Abstract
Protein Kinase C (PKC) is a family of serine/threonine-isozymes that are involved in many signaling events in normal and disease states. Previous studies from our lab have demonstrated that εPKC plays a pivotal role in neuroprotection induced by ischemic preconditioning. However, the role of εPKC during and after brain ischemia is not clearly defined. Therefore, in the present study, we tested the hypothesis that activation of εPKC during an ischemic event is neuroprotective. Furthermore, other studies have demonstrated that εPKC mediates cerebral ischemic tolerance in the rat brain by decreasing vascular tone. Thus, we also tested the effects of εPKC activation during ischemia on cerebral blood flow (CBF). We found that ψε-Receptors for activated C kinase (RACK), a εPKC-selective peptide activator, injected intravenously 30 minutes before induction of global cerebral ischemia conferred neuroprotection in the CA1 region of the rat hippocampus. Moreover, measurements of CBF before, during and after cerebral ischemia revealed a significant reduction in the reperfusion phase of rats pretreated with ψεRACK compared to Tat peptide (vehicle). Our results suggest that εPKC can protect the rat brain against ischemic damage by regulating CBF. Thus, εPKC may be one of the treatment modalities against ischemic injury.
Keywords: Ischemia, epsilon Protein Kinase C, Cerebral Blood Flow, Neuroprotection
Introduction
Several studies have demonstrated that protein kinase C epsilon (εPKC) is strongly involved in cardio- and neuroprotection; particularly those mechanisms induced by ischemic preconditioning (IPC) [3,4,21,20,1]. IPC is an endogenous mechanism of protection whereby brief sub-lethal periods of ischemia are able to reduce the deleterious effects of a subsequent, longer duration of ischemic episodes in the heart, brain, and other organs [10].
εPKC is a calcium-independent phorbol ester/diacylglycerol-sensitive serine/threonine kinase [13]. εPKC induces protection from ischemia by regulating many pathways such as: 1) phosphorylation of the mitochondria K+ATP channel [20], 2) increased synaptosomal mitochondrial respiration [7], 3) activation of extracellular signal-regulated kinase (ERK) pathway [15], via N-methyl-d-aspartate (NMDA) receptors [21], and 4) by regulating gamma-aminobutyric acid (GABA) synapses [9] among others. The neuroprotective properties of εPKC in preconditioning have led to the testing of agonists of εPKC (ψε-Receptors for activated C kinase (RACK), εPKC activator peptide; εPKC85–92:CHDAPIGYD) as possible therapeutic drugs against myocardial infarction [13], heart failure [17], and cerebral ischemia [1].
Cerebral blood flow (CBF) derangements also play key roles in the development of brain damage following cerebral ischemia (see [22]). Therefore, controlling CBF has been proposed as one of the main strategies for limiting ischemic/reperfusion injury. However, the mechanisms responsible for promoting CBF dysfucntion following cerebral ischemia are still not well-defined [22]. Recently, an in vivo study from Mochly-Rosen's laboratory suggested that ψεRACK protected the brain from damage after focal cerebral ischemia in rats [2]. In the present study, we examined whether ψεRACK treatment was neuroprotective following global cerebral ischemia. Moreover, we tested the hypothesis that ψεRACK improved CBF following ischemia.
