Abstract
Chronic pain is more common in the elderly and impairs functioning and quality of life. Though obesity, defined by body mass index (BMI), has been associated with pain prevalence among older adults, the mechanism of this association remains unclear. We examined components of the metabolic syndrome, insulin resistance, a marker of inflammation, and the presence of painful comorbidities as possible mediators of this association. Participants were 407 individuals age • 70 in the Einstein Aging Study. Chronic pain and pain over the last 3 months were defined using the Total Pain Index (TPI). Insulin resistance was modeled as fasting insulin, HOMA and QUICKI. High sensitivity C-reactive protein was used as a marker of inflammation. Cross-sectional logistic regression models were constructed to assess the associations of these factors with prevalent pain, adjusted for other known pain correlates. Prevalence of chronic pain was 52%. Of the clinical components of metabolic syndrome, central obesity was significantly associated with pain (OR 2.03, 95% CI 1.36-3.01). After adjustment for insulin resistance, inflammation, and pain-related comorbidities, central obesity predicted higher TPI scores (OR 1.55, 95% CI 1.04-2.33) and nearly doubled the risk of chronic pain (OR 1.70, 95% CI 1.05-2.75). Central obesity is the metabolic syndrome component showing the strongest independent association with pain, and the relationship is not explained by markers of insulin resistance or inflammation, nor by the presence of osteoarthritis or neuropathy.
Introduction
The burden of chronic pain among otherwise well, community residing older adults is substantial. In large U.S. community-based samples up to 61% of older adults have reported chronic pain [40]. The relationship of chronic pain to age is uncertain [5,6,34]. However, the negative impact of pain on functional performance and quality of life clearly and consistently increases with increasing age [34].
Pain prevalence and severity have been linked to obesity in adults [11]. This relationship is not limited to osteoarthritis pain, but also occurs in individuals with chronic headache [1] and neuropathic pain [22]. The impact of pain on functional status and health related quality of life is greater in obese individuals than in those with normal body mass index (BMI)[18].
The mechanisms underlying the relationship between pain and obesity remain unclear, particularly in older adults. Because obesity is a pro-inflammatory state and inflammatory mechanisms contribute to the development of pain, inflammation may be part of the causal pathway. Additionally, acute pain has been shown to cause transient insulin resistance in an experimental setting, suggesting a potential link through impaired glucose metabolism [8]. Finally, both obesity and chronic pain are associated with depression, and pain is worse among obese individuals with depression and anxiety [35]. A potential unifying mechanism may be found in the metabolic syndrome, which is known to be associated with chronic pain [17], inflammation [16], insulin resistance [15], and mood disorders [29].
The Einstein Aging Study (EAS) has reported that BMI based on self-reported weight and height was associated with increased prevalence of chronic pain at cross-section [19]. To help clarify mechanisms underlying this association, the present study used a sample from the EAS to investigate potential mediators of the relationship between pain and obesity in the elderly: (1) The metabolic syndrome and its individual clinical components; (2) Insulin resistance; (3) Inflammation as assessed by high sensitivity C-reactive protein (hsCRP) [33], and (4) The presence of highly prevalent painful comorbid conditions, osteoarthritis and neuropathy. Comorbid depression and anxiety and use of nonsteroidal anti-inflammatory medications (NSAIDs) were included in adjusted models to investigate their potential role as confounders of the pain-obesity relationship.
Methods
Study Population
The EAS is a longitudinal, community-based study of aging conducted in Bronx, NY. Subjects were recruited via systematic sampling from lists of Medicare-eligible persons in Bronx County, NY obtained from the Health Care Financing Administration (1998–2004), or from voter registration lists (since 2004). The demographic characteristics of these sampling frames are similar. All participants are community-dwelling, over age 70, ambulatory, fluent in English, and able to hear well enough to complete telephone screening. Subjects complete annual clinic assessments that include neurologic and cognitive testing, anthropometric measures, blood pressure, and collection of fasting blood samples. Within 3 months prior to each clinic visit subjects are contacted by phone to remind them of the visit and at that time are invited to participate in a computer-assisted telephone interview (CATI) [19]. The cross-sectional subsample included in this analysis consists of all subjects assessed from October 2004–July 2007 for whom complete data were available on pain as well as fasting glucose, insulin, hsCRP, triglycerides and HDL for the corresponding clinic visit (n=407). The EAS has been approved by the local institutional review board. Informed consent was obtained during participants' initial study center visit.
Measures
All medical history, epidemiologic, social and behavioral data were collected at annual clinic visits by structured interviews conducted by trained study staff. The only exception is the question “Has a doctor ever told you that you have or have had an anxiety disorder?” which was asked as part of the CATI. Neuropathy was ascertained through physical examination by study clinicians supervised by a board-certified neurologist. Depressive symptomatology was assessed using the 15-item Geriatric Depression Scale (GDS) [10] and clinically significant depression was defined using a cut-off of 6 or greater. Global cognitive function was assessed using the Blessed Information Memory Concentration test [2] (range 0-33 with higher score = greater impairment). Individuals with a score of 8 or greater were excluded (n=13). Race/ethnicity was self-classified by choosing from a list of options that included “Other.”
