B0 and B1 mappings, they show little geometric distortions and a homogeneous distribution of the image intensity across the phantom. Visual inspection reveals no difference between both system configurations. Likewise, the SFNR images (Fig. 4a, second row) reveal good signal stability and low noise levels across the phantom, without a noticeable impact of the PET insert. Figure 4b shows two typical signal time courses of the central ROI as well as the second order polynomial trend. Here, a clear-cut significant difference between the two configurations is visible, with the PET insert causing a slow decrease of the overall signal intensity with time. The results of the two exemplary runs depicted in Figure 4 are confirmed by the results pooled across all experimental runs acquired with (30 runs) and without (9 runs) the PET insert (Table 3). SFNR and also rdc are statistically significantly altered in the configuration with the PET insert installed. However, these values are even with the PET insert in an acceptable range that should allow in vivo fMRI experiments, especially when comparing them to SFNR and rdc values typically found in clinical MR systems that are in a similar range (18). Nevertheless, care must be taken in such comparisons because the used fBIRN scanning protocol and phantom was altered due to constraints imposed by the small animal scanner. Figure 5 shows a comparison of the average intensity, drift and RMS stability of the runs without and with the PET insert in place. A change in mean signal intensity is observed after a modification of the cooling system of the PET insert (depicted by arrows). This suggests that the mean signal intensity is mainly affected by the temperature of the PET insert. Separately obtained temperature measurements, using an alcohol thermometer, showed that the temperature inside the PET insert bore, when installed in the MR, increased from 19°C to approximately 27°C during the first hour of the PET operation. After approximately 4 hours, the temperature was relatively stable at 29°C. Further measures to stabilize the PET insert temperature are planned. Importantly, however, RMS stability remains unchanged.
Table 3.
Results of the fBIRN EPI stability measurements (in mean ± SD across runs). The only major difference between the runs with vs. without PET insert can be seen in the signal intensity drift. SFNR and rdc show a statistical significant difference between the two configurations, however, these values are still in a range where they are unlikely to impact in-vivo imaging studies. Average signal intensity and RMS stability are not influenced by the presence of the PET insert.
| number of runs | without PET | with PET | statistical significance |
|---|---|---|---|
| n = 9 | n = 30 | ||
| Average signal intensity [a.u.] | 2342 ± 6 | 2331 ± 89 | No (p > 0.05) |
| Signal intensity drift [%] | 0.062 ± 0.032 | 0.617 ± 0.346 | Yes (p < 0.05) |
| RMS stability [%] | 0.071 ± 0.004 | 0.070 ± 0.006 | No (p > 0.05) |
| SFNR | 333 ± 11 | 311 ± 18 | Yes (p < 0.05) |
| rdc [Pixel] | 4.01 ± 0.23 | 4.62 ± 0.42 | Yes (p < 0.05) |
FIG. 5.
Comparison between average signal intensity (top), signal intensity drift (center) and RMS stability without (left) and with (right) the PET insert installed. The signal intensity seems to be influenced by changes in the PET insert cooling system (arrows), which also affects the drift of the system. However, the RMS stability of the measurements is not impacted by the presence of the PET insert.
Discussion
In general, small but significant differences in the SNR and in the signal characteristics of a phantom filled with a homogeneous medium were detected with the PET insert installed in the magnet compared to measurements without the PET insert. The decrease in SNR and image homogeneity is most likely caused by the addition of conductive structures and some slightly magnetic components used for the PET insert. Moreover, it is difficult to exactly reposition the phantom used for the comparative measurements between the configurations with and without the PET insert. In addition, a fixed coil tuning was used for the experiments, and the addition of the PET insert might lead to a slight detuning of the RF coil. Because the maximum impact of the PET insert on the MR image SNR is less than 13%, it will not jeopardize in vivo MR imaging studies, where variations on this order of magnitude in SNR are likely due to shimming and inter-subject variations.
