Abstract
The purpose of this analysis was to develop an algorithm for the cost effective and accurate assessment of smoking during the previous few days by combining self report, breath carbon monoxide (BCO), and saliva cotinine (sCOT). These measurements are convenient, quantitative, and do not require invasive procedures. The data used to devise the algorithm were gathered during a treatment trial of participants seeking to stop smoking. Self report of smoking was determined using a written questionnaire, BCO was measured with a handheld breathalyzer, and sCOT was quantified using a high sensitivity ELISA. Participants were 130 males and 97 females between the ages of 19 and 67 years who reported smoking at least 15 cigarettes a day and had a BCO level ≥ 15 ppm. Self reports and BCO levels were collected at each of 6 visits (V0-V5) and sCOT levels were determined at V0 and V5. Based on the data collected, we recommend that the sequential determination of self reported smoking, BCO level, and sCOT level be employed to assess smoking during the previous few days to minimize the higher cost and longer turnaround time associated with the sCOT test while maximizing accuracy.
Keywords: breath carbon monoxide, saliva cotinine, cigarette smoking, human
1. Introduction
Breath carbon monoxide (BCO) and plasma (pCOT) or saliva cotinine (sCOT) levels are used for the objective assessment of smoking in clinical settings and research studies. Self report generally provides an accurate indication of whether someone is smoking or not, although up to 20% of people who say they have never smoked may be misreporting their exposure and smokers might underreport smoking as social acceptability decreases (Martinez et al., 2004). When a valid assessment of smoking is necessary, the measurement of biomarkers such as BCO and/or salivary cotinine (sCOT) is required. An algorithm to use BCO and sCOT as reflex tests from an initial self report of smoking might be a cost effective and accurate way to combine these smoking assessments. A reflex test is a follow up test performed based on the results of a previous, different type of test.
BCO accumulates during the inhalation of carbon monoxide from the incomplete burning of tobacco during smoking. The principal advantages of using BCO to indicate recent smoking are that it can be measured quickly with immediate results, sample collection is non-invasive, the test is inexpensive, and the results are not influenced by use of nicotine replacement therapy or smokeless nicotine preparations. The disadvantages of BCO are its short half life (2-6 h), which only allows for detecting smoking within the past 8 to 24 hours (Javors et al., 2005; Jatlow et al., 2008), and interference by environmental CO exposure (Benowitz et al., 2002). Nevertheless, BCO probably has been used more than any other marker to estimate smoking in research studies. The shortcomings of BCO suggest that combining BCO with sCOT as a reflex test would extend detection period while reducing the cost of testing.
sCOT has been widely used as a marker for cigarette smoking for at least two decades (Etzel, 1990; Curvall et al., 1990; Noland et al., 1988; Pierce et al., 1987) and has several advantages as a marker of smoking. sCOT has similar pharmacokinetics to pCOT and is as sensitive as pCOT at detecting smoking (Gorber et al., 2009; Jarvis et al., 2003; Curvall et al, 1990). Saliva samples are easily obtained and the half-life of sCOT is about 17 h (Curvall et al, 1990), so sCOT levels can detect smoking over the past several days. COT is the principal metabolite of nicotine and therefore a very specific marker for nicotine exposure. sCOT levels can be quantified accurately by ELISA or HPLC with UV detection, readily available laboratory techniques. The principal disadvantages of sCOT are that results are not immediately available, the cost is higher than for BCO, it cannot discriminate between short-term abstinence and continued smoking (Marrone et al., 2010), and cannot distinguish between smoking and smokeless tobacco or nicotine replacement therapy.
BCO and sCOT have been combined in previous studies (Gariti et al., 2002; Higgins et al., 2007), but no integrated approach has been proposed. We propose an algorithm for using BCO and sCOT as reflex tests following negative self report as a cost effective and accurate manner of assessing smoking status during the previous few days.