Resting blood pressure was manually recorded from seated participants as the average of 2 measurements taken from the same arm. Waist circumference was measured at the level of the umbilicus. Blood samples were centrifuged, transferred immediately to a -20° C storage facility, and processed in batches at the Albert Einstein College of Medicine General Clinical Research Center Core Laboratory on the same Olympus AU400 chemistry autoanalyzer. Glucose levels were assessed using the hexokinase G-6-PDH method. Blood samples for lipids were collected in heparinized tubes and analyzed by enzymatic methods. HsCRP was measured using a latex-agglutination immunoassay (Equal Diagnostics, Inc, Exton PA) with a sensitivity range from 0.05 mg/dL to 160.0 mg/dL. Insulin was measured by radioimmunoassay. Insulin resistance was estimated using 3 surrogate markers of the hyperinsulinemic euglycemic glucose clamp: fasting insulin concentration alone, homeostasis model assessment (HOMA, calculated as [fasting insulin (μIU/mL) * fasting glucose (mg/dL)]/405), and the quantitative insulin sensitivity check index (QUICKI, 1/[log (insulin μIU/mL) + log (glucose mg/dL)].
Body mass index (BMI) was calculated from weight measured on a manual scale and height measured using a wall-mounted ruler as weight (kg)/[height (m)]2. Metabolic syndrome (MetS) was defined according to National Cholesterol Education Panel's Adult Treatment Panel III [23] with the subsequently recommended revision of the threshold for abnormal fasting glucose [9]. The updated definition identifies 5 possible components as follows: elevated resting blood pressure (systolic •130 mmHg or diastolic •80 mmHg) or use of antihypertensive medication in a patient with a history of hypertension; fasting blood glucose •100 mg/dL (5.55 mmol/L) or treatment for elevated glucose; fasting triglycerides • 150 mg/dL (1.695 mmol/L) or use of triglyceride-lowering medication; HDL <40 mg/dL (1.036 mmol/L) in men or <50 mg/dL (1.295 mmol/L) in women or use of medication to increase HDL; and waist circumference •102 cm in men or •88 cm in women. Subjects met the definition of MetS if they were positive for •3 components.
Pain over the last three months was assessed by the Total Pain Index (TPI). The TPI is administered by phone and asks about pain in 8 locations (head; face; neck or shoulders; back; upper extremity including hands; lower extremity including hip, knees and feet; chest; abdomen or pelvis). For each location, subjects report the frequency of pain (none of the time, a slight bit of the time, some of the time, most of the time, or all of the time) and severity (0–10) over the past three months [19]. Summary scores for chronic pain and total pain (as a continuous variable) were derived as follows [19]. Chronic pain was defined as pain in at least one area that occurs at least some of the time with at least moderate severity (• 4/10) over the past 3 months. This definition is based on the International Association for the Study of Pain (IASP). The TPI was constructed as the product of pain frequency and pain severity at each location summed over the eight pain locations for a total possible score of 80. Quartiles were generated from the distribution of the TPI within this subsample. The TPI has been validated within the EAS and shown to have high test-retest reliability and high correlation with other pain indices including the Bodily Pain Index from the Short Form-36 [39].
Statistical Analysis
Dependent variables were chronic pain and quartiles of TPI as defined above. All variables were examined for outliers, missing data, and entry errors. Odds ratios and confidence intervals for bivariate associations with chronic pain were derived using univariate logistic regression models. Bivariate trends over ordered quartiles of pain were tested using Stata's nonparametric test of trend, an extension of the Wilcoxon rank-sum test. Multivariable modeling of chronic pain was performed using logistic regression. Quartiles of TPI were modeled using ordinal logistic regression. Variables were added to models in a stepwise fashion while the coefficient for abdominal obesity was monitored for a change • 15% (an indication of confounding). For ordinal logistic regression models, the assumption of proportional odds was tested using a likelihood ratio test as well as the Brant test of the parallel regression assumption. The strength of the final logistic models was assessed using the Hosmer-Lemeshow goodness-of-fit summary statistic. Regression residuals were examined to check for covariate patterns exerting undue influence. All analyses were performed by a single author (LMR) using Stata 9.2 (Statacorp LP, College Station, TX).
To explore the hypothesis suggested by the results of bivariate analysis in Table 1 that the relationship of metabolic syndrome to pain is primarily caused by abdominal obesity, the five MetS components were entered simultaneously into a set of regression models. Models are presented with and without adjustment for covariates known to be associated with pain. Diagnostic tests for collinearity and model fit were performed. Next, a set of models for each of the two pain outcome measures was created to investigate the effects of measures of disordered glucose handling (diabetes, fasting glucose, and insulin resistance); inflammation measured by hsCRP; and painful comorbidities (osteoarthritis and neuropathy) on the association of abdominal obesity with pain. All models were adjusted for age, sex, education, white race, alcohol use (at least one drink per week), NSAID use, depression and anxiety. Each of our measures of insulin resistance was tested in individual models. Results were similar for each, and those for fasting insulin are reported. Finally, the odds ratios and exact confidence limits for site-specific pain in relation to abdominal obesity were estimated using logistic regression models to develop Figure 1.
Table 1.