The main magnetic field homogeneity (B0) seems not to be fundamentally influenced by the presence of the PET insert. The cap of the phantom tube, which is made of a different plastic material compared to the rest of the tube, seems to be the main reason for the static field inhomogeneities at the bottom side of the phantom. The differences in B0 between the configuration with and without the PET insert are smaller than the scanner main magnetic field homogeneity specifications. Altogether, image quality is not affected by the slight B0 changes caused by the PET insert, which are clearly smaller than the B0 variations to be expected from the addition of a living subject such as a mouse.
The B1 mapping showed RF field variations to be similar for configurations with or without the PET insert. This is especially important for quantitative MR studies, in which the signal intensity is dependent on the excitation flip angle. Even comparative studies can be performed between scans performed with and without the PET insert.
The RF noise measurements do not indicate any prominent RF interference signals when the PET insert is installed. This finding confirms that RF signals produced by the PET electronics are sufficiently shielded from the RF components of the MR system. The mean RF noise levels and the standard deviation of the RF noise measurement over the assessed frequency range of (300.4 ± 0.25) MHz showed no fundamental alteration due to the installation of the PET insert. Also, the inclusion of a [18F]fluoride radioactivity filled phantom in the PET/MR FOV that produced strong signals in the PET detection system, did not show an increase in the mean or standard deviation of the RF noise measurement. However, the measurement with the insufficiently shielded PET detector (Fig. 3d) clearly indicates that the used method is sensitive in detecting RF interferences.
The fBIRN EPI quality assurance showed an increased low frequency drift of the mean signal intensity when the PET insert was installed. This is most likely caused by temperature variations that are imposed by the PET insert and its cooling mechanism. The drift with the PET insert installed is about 10 times larger compared to the configuration without the PET insert. This drift can easily be compensated for in normal fMRI experiments because it is nearly linear. Usually a high-pass filter is applied in fMRI data post-processing to remove slow signal variations. The slightly lower rdc in our measurements is most likely caused by the use of a phantom containing free water for the measurements, so that small water motion, e.g., motion induced by vibrations due to gradient switching, might have reduced the rdc value. Moreover, the recommended rdc values of 5 are for human systems (20); alterations in this value due to the adaptations needed for a small animal scanner have to be expected. The statistically significant difference between the rdc measurements with and without the PET insert installed in the MR is an effect of the repositioning of the phantom between the runs with and without the PET insert. The SFNR is significantly lower in the configuration with the PET insert installed in the MR. This decrease in SFNR can be attributed to the increased signal intensity drift and the repositioning of the phantom. However, it is unlikely that such a small decrease in SFNR will fundamentally impact fMRI studies in small animals because such signal alterations have to be expected on the basis of an inter-subject variation. Furthermore, the RMS stability, which is an indicator of the signal fluctuation during an fMRI experiment, is not altered by the presence of the PET insert. The RMS value of 0.07% is approximately 12 times smaller than the 1% or more (21) activation to be expected in a typical fMRI small animal experiment at 7 T. For this reason, the RMS stability is sufficient to perform fMRI studies in the presence of a PET insert.
Conclusion
In summary, the evaluation of the MR compatibility of a small animal PET insert has shown that there are only minor impacts on the signal to noise ratio of the MR images and increased signal intensity drift caused by the operation of an additionally installed and active PET insert. B0 and B1 image homogeneity as well as EPI imaging stability are not tremendously affected by the addition of a PET insert. These results are very positive, especially because the PET insert houses the entire electronics at the detector front-end and exhibits therefore a worst-case scenario compared to other realizations of PET/MR scanners (2,6,13,22).
The ability to perform advanced functional MR protocols such as BOLD imaging, even in the presence of a PET insert, opens new possibilities for multimodal imaging. Using simultaneous PET/MR as a tool allows the study of biological processes with functional data derived from the MR as well as from the PET to complement each other. Therefore, new insights can be gained from such a new, multimodal imaging approach.
Acknowledgments
We would like to thank Michael Erb from the University of Tuebingen, Section for Experimental MR of the CNS, and Markus Becker from Bruker BioSpin MRI for useful discussions. This work was supported by grants from DFG PI771 and NIH 1R21EB004483.