2. Methods
2.1 Subjects
Subjects were participants in a smoking cessation research program where inclusion criteria were reported smoking at least 15 cigarettes a day, a BCO level ≥15 ppm at intake, and desire to stop smoking. During this behavioral treatment trial, participants were paid to deliver BCO levels <3 ppm, but sCOT was not used as a contingency. Informed consent was obtained by a trained research assistant and the study design was approved by the IRB of the University of Texas Health Science Center.
Age, gender, education, smoking history, and smoking behavior were determined by questionnaire. Measures of smoking behavior included the self-reported average number of cigarettes smoked daily prior to study entry (V0), number of cigarettes smoked during the previous day (V0-V5), and the Fagerstrom Test for Nicotine Dependence index (V0).
Non-smoking days were days when the self report indicated zero cigarettes.
2.2 Measurement of Breath Carbon Monoxide (BCO)
Participants visited the research site for an initial visit (V0) and then five subsequent visits on weekdays only (V1-V5) between the hours of 7 and 10 AM to deliver a breath sample. A Vitalograph CO monitor (Vitalograph Inc. Lenexa, KS) was used to measure BCO at all visits. BCO and BCO cut-off level were expressed as integers in parts per million (ppm).
2.3 Measurement of Saliva Cotinine (sCOT)
Saliva samples were collected at V0 and V5 using plain cotton Salivette tubes (Sarstedt) and then stored immediately at -80°C until the assay for cotinine. sCOT was assessed according to the instructions for the High Sensitivity Salivary Cotinine Quantitative Enzyme Immunoassay kits (Salimetrics LLC, State College, PA; No. 1-2002/1-20121) using a 96 well plate reader with UV detection (Spectramax 384, Molecular Devices). The cross reactivity of the assay indicated that the results include 100% of cotinine, 55% of 3-hydroxycotinine, and 1.4% of nicotine. All samples were run in duplicate. Analytical results were adjusted to account for dilution, so the lower limit of quantification was 1 ng/ml, intra- and inter-assay precision was ≤8.2%, and final results were reported in ng/ml.
3. Results
3.1 Demographics and Smoking Parameters at Study Entry (V0)
Data were collected at V0 from 130 male and 97 female participants. The mean ± SD age was 42.2 ± 12.1 years (range: 19–67), Fagerstrom score was 6.0 ± 2.0, average daily reported cigarettes was 23.6 ± 7.3, BCO was 24.4 ± 9.4 ppm, and sCOT was 327 ± 194 ng/ml.
3.2 Marker Levels, Cutoffs, and Days of Abstinence
Table 1 shows the distribution of BCO and sCOT tests above and below various cutoffs. Positive BCO tests at a cutoff of ≥3 ppm correctly identified 76% (105/138) of participants reporting smoking the previous day. This cutoff also correctly identified as abstinent 91% (21/23) reporting not smoking on the previous day (1 day of abstinence) and 89% (59/66) maintaining at least 2 to 5 days of abstinence. It is important to note that the 91% of participants maintaining at least one day of abstinence, and having a negative BCO test, had smoked within the past few days. Positive BCO tests at a cutoff of ≥9 ppm identified only 37% (51/138) of those smoking during the previous day, misidentifying 63% (87/138) of smokers as abstinent. On the other hand, the percentage of true positive tests using a BCO cutoff of ≥9 ppm was 100% (51/51; Table 1).
Table 1.
Distribution of Participants According to Minimum Sequential Days Abstinent, BCO Cutoff, and sCOT Cutoff
Number of Participants in Each Category*
| Cutoffs | Minimum Sequential Days Abstinent | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| BCO Cutoff | ||||||
| <3 | 33 | 21 | 32 | 10 | 11 | 6 |
| ≥3 | 105 | 2 | 3 | 3 | 1 | 0 |
| <9 | 87 | 23 | 35 | 13 | 12 | 6 |
| ≥9 | 51 | 0 | 0 | 0 | 0 | 0 |
| <15 | 119 | 23 | 35 | 13 | 12 | 6 |
| ≥15 | 19 | 0 | 0 | 0 | 0 | 0 |
| sCOT Cutoff | ||||||
| <1 | 0 | 2 | 5 | 0 | 3 | 1 |
| ≥1 | 116 | 16 | 21 | 9 | 4 | 3 |
| <3 | 0 | 4 | 6 | 1 | 4 | 2 |
| ≥3 | 116 | 14 | 20 | 8 | 3 | 2 |
| <10 | 3 | 4 | 16 | 3 | 6 | 3 |
| ≥10 | 113 | 14 | 10 | 6 | 1 | 1 |
| <30 | 6 | 12 | 22 | 4 | 6 | 4 |
| ≥30 | 110 | 6 | 4 | 5 | 1 | 0 |
| <100 | 32 | 14 | 26 | 7 | 7 | 4 |
| ≥100 | 84 | 4 | 0 | 2 | 0 | 0 |
BCO was measured for all 227 participants at V5, but saliva samples were only collected for 180 participants at V5.