Subject characteristics by pain quartile and bivariate odds ratios for chronic pain, Einstein Aging Study (N=407).
| Quartile of Pain (low to high) Mean or % | Chronic Pain | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean (SD) N (%) | 1 | 2 | 3 | 4 | p-value for trend | OR (95% CI) | p-value |
| Age, years | 80.0 (5.3) | 79.7 | 80.1 | 80.1 | 80.0 | 0.738 | 1.02 (0.98–1.06) | 0.362 |
| Female, % | 252 (61.9) | 53.0 | 58.7 | 60.2 | 76.8 | 0.001 | 1.81 (1.21–2.71) | 0.004 |
| Education, yr | 13.9 (3.5) | 14.0 | 14.2 | 13.8 | 13.8 | 0.510 | 0.95 (0.90–1.01) | 0.099 |
| Race, % white | 284 (70.1) | 73.0 | 75.0 | 72.0 | 60.2 | 0.051 | 0.66 (0.43–1.02) | 0.061 |
| Alcohol ≥1 drink/wk, % | 143 (35.2) | 43.0 | 43.7 | 32.4 | 21.1 | <0.001 | 0.52 (0.34–0.79) | 0.002 |
| GDS ≥ 6, % | 31 (7.6) | 6.0 | 5.8 | 7.4 | 11.6 | 0.130 | 1.29 (0.61–2.70) | 0.507 |
| Self-Reported Anxiety Disorder, % | 28 (6.9) | 5.0 | 4.8 | 7.4 | 10.8 | 0.087 | 1.46 (0.67–3.20) | 0.347 |
| BIMC | 1.9 (1.8) | 1.66 | 1.85 | 2.0 | 1.91 | 0.291 | 1.07 (0.95–1.19) | 0.263 |
| Metabolic Syndrome, % | 138 (33.9) | 30.0 | 29.8 | 30.6 | 46.3 | 0.023 | 1.41 (0.93–2.13) | 0.103 |
| HBP, % | 353 (86.7) | 93.0 | 82.7 | 82.4 | 89.5 | 0.447 | 0.86 (0.48-1.53) | 0.611 |
| Triglycerides ≥ 150 mg/dl, % | 98 (24.1) | 21.0 | 23.1 | 24.1 | 28.4 | 0.233 | 1.22 (0.77–1.93) | 0.389 |
| Low HDL, % | 71 (17.4) | 15.0 | 16.3 | 19.4 | 18.9 | 0.376 | 1.06 (0.63–1.77) | 0.826 |
| Glucose ≥ 100 mg/dl or Diabetes, % | 139 (34.2) | 31.0 | 27.9 | 35.2 | 43.2 | 0.042 | 1.26 (0.83–1.90) | 0.272 |
| Abdominal Obesity, % | 188 (46.2) | 34.0 | 43.3 | 45.4 | 63.2 | <0.001 | 2.03 (1.36–3.01) | <0.001 |
| BMI, kg/m2 | 27.0 (4.6) | 26.2 | 25.9 | 27.6 | 28.1 | 0.001 | 1.09 (1.04–1.14) | 0.000 |
| Insulin, uU/ml | 33.0 (51.2) | 31.7 | 31.9 | 29.9 | 39.0 | 0.172 | 1.00 (1.00–1.00) | 0.772 |
| HOMA | 9.6 (20.3) | 8.5 | 8.7 | 8.3 | 13.1 | 0.067 | 1.00 (0.99–1.01) | 0.454 |
| QUICKI | 0.6 (0.2) | 0.63 | 0.59 | 0.58 | 0.57 | 0.116 | 0.75 (0.30–1.82) | 0.520 |
| HsCRP | 3.5 (5.2) | 2.7 | 3.0 | 4.0 | 4.3 | 0.001 | 1.06 (1.01–1.11) | 0.016 |
| Osteoarthritis | 279 (70.5) | 50.0 | 60.6 | 83.2 | 88.0 | <0.001 | 3.88 (2.44–6.17) | <0.001 |
| Neuropathy | 86 (23.8) | 23.7 | 19.8 | 21.9 | 30.9 | 0.277 | 1.18 (0.73–1.92) | 0.506 |
| NSAID use, % | 20 (5.0) | 3.0 | 2.9 | 7.4 | 6.3 | 0.130 | 3.86 (1.27–11.75) | 0.017 |
HBP – High Blood Pressure – Systolic ≥ 130 mmHg or diastolic ≥ 80 mmHg or antihypertensive medication use.
Abdominal Obesity – waist circumference ≥ 88 cm (females), ≥ 102 cm (males).
GDS – Geriatric Depression Scale.
BIMC – Blessed Information Memory Concentration Test.
BMI –Body mass index, weight (kg)/height (m2).
HOMA – Homeostasis Model Assessment ([fasting insulin (μU/mL) * fasting glucose (mg/dL)]/405)
Low HDL – < 40 mg/dl male, < 50 mg/dl Female.
QUICKI – Quantitative Insulin Sensitivity Check Index (1/[log (insulin μU/mL) + log (glucose mg/dL)].