Footnotes
| List of Symbols: | |
| cos α | :roman cos for the mathematical symbol cosine, and italic greek lower case alpha as the argument of the cosine (here used for the flip angle) |
| ΔB0(r) | :greek lower case letter delta, italic upper case B (“bee”), subscript “zero”, in parentheses bold lower case roman r (“err”) (indicating a vector) |
| exp(t/T1) | :roman exp (for exponential) and in parentheses lower case t (“tee”), dash (/) for divided by, upper case italic T (“tee”) and subscript 1 “one” |
| γ | :greek lower case letter gamma (here used for the gyromagnetic ratio) |
| Φ | :italic greek upper case letter Phi (here used for the image phase) |
| Φ(rTE1) | :italic greek upper case letter Phi, in parentheses bold lower case roman r (indicating a vector), upper case italic TE and subscript 1 “one” |
| rdc | :roman lower case r “err”, subscript roman lower case d “dee” and c “cee” |
| σ | :italic greek lower case letter sigma (here used for the image noise) |
| SSE | :italic capital letter S, subscript italic capital letter S, subscript italic capital letter E |
| SSTE | :italic capital letter S, subscript italic capital letter S, subscript italic capital letter T, subscript italic capital letter E |
| T1 | :italic capital letter T, and subscript italic 1 “one” (for the relaxation time) |
| T2 | :italic capital letter T, and subscript italic 2 “two” (for the relaxation time) |
References
- 1.Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, Thielscher A, Kneilling M, Lichy MP, Eichner M, Klingel K, Reischl G, Widmaier S, Rocken M, Nutt RE, Machulla HJ, Uludag K, Cherry SR, Claussen CD, Pichler BJ. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14(4):459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 2.Catana C, Procissi D, Wu Y, Judenhofer MS, Qi J, Pichler BJ, Jacobs RE, Cherry SR. Simultaneous in vivo positron emission tomography and magnetic resonance imaging. Proc Natl Acad Sci U S A. 2008;105(10):3705–3710. doi: 10.1073/pnas.0711622105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlemmer HP, Pichler BJ, Schmand M, Burbar Z, Michel C, Ladebeck R, Jattke K, Townsend D, Nahmias C, Jacob PK, Heiss WD, Claussen CD. Simultaneous MR/PET imaging of the human brain: feasibility study. Radiology. 2008;248(3):1028–1035. doi: 10.1148/radiol.2483071927. [DOI] [PubMed] [Google Scholar]
- 4.Wehrl HF, Judenhofer MS, Wiehr S, Pichler BJ. Pre-clinical PET/MR: technological advances and new perspectives in biomedical research. Eur J Nucl Med Mol Imaging. 2009;36 1:S56–68. doi: 10.1007/s00259-009-1078-0. [DOI] [PubMed] [Google Scholar]
- 5.Marsden PK, Strul D, Keevil SF, Williams SC, Cash D. Simultaneous PET and NMR. Br J Radiol. 2002;75 doi: 10.1259/bjr.75.suppl_9.750053. Spec No:S53-59. [DOI] [PubMed] [Google Scholar]
- 6.Raylman RR, Majewski S, Lemieux SK, Velan SS, Kross B, Popov V, Smith MF, Weisenberger AG, Zorn C, Marano GD. Simultaneous MRI and PET imaging of a rat brain. Phys Med Biol. 2006;51(24):6371–6379. doi: 10.1088/0031-9155/51/24/006. [DOI] [PubMed] [Google Scholar]
- 7.Shao Y, Cherry SR, Farahani K, Slates R, Silverman RW, Meadors K, Bowery A, Siegel S, Marsden PK, Garlick PB. Development of a PET detector system compatible with MRI/NMR systems. Nuclear Science, IEEE Transactions. 1997;44(3):1167–1171. on. [Google Scholar]