Positive tests for sCOT at a cutoff of ≥3 ng/ml identified 100% (116/116) of subjects smoking during the previous day and 78% (14/18), 77% (20/26), and 89% (8/9) of those reporting at least 1, 2, or 3 days abstinence, respectively, but who had smoked on earlier days. Conversely, negative sCOT levels (≥3 ng/ml cutoff) occurred in 28% (13/46) of participants with 2 to 5 days of abstinence after previous smoking. The data shown in Table 1 suggest that either ≥3 ng/ml or ≥10 ng/ml might be used as a sCOT cutoff in the algorithm and do not support one over the other. A sCOT cutoff of ≥10 ng/ml was chosen for the algorithm to account for the possibility of environmental exposure (second hand smoke). Determining the better cutoff will require studies in larger populations of non-smokers.
3.3 Combined Use of Reported Smoking, BCO, and sCOT
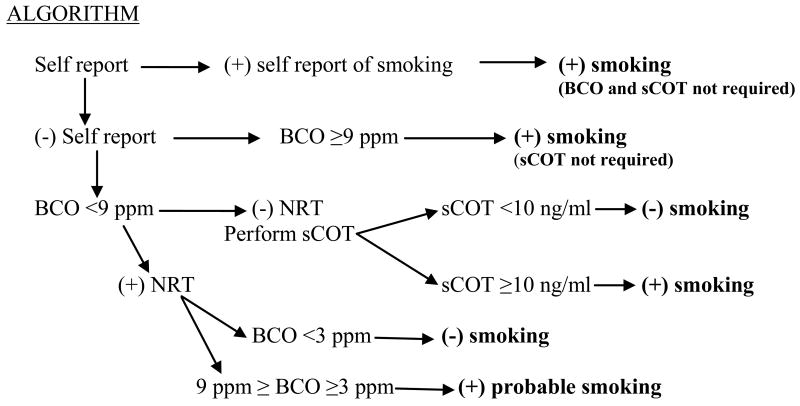
Our data suggest that the sequential combination of self report, BCO levels, and sCOT levels would be an accurate, sensitive, and cost effective way of assessing recent smoking. The algorithm in Fig. 1 shows a step wise decision tree that could be employed. The first step is to ask the participant if he/she is smoking. A positive response indicates smoking and would require no further testing. If the participant denies smoking, then a BCO test would be performed as confirmation of abstinence. A BCO test result ≥9 ppm would indicate smoking at least during the previous day and sCOT would not be required. A BCO result <9 ppm in the absence of NRT and smokeless tobacco use would trigger the collection of saliva and a sCOT test. A negative sCOT test (<10 ng/ml) would confirm abstinence for the past few days, whereas a positive sCOT test (≥10 ng/ml) would indicate smoking in the previous few days. The special case in which sCOT was <10 ng/ml (negative) but BCO was ≥3 but <9 ppm would suggest probable smoking, but not necessarily of tobacco.
Figure 1. Algorithm for Use of Self Report, BCO, and sCOT for Assessment of Smoking Status.
Abbreviations: Self report is obtained by verbal or written communication of whether or not the participant is currently smoking; BCO is breath carbon monoxide level in parts per million (ppm); sCOT is saliva cotinine level in ng/ml; NRT is nicotine replacement therapy.