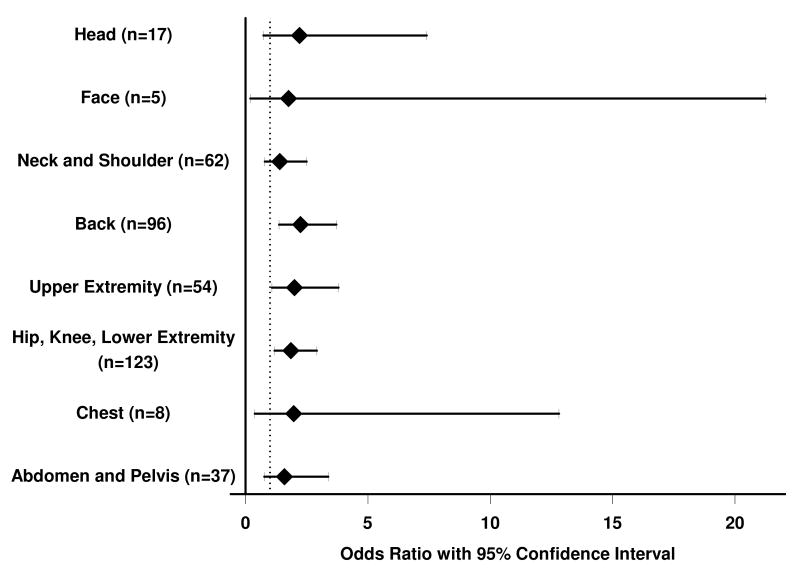
Figure 1.
Odds ratios (95% confidence limits) for the association of abdominal obesity with pain in specific body sites (N=407). Dashed vertical line represents Odds Ratio= 1.0. Body site and number of participants reporting pain in each site is indicated on the Y-axis.
Results
Demographic and psychiatric variables as well as bivariate associations by chronic pain status and quartiles of TPI are provided in Table 1. Subjects were predominately female (61.9%) and white (70.1%), and ranged in age from 70 to 96 years of age (average 80 years). Pain of any severity during the prior 3 months at one or more locations was reported by 308 individuals (76%), and 213 (52.3%) met the definition of chronic pain. Moderate or severe pain was reported by 58% (n=237). The overall average score on the TPI was 3.8.
Females were significantly more likely to have chronic pain than males (OR 1.81, 95% CI 1.21-2.71). Caucasians reported less pain than non-white subjects (p = 0.051), but the association was not significant among individuals with chronic pain. Education was not associated with pain. A strong inverse relationship with alcohol was found, with those reporting at least one drink per week experiencing less pain (p-value for trend <0.001) and being less likely to report chronic pain (OR 0.52, 95% CI 0.34-0.79). History of smoking was not associated with pain (data not shown). Chronic pain was significantly associated with depression score (OR 1.12, 95% CI 1.01-1.24), and depression score increased significantly with quartile of TPI (p-value for trend <0.001, data not shown). However, individuals categorized as having depression based on a GDS score ≥6 were not more likely to have chronic pain, nor were subjects with a self-reported, physician-diagnosed history of anxiety. There was no association between poorer performance on the test of global cognitive function and pain in this group.
Both BMI and abdominal obesity were associated with chronic pain and quartile of TPI. The relative odds of chronic pain increased with each unit of BMI (OR 1.09, 95% CI 1.04-1.14; test for trend, p=0.001) and individuals with abdominal obesity were twice as likely to report chronic pain (OR 2.03, 95% CI 1.36-3.01; test for trend <0.001). Regardless of whether obesity was defined as a dichotomous variable (BMI •30) or based on a waist circumference cut-score, the relationships between obesity and dichotomous chronic pain were very similar.
Thirty-four percent (n=138) of the sample met the definition for metabolic syndrome. Metabolic syndrome increased in prevalence with quartile of TPI but showed only a trend toward greater frequency among those with chronic pain (test for trend, p = 0.023; OR 1.41, 95% CI 0.93-2.13). Upon examination of the components of metabolic syndrome (tested as continuous predictors and as dichotomous variables using defined cut-offs), only abdominal obesity was consistently associated with pain.
Neither of the two measures of insulin resistance (fasting insulin, p-value for trend = 0.172, and HOMA, p-value for trend = 0.067), nor the measure of insulin sensitivity (QUICKI, p-value for trend = 0.116) reflected increasing insulin resistance across quartiles of pain, although values at the extremes suggested a trend in the expected direction. None of these measures was associated with dichotomous chronic pain.
HsCRP was higher in individuals with pain (Table 1). Thirty-three percent (n =133) had a hsCRP level greater than 3, a value known to confer increased risk for new cardiovascular events. Seven percent (n = 31) had hsCRP levels greater than 10, suggesting the presence of an underlying inflammatory disease. Bivarate analyses with the latter group excluded (data not shown) revealed a consistent pattern of increasing hsCRP with quartiles of pain (p for trend = 0.026) but the association with chronic pain was attenuated in this subgroup (OR 1.08, 95% CI 0.98-1.19, p = 0.104).
Osteoarthritis was highly prevalent in this sample (n = 279, 70%) and was strongly associated with both pain measures. Neuropathy was present in 86 of 361 (24%) individuals for whom clinical examinations were performed (89% of subjects had nonmissing data). Neuropathy was not associated with pain in bivariate analyses. Only 5% of the sample reported regular use of NSAIDs, but these individuals were more likely to report chronic pain.