- 8.Lucas AJ, Hawkes RC, Ansorge RE, Williams GB, Nutt RE, Clark JC, Fryer TD, Carpenter TA. Development of a combined microPET-MR system. Technol Cancer Res Treat. 2006;5(4):337–341. doi: 10.1177/153303460600500405. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert KM, Handler WB, Scholl TJ, Odegaard JW, Chronik BA. Design of field-cycled magnetic resonance systems for small animal imaging. Phys Med Biol. 2006;51(11):2825–2841. doi: 10.1088/0031-9155/51/11/010. [DOI] [PubMed] [Google Scholar]
- 10.Handler WB, Gilbert KM, Peng H, Chronik BA. Simulation of scattering and attenuation of 511 keV photons in a combined PET/field-cycled MRI system. Phys Med Biol. 2006;51(10):2479–2491. doi: 10.1088/0031-9155/51/10/008. [DOI] [PubMed] [Google Scholar]
- 11.Catana C, Wu Y, Judenhofer MS, Qi J, Pichler BJ, Cherry SR. Simultaneous Acquisition of Multislice PET and MR Images: Initial Results with a MR-Compatible PET Scanner. J Nucl Med. 2006;47(12):1968–1976. [PubMed] [Google Scholar]
- 12.Judenhofer MS, Catana C, Swann BK, Siegel S, Jung WI, Nutt R, Cherry SR, Claussen CD, Pichler BJ. Simultaneous PET/MR Images, acquired with a Compact MRI Compatible PET Detector in a 7 Tesla Magnet. Radiology. 2007;244(3):807–814. doi: 10.1148/radiol.2443061756. [DOI] [PubMed] [Google Scholar]
- 13.Slates RB, Farahani K, Shao Y, Marsden PK, Taylor J, Summers PE, Williams S, Beech J, Cherry SR. A study of artefacts in simultaneous PET and MR imaging using a prototype MR compatible PET scanner. Phys Med Biol. 1999;44(8):2015–2027. doi: 10.1088/0031-9155/44/8/312. [DOI] [PubMed] [Google Scholar]
- 14.Henkelman RM. Measurement of signal intensities in the presence of noise in MR images. Med Phys. 1985;12(2):232–233. doi: 10.1118/1.595711. [DOI] [PubMed] [Google Scholar]
- 15.Price RR, Axel L, Morgan T, Newman R, Perman W, Schneiders N, Selikson M, Wood M, Thomas SR. Quality assurance methods and phantoms for magnetic resonance imaging: report of AAPM nuclear magnetic resonance Task Group No. 1. Med Phys. 1990;17(2):287–295. doi: 10.1118/1.596566. [DOI] [PubMed] [Google Scholar]
- 16.Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic Resonance Imaging: Physical Principles and Sequence Design. Wiley & Sons; 1999. p. 914. [Google Scholar]
- 17.Witoszynskyj S, Rauscher A, Reichenbach JR, Barth M. Phase unwrapping of MR images using Phi UN--a fast and robust region growing algorithm. Med Image Anal. 2009;13(2):257–268. doi: 10.1016/j.media.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Friedman L, Glover GH. Report on a multicenter fMRI quality assurance protocol. J Magn Reson Imaging. 2006;23(6):827–839. doi: 10.1002/jmri.20583. [DOI] [PubMed] [Google Scholar]
- 19.Weisskoff RM. Simple measurement of scanner stability for functional NMR imaging of activation in the brain. Magn Reson Med. 1996;36(4):643–645. doi: 10.1002/mrm.1910360422. [DOI] [PubMed] [Google Scholar]
- 20.Uludag K, Dubowith DJ, Buxton RB. Clinical MRI. San Diego: Elsevier; 2005. Basic principles of functional MRI; pp. 249–287. [Google Scholar]
- 21.Sommers MG, van Egmond J, Booij LH, Heerschap A. Isoflurane anesthesia is a valuable alternative for alpha-chloralose anesthesia in the forepaw stimulation model in rats. NMR Biomed. 2009;22(4):414–418. doi: 10.1002/nbm.1351. [DOI] [PubMed] [Google Scholar]
- 22.Olcott PD, Peng H, Levin CS. Novel electro-optical coupling technique for magnetic resonance-compatible positron emission tomography detectors. Mol Imaging. 2009;8(2):74–86. [PubMed] [Google Scholar]