Methods
εPKC agonist (ψεRACK) [εPKC activator, amino acids 85–92 (HDAPIGYD)] and Tat protein (control) [carrier peptide, amino acids 47–57 (YGRKKRRQRRR)] were dissolved in NaCl (0.9%). The drugs were obtained from KAI Pharmaceuticals Inc. (South San Francisco, CA). An injection volume of 0.2 mg/kg was injected intravenous (IV) 30 min before induction of global cerebral ischemia [2,12]. ψεRACK may induce neuroprotection when injected 24 hours before or 3 minutes after cerebral ischemia [2]. All animal procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and were approved by the Animal Care and Use Committee of the University of Miami. Sprague Dawley (SD) rats weighing 250 to 300 g were fasted overnight and then anesthetized with 3% halothane and 70% nitrous oxide (in a balance of oxygen) by inhalation. The femoral arteries were cannulated for blood pressure measurements and for arterial sampling of blood gases. Arterial blood gases (178 pH/blood gas analyzer, Ciba-Corning), plasma glucose levels (One Touch glucose monitor, Lifescan), and hematocrit were measured intermittently throughout the experiment. Goals were to maintain blood gases in the normal range. If this range was not maintained throughout the period of surgery and data collection, the rats were discarded and data were not further analyzed. Rats underwent endotracheal intubation and were artificially ventilated with 0.5% halothane and 70% nitrous oxide (in a balance of oxygen). Rats were immobilized with pancuronium bromide (0.75 mg/kg intravenously). Both common carotid arteries were exposed by a midline ventral incision and gently dissected free of surrounding nerve fibers. Ligatures of polyethylene (PE-10) tubing, contained within a double-lumen Silastic tubing, were passed around each carotid artery. Brain temperature was monitored with a 33-gauge thermocouple implanted in the temporalis muscle [23]. The temperature was maintained at 36° to 37°C throughout the experiment by a small warming lamp placed above the animal's head. Before each ischemic insult, blood was gradually withdrawn from the femoral vein into a heparinized syringe to reduce systemic blood pressure to 50 mmHg. Cerebral ischemia was then produced by tightening the carotid ligatures bilaterally for 10 minutes (two vessel occlusion (TVO)) [23]. The brain was reperfused post-ischemia by removing the carotid ligatures, and the shed blood was reinjected into the femoral vein. This infusion usually restored mean arterial blood pressure to 130 to 140 mm Hg. The vessels were inspected to verify that perfusion was re-established.
Descriptions for all groups are as follows: Group 1 - Sham (n=5): Sham surgery was performed on the animals. Group 2 - Vehicle + Ischemia (n=5): 700 μl of saline NaCl 0.9% were injected IV in male SD rats 30 minutes before to induce 10 min of global cerebral ischemia. Group 3 - ψεRACK + Ischemia (n=5): this group differed from group 2 in that ψεRACK was injected IV 30 minutes before induction of cerebral ischemia. Group 4 - Tat Peptide Protein + Ischemia (n=5): Tat peptide in 700 μl of saline (NaCl, 0.9%) was injected IV into male SD rats 30 minutes before the induction of global cerebral ischemia.
At the end of 7 days of reperfusion, rats were anesthetized with isoflurane and perfused with FAM (a mixture of 40 % formaldehyde, glacial acetic acid, and methanol, 1:1:8 by volume) for 19 min following a 1 min initial perfusion with physiological saline. The perfusate was delivered into the root of the ascending aorta at a constant pressure of 110–120 mmHg as previously described [19]. All details for histological process are explained by Dave et al. [8]. The brains were then removed from the skull, and coronal brain blocks were prepared and embedded in paraffin. Coronal sections of 10 μm thicknesses were taken at 200 μm intervals spanning entire hippocampus (anterior to posterior) and were stained with hematoxylin and eosin. The stained sections were visualized at 40 × magnification with a Nikon Microphot-SA microscope equipped with a Sony 3 CCD camera interfaced to a MCID image analyzer (Imaging Research, St. Catherines, Ontario, Canada). Counts of normal neurons were made within the CA1 region of hippocampus at 3.8 mm posterior to bregma. Neurons were counted in eighteen fields per section, along the medial to lateral extent of the CA1 region of the hippocampus. The neuronal counts are expressed as number of normal neurons per CA1 region of hippocampus.
Laser Doppler flowmetry measurements were obtained to determine flow dynamics of cortical blood vessels in treatment groups 3 and 4 from 30 minutes before cerebral ischemia to 1 hour after cerebral ischemia. To monitoring blood perfusion in the cerebral cortex, a 2 mm2 burr hole was made over the left frontoparietal cortex approximately 5.0 mm posterior and 3.5 mm lateral to bregma. Under a Zeiss operating microscope, the bone was drilled to a thin layer with a cutting burr under saline irrigation, and a cortical area with blood vessels less than 50 μm diameter was selected by visualization through the thin bone layer, and a fiber optic probe (1 mm) was placed thereupon. The fiber optic probe when coupled to a PeriFlux 4001 Master laser Doppler blood perfusion monitor (Perimed Inc.) measures cerebral blood perfusion in a 1 mm2 tissue region. The Doppler signals were routed to a polygraphic recording system (Perisoft for Windows) interfaced to a personal computer, via an A to D converter, utilizing data acquisition software (Perisoft for Windows). All data are expressed as mean ± SEM. Statistical evaluation of the data was performed using ANOVA test, followed by Bonferroni's post hoc test. P<0.05 was considered significant.