To further examine the role of abdominal obesity as the potential driver of the relationship between metabolic syndrome and pain, metabolic syndrome components were simultaneously entered into models with pain as the dependent variable (Table 2). Abdominal obesity was the only component that significantly predicted pain in these models. Checks for multicollinearity in the models shown in Table 2 revealed standard errors that were reasonable relative to the regression coefficients, and collinearity diagnostics showed that the tolerance and variance inflation factors were close to 1.
Table 2.
Estimated odds ratios (95% CI) for pain associated with components of metabolic syndrome, Einstein Aging Study, N=407.
| Pain Outcome | ||||
|---|---|---|---|---|
| Trend over Pain Quartiles | Chronic Pain | |||
| Parameter | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value |
| Model 11 | ||||
| Abdominal Obesity | 1.95 (1.33–2.84) | 0.001 | 2.03 (1.33–3.11) | 0.001 |
| Hypertension3 | 0.74 (0.45–1.22) | 0.241 | 0.80 (0.44–1.45) | 0.470 |
| High triglyceride | 0.98 (0.62–1.54) | 0.921 | 0.98 (0.59–1.64) | 0.942 |
| Low HDL | 1.11 (0.68–1.81) | 0.662 | 0.96 (0.55–1.67) | 0.886 |
| Elevated fasting glucose, history of diabetes4 | 1.27 (0.86–1.87) | 0.235 | 1.06 (0.68–1.65) | 0.789 |
| Model 21 | ||||
| Abdominal Obesity | 1.67 (1.13–2.47) | 0.010 | 1.71 (1.09-2.68) | 0.019 |
| Hypertension3 | 0.69 (0.42–1.15) | 0.152 | 0.72 (0.39-1.34) | 0.300 |
| High triglyceride | 1.01 (0.63–1.62) | 0.961 | 1.05 (0.61-1.80) | 0.871 |
| Low HDL | 1.00 (0.60–1.66) | 0.999 | 0.84 (0.47-1.51) | 0.556 |
| Elevated fasting glucose, history of diabetes4 | 1.36 (0.90–2.07) | 0.144 | 1.19 (0.75-1.91) | 0.461 |
Model 1 included all components of the metabolic syndrome simultaneously, thus parameters reported are adjusted for each of the other components.
Model 2 included all parameters in Model 1 plus sex, education, race, depression, anxiety, NSAIDs and alcohol use.
Systolic ≥ 130 mmHg or diastolic ≥ 80 mmHg or antihypertensive medication use.
Or use of glucose-lowering medication.
Further analyses focused on central obesity as the salient clinical factor linking metabolic syndrome to pain. Table 3 shows results for the two sets of models used to explore potential mediators of the relationship between abdominal obesity and either quartiles of pain or presence of chronic pain. In the first model (Model 1), abdominal obesity remains a significant independent predictor of pain after adjustment for demographic characteristics and other factors that were associated with pain on bivariate analysis. Individuals with abdominal obesity show an 83% increased likelihood (OR for chronic pain 1.83, 95% CI 1.20-2.79) of having chronic pain as compared to non-obese subjects. The second set of models (Model 2) include adjustment for fasting insulin level, fasting glucose and history of diabetes in order to isolate the effect of insulin resistance among non-diabetics on the presence of pain. None of these measures diminished the strong, independent association of abdominal obesity with pain (OR for chronic pain 1.82, 95%CI 1.17-2.84) for a model that included fasting insulin as a covariate and chronic pain as the dependent variable). Models using alternative measures of insulin resistance (QUICKI or HOMA) rather than insulin level yielded similar results (data not shown). The inflammatory marker hsCRP was a significant, independent predictor of pain in this sample, but its presence in the model did not attenuate the relationship between abdominal obesity and pain (Model 3). In Model 4, the association of abdominal obesity with pain remains significant even when osteoarthritis and neuropathy are taken into account. In this model individuals with abdominal obesity continue to have a 70% increased likelihood of having chronic pain that is independent of the presence of painful comorbid conditions.
Table 3. Adjusted1 odds ratios (95% confidence intervals) for pain associated with abdominal obesity, Einstein Aging Study, N=407.