Results
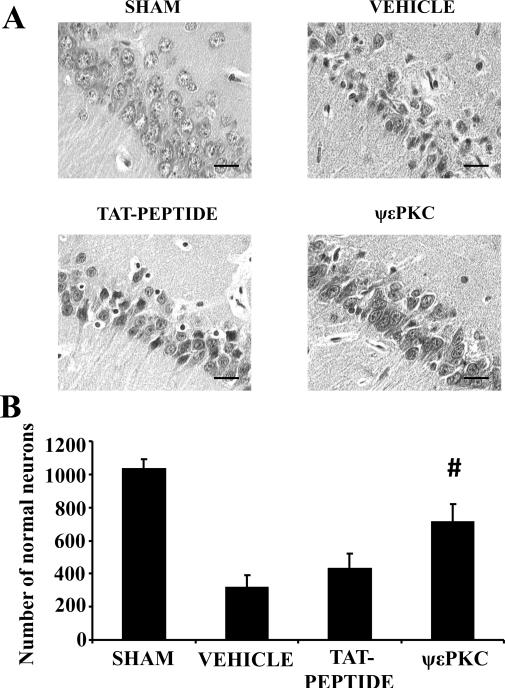
We tested the hypothesis that rats pretreated with ψεRACK (εPKC agonist) could protect the brain against ischemic damage. Before and after the induction of global cerebral ischemia or sham TVO, physiological parameters including pCO2, pO2, HCO3 and plasma glucose concentrations were similar among all experimental groups (Table 1). No significant differences in physiological parameters were found between groups. We performed a histological assessment of neuronal cell death in the CA1 region of the hippocampus 7 days after induction of ischemia/reperfusion (Figs. 1A, B). The number of normal neurons in the CA1 of sham operated rats was 1034 ± 58 (n = 5). After global cerebral ischemia, the number of normal neurons decreased to 314 ± 78 (n = 5). Injection of Tat peptide (n = 5) before cerebral ischemia did not significantly alter the number of normal neurons (429 ± 90) when compared to vehicle group (Fig. 1A, B). ψεRACK (n = 5) significantly increased the number of normal neurons (712 ± 109, p<0.05) by 38% and 25% (Fig. 1A,B) as compared to vehicle and Tat peptide treated groups, respectively.
Table I.
Physiological variables
| GROUP | VARIABLE | |||
|---|---|---|---|---|
| Before | During | After | ||
| Sham TVO (n = 5) | Body weight | 315 ± 14 | ||
| pH | 7.4 ± 1.3 | 7.4 ± 0.02 | 7.37 ± 0.02 | |
| pCO2 mm Hg | 38 ± 1 | 27 ± 2 | 39 ± 1 | |
| pO2 mm Hg | 146 ± 9 | 131 ± 10 | 132 ± 8 | |
| Plasma glucose | 133 ± 2 | |||
| Vehicle + TVO (n = 5) | Body weight | 322 ± 11 | ||
| pH | 7.4 ± 0.1 | 7.46 ± 0.09 | 7.39 ± 0.01 | |
| pCO2 mm Hg | 40 ± 1 | 29 ± 1 | 31 ± 2 | |
| pO2 mm Hg | 141 ± 6 | 125 ± 9.06 | 127 ± 7 | |
| Plasma glucose | 126 ± 3 | |||
| Tat peptide + TVO (n = 5) | Body weight | 318 ± 12 | ||
| pH | 7.43 ± 0.1 | 7.42 ± 0.1 | 7.41 ± 0.02 | |
| pCO2 mm Hg | 41 ± 5 | 28 ± 2 | 39 ± 1 | |
| pO2 mm Hg | 117 ± 6 | 125 ± 9 | 129 ± 7 | |
| Plasma glucose | 155 ± 26 | |||
| ψεRACK + TVO (n = 5) | Body weight | 319 ± 8 | ||
| pH | 7.44 ± 1 | 7.48 ± 0.08 | 7.4 ± 0.03 | |
| pCO2 mm Hg | 26 ± 1 | 27 ± 1.1 | 27 ± 0.5 | |
| pO2 mm Hg | 130 ± 10 | 126 ± 8 | 131 ± 4 | |
| Plasma glucose | 129 ± 6 |
Figure 1. In vivo pretreatment with ψεRACK protected the hippocampal CA1 region against cerebral ischemia.