| Ordinal Logistic Regression with ordered Quartiles of Pain | Logistic Regression with Chronic Pain | |||
|---|---|---|---|---|
| Parameter | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value |
| (1) Basic Model | ||||
| Abdominal Obesity | 1.82 (1.26–2.63) | 0.002 | 1.83 (1.20-2.79) | 0.005 |
| (2) Glucose Metabolism | ||||
| Abdominal Obesity | 1.71 (1.16–2.53) | 0.007 | 1.82 (1.17-2.84) | 0.008 |
| History of Diabetes | 1.58 (0.88–2.84) | 0.123 | 1.08 (0.57-2.05) | 0.804 |
| Fasting Glucose (mg/dL) | 1.00 (0.99-1.01) | 0.932 | 1.00 (0.99-1.01) | 0.854 |
| Fasting Insulin (uIU/mL) | 1.00 (1.00–1.00) | 0.850 | 1.00 (1.00-1.00) | 0.974 |
| (3) Inflammation | ||||
| Abdominal Obesity | 1.69 (1.16–2.47) | 0.006 | 1.70 (1.10-2.64) | 0.017 |
| High sensitivity CRP (mg/L) | 1.04 (1.01–1.08) | 0.020 | 1.05 (1.00-1.10) | 0.038 |
| (4) Painful Comorbitidies2 | ||||
| Abdominal Obesity | 1.55 (1.04-2.33) | 0.033 | 1.70 (1.05-2.75) | 0.032 |
| Osteoarthritis | 4.25 (2.7-6.70) | <0.001 | 4.32 (2.53-7.38) | <0.001 |
| Neuropathy | 1.22 (0.77-1.95) | 0.391 | 1.17 (0.67-2.02) | 0.581 |
All models adjusted for age, sex, education, race, depression, anxiety and alcohol use.
Subset of 348 individuals for whom neuropathy and all other variables are complete.
Figure 1 shows unadjusted odds ratios and 95% CI for the relationship between abdominal obesity and chronic pain by body location. Subjects with abdominal obesity were approximately twice as likely to have chronic back pain (OR 2.25, 95% CI 1.37-3.17), chronic hip, knee, or leg pain (OR 1.86, 95% CI 1.18-2.92), or upper extremity pain (OR 2.01, 95% CI 1.08-3.80). Additional analyses showed a trend toward increased number of pain locations reported among those with abdominal obesity (Rank-sum test p< 0.001). Because the number of individuals reporting pain in each site was small, these analyses are considered exploratory and were not adjusted for covariates.
All ordinal logistic models satisfied the likelihood ratio and Brant tests of the proportional odds assumption. No interaction terms met the predetermined cut-off of <0.1 for significance. Regression residuals yielded one covariate pattern suspicious for excessive influence on model estimates. Exploration of the data for that individual did not reveal unusual values. Models were reconstructed after removing the influential subject. The Hosmer-Lemeshow goodness-of-fit statistic was only minimally changed, and regression coefficients were changed by less than 5%. Therefore, that subject was retained in the final models.
Discussion
Our findings contribute to the growing body of evidence that pain among community-dwelling, well elderly individuals is associated with obesity. Thirty-four percent of the sample had metabolic syndrome, the presence of which was associated with higher pain scores even after adjustment for demographic and other factors related to pain. Of the individual components of metabolic syndrome, only abdominal obesity was linked to the presence of chronic pain and higher pain scores. BMI bore the same relationship to pain as central obesity in this sample. Potential mechanistic links between the co-occurrence of obesity and pain were explored in multivariable analyses, yet none of these potential mediators fully accounted for the relationship. Insulin resistance showed no consistent association with pain. The inflammatory marker hsCRP was linked to pain in adjusted models but was independent of the association of pain and abdominal girth (OR for obesity after adjustment for hsCRP and basic model covariates = 1.70, 95% CI 1.10-2.64). Osteoarthritis was highly prevalent in this sample, with 70% of the sample affected, and of all the covariates studied had the strongest relationship to pain. Yet even after adjustment for the presence of osteoarthritis, neuropathy, and NSAID use, individuals with abdominal obesity were estimated to have a 70% increased likelihood of having pain (OR for chronic pain 1.70, 95% CI 1.05-2.75).
Increased waist circumference has been increasingly recognized as a predictor of adverse health outcomes in the elderly, including cardiovascular diseases such as congestive heart failure, coronary heart disease, hypertension, and dyslipidemia, as well as diabetes, all-cause mortality, and cancer. Central fat has been found in a number of reports to better reflect the risks of obesity in the elderly than BMI [42]. It has also been linked to the prevalence of neuropathy in pre-diabetic humans [43] and mice [27].
Mechanistic explanations for the relationship of abdominal obesity to cardiovascular and metabolic outcomes point to the release of pro-inflammatory and insulin resistance-inducing substances from visceral adipose tissue [13,4]. In the present study, the association between abdominal obesity and pain was not attenuated by any measure of insulin resistance or by inflammation as measured by hsCRP. Neuropathic pain is frequent among elderly individuals, and may be linked to obesity through diabetes. In these analyses neuropathic pain also failed to account for the observed link between central obesity and pain. As summarized by Bray and Bellanger [4], the health consequences of overweight may be categorized as (1) those associated with the secretory products of adipose tissue, and (2) those caused by increased fat cell size and the resulting increase in body mass. Our models also adjusted for the presence of osteoarthritis, a primary consequence of altered body mechanics and joint load associated with increased girth, which was highly prevalent in this sample and strongly associated to pain. The finding that central adiposity was independent even of painful comorbitidies suggests that an alternative pathway accounts for the pain-obesity association, which may be bidirectional. For example, pain may lead to decreased physical activity, depression, and obesity. In particular, chronic pain may lead to cortisol secretion that contributes to truncal obesity. Conversely, the metabolic derangements of obesity may predispose to pain. These potential relationships should be addressed in longitudinal studies.
The potential benefits of weight loss as a strategy for reducing or preventing chronic pain have yet to be demonstrated. Recommendations that elderly adults restrict calories must take into consideration the potential risk of weight loss in this population. Several authors have raised concerns about the potential for losing crucial lean body mass along with fat mass through weight loss efforts, [21] and weight loss has been associated with increased risk for mortality in elderly samples [25]. Fat mass (along with lean mass) is also positively correlated to bone mineral density in the elderly [12]. However, a number of reports have found that exercise interventions with and without caloric restriction lead to decrements in abdominal obesity [28], other cardiovascular risk factors [38], and insulin resistance [41] without significant losses of bone mineral density [31] or lean mass [26,28,32] and without increasing mortality [30]. Lifestyle modifications have also been shown to decrease inflammatory markers [14,20], increase physical function [20], and prevent frailty [37] in the elderly. Consequently, there are now 2 published consensus statements supporting weight loss interventions that minimize loss of lean body mass in obese elderly [24,36].
The results of this study are strengthened by the fact that our sample consisted of relatively healthy, community-dwelling individuals who were not self-selected on the basis of seeking treatment for pain. Thus, results can be more safely extrapolated to the general elderly population. Our findings are limited by the cross-sectional design, which prevents the determination of temporal sequence and thus causation. Future studies of the association between metabolic dysregulation, visceral adiposity and pain may also benefit from inclusion of a range of inflammatory markers. Although often linked to acute pain, hsCRP has been less consistently associated with chronic pain [7]. We tested 3 separate surrogate markers of insulin resistance in our models, but recognize that these measures are only approximations of the euglycemic clamp and may have underestimated the role of insulin resistance in determining the probability of having pain [3].
In summary, we found a nearly twofold increase in the probability of chronic pain among elderly individuals with abdominal obesity that was independent of the other components of metabolic syndrome, hsCRP, established surrogate markers for insulin resistance, depression, anxiety, and the presence of painful comorbid conditions. Although this relationship has been observed at cross-section only, these results suggest an alternative etiologic mechanism linking obesity to pain.
Acknowledgments
This publication was made possible by NIH/NIA grant P01AG03949 and by CTSA Grant UL1 RR025750, from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. The contents are solely the responsibility of the authors and do not necessary represent the official view of the NCRR or NIH. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Financial Disclosures: None
Abdominal obesity was associated with a nearly 2-fold increase in the probability of chronic pain in older adults, independent of other metabolic syndrome components.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bigal ME, Tsang A, Loder E, Serrano D, Reed ML, Lipton RB. Body mass index and episodic headaches: a population-based study. Arch Intern Med. 2007;167(18):1964–1970. doi: 10.1001/archinte.167.18.1964. [DOI] [PubMed] [Google Scholar]
- 2.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968 Jul;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 3.Bloomgarden Z. Third world congress on the insulin resistance syndrome: mediators, antecedents, and measurement. Diabetes Care. 2006;29(7):1700–1706. doi: 10.2337/dc06-zb07. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29(1):109–117. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- 5.Carmaciu C, Iliffe S, Kharicha K. Health risk appraisal in older people 3: prevalence, impact, and context of pain and their implications for GPs. Br J Gen Pract. 2007;57(541):630–635. [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott AM, Smith BH, Penny KI, Smith WC, Chambers WA. The epidemiology of chronic pain in the community. Lancet. 1999;354(9186):1248–1252. doi: 10.1016/s0140-6736(99)03057-3. [DOI] [PubMed] [Google Scholar]
- 7.Gebhardt K, Brenner H, Stürmer T, Raum E, Richter W, Schiltenwolf M, Buchner M. The course of high-sensitive C-reactive protein in correlation with pain and clinical function in patients with acute lumbosciatic pain and chronic low back pain—a 6 months prospective longitudinal study. Eur J Pain. 2006;10(8):711–719. doi: 10.1016/j.ejpain.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Greisen J, Juhl CB, Grøfte T, Vilstrup H, Jensen TS, Schmitz O. Acute pain induces insulin resistance in humans. Anesthesiology. 2001;95(3):573–574. doi: 10.1097/00000542-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F, American Heart Association; National Heart, Lung, and Blood Institute Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann N, Mittmann N, Silver IL, Shulman KI, Busto UA, Shear NH, Naranjo CA. A validation study of The Geriatric Depression Scale short form. Int J Geriatr Psychiatry. 1996;11(5):457–460. [Google Scholar]
- 11.Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of obesity and pain in a general population: results from the Southern Pain Prevalence Study. J Pain. 2007;8(5):430–436. doi: 10.1016/j.jpain.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Ho-Pham LT, Nguyen ND, Lai TQ, Nguyen TV. Contributions of lean mass and fat mass to bone mineral density: a study in postmenopausal women. BMC Musculoskelet Disord. 2010 Mar;:11–59. doi: 10.1186/1471-2474-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R, Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; American Society for Nutrition; American Diabetes Association Waist circumference and cardiometabolic risk: a consensus statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr. 2007;85(5):1197–1202. doi: 10.1093/ajcn/85.5.1197. [DOI] [PubMed] [Google Scholar]