(A) Representative histological images and (B) number of normal neurons in the CA1 region of hippocampus are presented. Scale bar indicates 30 μm. #P<0.05 compared to vehicle, and Tat peptide.
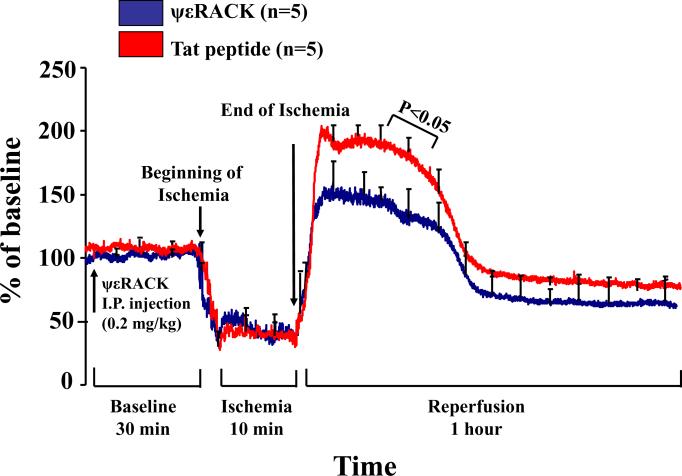
Next, we tested whether IV injection of ψεRACK altered CBF before, during and after global cerebral ischemia. We measured CBF with laser-Doppler flowmetry 30 minutes before induction of ischemia (drug treatment time-point), during 10 minutes of ischemia, and 1 hour following brain reperfusion. During the first 30 minutes after injection of ψεRACK, Tat peptide, or during the ischemic insult, the recordings did not show any significant difference between the two treated groups in cerebral perfusion (Fig. 2). Instead, during the first 25 minutes of reperfusion time, following 10 min global cerebral ischemia, we found a 30% reduction in CBF (p<0.05) between ψεRACK treated rats compared with Tat peptide treated rats (Fig. 2).
Figure 2. In vivo pretreatment with ψεRACK reduces cerebral reperfusion after ischemia.
A ψεRACK bolus 30 min before cerebral ischemia reduced post-ischemic cerebral blood flow (CBF). CBF (% of baseline) curves of laser-Doppler flowmetry tracing before, during, and one hour after an ischemic insult produced by bilateral carotid occlusion and systemic hypotension. P<0.05 compared to Tat peptide.
Discussion
Previous studies have shown that εPKC protect the heart and brain from ischemia [12,2,6,9]. Here, we show that ψεRACK, injected IV 30 minutes before induction of ischemia, protected CA1 neurons in the rat hippocampus from ischemic damage. Moreover, we demonstrated a significant reduction of hyperemia in the first 25 minutes of reperfusion after ischemia in rats pretreated with ψεRACK as compared to Tat peptide.
εPKC is associated with mechanisms leading to neuroprotection against ischemia. In previous studies, our group focused on defining the role of εPKC on IPC-mediated neuroprotection. In the brain, IPC leads to activation of εPKC in the hippocampus [11]. Using hippocampal organotypic slices and oxygen-glucose deprivation (OGD) method to induce ischemia, we previously showed that IPC can be emulated with an agonist of εPKC (ψεRACK) and blocked by εV1-2 [21]. εPKC also modulatess GABAergic synaptic activity, which suggested that an effect of εPKC on GABAergic neurons could be involved with IPC-mediated neuroprotection against ischemia [9]. In support of these findings, application of the GABA(A) receptor agonist (muscimol) blocked the increase in cerebellar oxygen consumption evoked by synaptic excitation concomitant with an attenuation of CBF responses [5]. These findings suggest a possible link between GABA and εPKC neurogenically controlling CBF. Other in vitro studies further confirmed that εPKC plays a pivotal role in the induction of ischemic tolerance through its activation of different cellular pathways [15,14,20]. Using hippocampal synaptosomes isolated from the rat brain after IPC and lethal ischemia induced by the TVO model, we previously demonstrated that activation of εPKC improves the rate of mitochondrial respiration not only during the induction of IPC, but also at the time of ischemia [7]. Interestingly, in a recent study, arctic ground squirrels exhibited little neuronal damage following asphyxial cardiac arrest and that this protection mediated by εPKC activation [6]. In the present study, we demonstrated, using an in vivo model to induce global cerebral ischemia, that treatment with εPKC agonist 30 minutes before cerebral ischemia confers neuroprotection.
A recent in vivo study from the Mochly-Rosen laboratory suggested a role for εPKC in regulating CBF. ψεRACK (0.2 mg/kg in 1 mL) delivered immediately before measurement with laser-Doppler flowmetry, reduced CBF by an average of 15±6% compared to Tat peptide, which did not significantly alter CBF. The onset of flow reduction following ψεRACK delivery was variable between animals from 5 to 30 min. The εPKC-mediated decrease in CBF was maintained for the duration of the study (up to 60 min post-ψεRACK delivery). Interestingly, this reduction in flow occurs in the absence of any ischemic stimulus [2]. Therefore, ψεRACK may have direct effects on vascular cell function, or may play an indirect role on neuronal regulation of cerebral blood flow. Although the role of PKC on vasoreactivity has a long history of investigation [16], the exact role of different PKC isoforms in regulating CBF is still unknown. In our study, we did not find significant difference in CBF after ψεRACK or Tat peptide injection, while we found a significant reduction in CBF in rats treated with ψεRACK during the first 25 min. of reperfusion. This suggests that ψεRACK effects on CBF following cerebral ischemia may be indirect and linked to the brain parenchyma.
To our knowledge, this is the first study that has investigated the effects of εPKC on CBF, before, during and after ischemia. Our findings suggest that one of the mechanisms by which εPKC induces neuroprotection against cerebral ischemia could be its ability to reduce CBF during the first phase of reperfusion. The capacity of εPKC to regulate nitric oxide synthase may also be associated with the vascular tone response [18]. However, as mentioned above, the role of εPKC in regulating vascular diameters and in CBF regulation needs further exploration.
In conclusion, we demonstrated a protective effect of εPKC against global cerebral ischemia in the CA1 region of the rat hippocampus. We also demonstrated a reduction in CBF, as measured with laser-Doppler flowmetry, during the first phase of reperfusion following an ischemic episode in rats pretreated with εPKC agonist. εPKC has been proposed as a potential therapeutic agent against ischemic/reperfusion injury. This study provides further support for this use; however, more research into the neuroprotective roles of εPKC is needed.
Highlights.
-
➣
εPKC plays a pivotal role in neuroprotection induced by ischemic preconditioning.
-
➣
εPKC agonist protects against global cerebral ischemia in the rat hippocampus.
-
➣
εPKC agonist reduces cerebral blood flow in the reperfusion phase following ischemia.
Acknowledgments
This study was supported by PHS grants NS34773, NS05820, NS045676, NS054147 and AHA grant 0725314B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bright R, Mochly-Rosen D. The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke. 2005;36:2781–2790. doi: 10.1161/01.STR.0000189996.71237.f7. [DOI] [PubMed] [Google Scholar]
- [2].Bright R, Sun GH, Yenari MA, Steinberg GK, Mochly-Rosen D. epsilonPKC confers acute tolerance to cerebral ischemic reperfusion injury. Neurosci Lett. 2008;441:120–124. doi: 10.1016/j.neulet.2008.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Budas GR, Churchill EN, Mochly-Rosen D. Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res. 2007;55:523–536. doi: 10.1016/j.phrs.2007.04.005. [DOI] [PubMed] [Google Scholar]
- [4].Budas GR, Mochly-Rosen D. Mitochondrial protein kinase Cepsilon (PKCepsilon): emerging role in cardiac protection from ischaemic damage. Biochem Soc Trans. 2007;35:1052–1054. doi: 10.1042/BST0351052. [DOI] [PubMed] [Google Scholar]
- [5].Caesar K, Offenhauser N, Lauritzen M. Gamma-aminobutyric acid modulates local brain oxygen consumption and blood flow in rat cerebellar cortex. J Cereb Blood Flow Metab. 2008;28:906–915. doi: 10.1038/sj.jcbfm.9600581. [DOI] [PubMed] [Google Scholar]