- 14.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol. 2008 Aug;105(2):473–8. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am. 2007;91(6):1063–1077. doi: 10.1016/j.mcna.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Lee IT, Lee WJ, Huang CN, H-H Sheu W. The association of low-grade inflammation, urinary albumin, and insulin resistance with metabolic syndrome in nondiabetic Taiwanese. Metabolism. 2007;56(12):1708–1713. doi: 10.1016/j.metabol.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Loevinger BL, Muller D, Alonso C, Coe CL. Metabolic syndrome in women with chronic pain. Metabolism. 2007;56(1):87–93. doi: 10.1016/j.metabol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Marcus DA. Obesity and the impact of chronic pain. Clin J Pain. 2004;20(3):186–91. doi: 10.1097/00002508-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy LH, Bigal ME, Katz M, Derby C, Lipton RB. Chronic pain and obesity in elderly people: results from the Einstein aging study. J Am Geriatr Soc. 2009;57(1):115–119. doi: 10.1111/j.1532-5415.2008.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller GD, Nicklas BJ, Loeser RF. Inflammatory biomarkers and physical function in older, obese adults with knee pain and self-reported osteoarthritis after intensive weight-loss therapy. J Am Geriatr Soc. 2008 Apr;56(4):644–51. doi: 10.1111/j.1532-5415.2007.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008 Aug-Sep;12(7):487–91. doi: 10.1007/BF02982710. [DOI] [PubMed] [Google Scholar]
- 22.Miscio G, Guastamacchia G, Brunani A, Priano L, Baudo S, Mauro A. Obesity and peripheral neuropathy risk: a dangerous liaison. J Peripher Nerv Syst. 2005;10(4):354–358. doi: 10.1111/j.1085-9489.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- 23.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 24.National Institutes of Health. The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: NIH Publication No. 00-4084 Department of Health and Human Services. National Institutes of Health, National Heart, Lung, and Blood Institute; Bethesda, MD: 2000. [Google Scholar]
- 25.Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP, the Cardiovascular Study Research Group Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 26.Nicklas BJ, Wang X, You T, Lyles MF, Demons J, Easter L, Berry MJ, Lenchik L, Carr JJ. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009 Apr;89(4):1043–52. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, Drel VR. High-fat diet-induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56(10):2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- 28.O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006 May;100(5):1584–9. doi: 10.1152/japplphysiol.01336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Räikkönen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care. 2007;30(4):872–877. doi: 10.2337/dc06-1857. [DOI] [PubMed] [Google Scholar]
- 30.Shea MK, Houston DK, Nicklas BJ, Messier SP, Davis CC, Miller ME, Harris TB, Kitzman DW, Kennedy K, Kritchevsky SB. The effect of randomization to weight loss on total mortality in older overweight and obese adults: the ADAPT Study. J Gerontol A Biol Sci Med Sci. 2010 May;65(5):519–25. doi: 10.1093/gerona/glp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverman NE, Nicklas BJ, Ryan AS. Addition of aerobic exercise to a weight loss program increases BMD, with an associated reduction in inflammation in overweight postmenopausal women. Calcif Tissue Int. 2009 Apr;84(4):257–65. doi: 10.1007/s00223-009-9232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart KJ, Bacher AC, Turner K, Lim JG, Hees PS, Shapiro EP, Tayback M, Ouyang P. Exercise and risk factors associated with metabolic syndrome in older adults. Am J Prev Med. 2005 Jan;28(1):9–18. doi: 10.1016/j.amepre.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Stürmer T, Raum E, Buchner M, Gebhardt K, Schiltenwolf M, Richter W, Brenner H. Pain and high sensitivity C reactive protein in patients with chronic low back pain and acute sciatic pain. Ann Rheum Dis. 2005;64(6):921–924. doi: 10.1136/ard.2004.027045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2004;110(1-2):361–368. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Tietjen GE, Peterlin BL, Brandes JL, Hafeez F, Hutchinson S, Martin VT, Dafer RM, Aurora SK, Stein MR, Herial NA, Utley C, White L, Khuder SA. Depression and anxiety: effect on the migraine-obesity relationship. Headache. 2007;47(6):866–875. doi: 10.1111/j.1526-4610.2007.00810.x. [DOI] [PubMed] [Google Scholar]
- 36.Villareal DT, Apovian CM, Kushner RF, Klein S, American Society for Nutrition; NAASO, The Obesity Society Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005 Nov;13(11):1849–63. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 37.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006 Apr 24;166(8):860–6. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 38.Villareal DT, Miller BV, 3rd, Banks M, Fontana L, Sinacore DR, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006 Dec;84(6):1257–8. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- 39.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 40.Watkins EA, Wollan PC, Melton LJ, 3rd, Yawn BP. A population in pain: report from the Olmsted County health study. Pain Med. 2008;9(2):166–174. doi: 10.1111/j.1526-4637.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 41.Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults—a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2009 Jan;64(1):90–5. doi: 10.1093/gerona/gln032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zamboni M, Mazzali G, Zoico E, Harris TB, Meigs JB, Di Francesco V, Fantin F, Bissoli L, Bosello O. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond) 2005 Sep;29(9):1011–29. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. Prevalence of polyneuropathy in prediabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31(3):464–469. doi: 10.2337/dc07-1796. [DOI] [PubMed] [Google Scholar]