- [6].Dave KR, Anthony Defazio R, Raval AP, Dashkin O, Saul I, Iceman KE, Perez-Pinzon MA, Drew KL. Protein kinase C epsilon activation delays neuronal depolarization during cardiac arrest in the euthermic arctic ground squirrel. J Neurochem. 2009;110:1170–1179. doi: 10.1111/j.1471-4159.2009.06196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dave KR, DeFazio RA, Raval AP, Torraco A, Saul I, Barrientos A, Perez-Pinzon MA. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J Neurosci. 2008;28:4172–4182. doi: 10.1523/JNEUROSCI.5471-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dave KR, Raval AP, Prado R, Katz LM, Sick TJ, Ginsberg MD, Busto R, Perez-Pinzon MA. Mild cardiopulmonary arrest promotes synaptic dysfunction in rat hippocampus. Brain Res. 2004;1024:89–96. doi: 10.1016/j.brainres.2004.07.050. [DOI] [PubMed] [Google Scholar]
- [9].DeFazio RA, Raval AP, Lin HW, Dave KR, Della-Morte D, Perez-Pinzon MA. GABA synapses mediate neuroprotection after ischemic and epsilonPKC preconditioning in rat hippocampal slice cultures. J Cereb Blood Flow Metab. 2009;29:375–384. doi: 10.1038/jcbfm.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- [11].Hatakeyama T, Matsumoto M, Brengman JM, Yanagihara T. Immunohistochemical investigation of ischemic and postischemic damage after bilateral carotid occlusion in gerbils. Stroke. 1988;19:1526–1534. doi: 10.1161/01.str.19.12.1526. [DOI] [PubMed] [Google Scholar]
- [12].Inagaki K, Begley R, Ikeno F, Mochly-Rosen D. Cardioprotection by epsilon-protein kinase C activation from ischemia: continuous delivery and antiarrhythmic effect of an epsilon-protein kinase C-activating peptide. Circulation. 2005;111:44–50. doi: 10.1161/01.CIR.0000151614.22282.F1. [DOI] [PubMed] [Google Scholar]
- [13].Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70:222–230. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- [14].Kim E, Raval AP, Defazio RA, Perez-Pinzon MA. Ischemic preconditioning via epsilon protein kinase C activation requires cyclooxygenase-2 activation in vitro. Neuroscience. 2007;145:931–941. doi: 10.1016/j.neuroscience.2006.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lange-Asschenfeldt C, Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon protein kinase C mediated ischemic tolerance requires activation of the extracellular regulated kinase pathway in the organotypic hippocampal slice. J Cereb Blood Flow Metab. 2004;24:636–645. doi: 10.1097/01.WCB.0000121235.42748.BF. [DOI] [PubMed] [Google Scholar]
- [16].Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res. 1991;68:359–367. doi: 10.1161/01.res.68.2.359. [DOI] [PubMed] [Google Scholar]
- [17].Palaniyandi SS, Sun L, Ferreira JC, Mochly-Rosen D. Protein kinase C in heart failure: a therapeutic target? Cardiovasc Res. 2009;82:229–239. doi: 10.1093/cvr/cvp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Paul A, Doherty K, Plevin R. Differential regulation by protein kinase C isoforms of nitric oxide synthase induction in RAW 264.7 macrophages and rat aortic smooth muscle cells. Br J Pharmacol. 1997;120:940–946. doi: 10.1038/sj.bjp.0700976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Perez-Pinzon MA, Xu GP, Dietrich WD, Rosenthal M, Sick TJ. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:175–182. doi: 10.1097/00004647-199702000-00007. [DOI] [PubMed] [Google Scholar]
- [20].Raval AP, Dave KR, DeFazio RA, Perez-Pinzon MA. epsilonPKC phosphorylates the mitochondrial K(+) (ATP) channel during induction of ischemic preconditioning in the rat hippocampus. Brain Res. 2007;1184:345–353. doi: 10.1016/j.brainres.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003;23:384–391. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rudzinski W, Swiat M, Tomaszewski M, Krejza J. Cerebral hemodynamics and investigations of cerebral blood flow regulation. Nucl Med Rev Cent East Eur. 2007;10:29–42. [PubMed] [Google Scholar]
- [23].Truettner JS, Hu K, Liu CL, Dietrich WD, Hu B. Subcellular stress response and induction of molecular chaperones and folding proteins after transient global ischemia in rats. Brain Res. 2009;1249:9–18. doi: 10.1016/j.brainres.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]