4. Discussion
This study supports the combined use of BCO and sCOT as markers for verifying reported smoking. By using BCO and sCOT as reflex tests following negative self report, a cost effective and accurate means of assessing smoking during the previous few days is possible.
Marker results of 89 participants who maintained abstinence for at least 1 to 5 sequential days prior to V5 highlights the notion that BCO levels are best suited to assess smoking in the past day and that sCOT levels remain detectable following up to 5 days of abstinence (Table 1). A single day of smoking abstinence reduced BCO levels in most participants to less than 3 ppm, the most accurate cutoff to distinguish between smoking and not smoking during the previous 24 hours (Javors et al, 2005). On the other hand, detectable sCOT levels persisted for up to 5 days of abstinence in most, but not all, subjects. These data conform to and are largely explained by the known half lives of BCO and sCOT. A questionnaire for reported smoking and NRT use combined with BCO and sCOT levels according to the algorithm in Fig. 1 might provide an accurate and cost effective approach for assessing smoking status over the previous few days.
The algorithm in Fig. 1 is based on the results in Table 1 and is designed to facilitate an assessment of whether any smoking occurred during the previous few days. For those reporting smoking, there is no reason for further testing. If the participant denies smoking, then a BCO test would be performed for confirmation. A BCO level of ≥9 ppm would indicate smoking because our data showed that 100% of BCO tests ≥9 ppm were true positives. If the BCO were <9 ppm, information about use of NRT, smokeless tobacco, etc., would determine whether or not a saliva sample for sCOT would be collected. That is, if the participant were using NRT or smokeless tobacco, there would be no utility of a sCOT test. However, in the absence of NRT and smokeless tobacco, a saliva sample would increase certainty about smoking status. A positive sCOT test would indicate recent smoking. The special case in which sCOT was <3 ng/ml (negative) but BCO was ≥3 but <9 ppm would suggest probable smoking. This proposed algorithm provides an effective and accurate sequential process to combine self report, BCO, and sCOT that could be used to assess smoking during the previous few days.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benowitz NL, Jacob P, Ahijevych K, Jarvis MF, Hall S, LeHouezec J, Hansson A, Lichtenstein E, Henningfield J, Tsoh J, Hurt RD, Velicer W. Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4:149–159. [Google Scholar]
- Curvall M, Elwin CE, Kazemi-Vala E, Warholm C, Enzell CR. The pharmacokinetics of cotinine in plasma and saliva from non-smoking healthy volunteers. European Journal of Clinical Pharmacology. 1990;38:281–287. doi: 10.1007/BF00315031. [DOI] [PubMed] [Google Scholar]
- Etzel RA. A review of the use of saliva cotinine as a marker of tobacco smoke exposure. Preventive Medicine. 1990;19:190–197. doi: 10.1016/0091-7435(90)90020-k. [DOI] [PubMed] [Google Scholar]
- Gariti P, Alterman AI, Ehrman R, Mulvaney FD, O'Brien CP. Detecting smoking following smoking cessation treatment. Drug and Alcohol Dependence. 2002;65:191–196. doi: 10.1016/s0376-8716(01)00162-4. [DOI] [PubMed] [Google Scholar]
- Gorber SC, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine and Tobacco Research. 2009;11:12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Badger GJ, Mongeon JA, Soloman LJ, McHale L, Bernstein IM. Biochemical verification of smoking status in pregnant and recently postpartum women. Experimental and Clinical Psychopharmacology. 2007;15:58–66. doi: 10.1037/1064-1297.15.1.58. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Primatesta P, Erens B, Feyerabend C, Bryant A. Measuring nicotine intake in population surveys: Comparability of saliva cotinine and plasma cotinine estimates. Nicotine and Tobacco Research. 2003;5:349–355. doi: 10.1080/1462220031000094213. [DOI] [PubMed] [Google Scholar]
- Jatlow P, Toll BA, Leary V, Krishnan-Sarin S, O'Malley SS. Comparison of expired carbon monoxide and plasma cotinine as markers of cigarette abstinence. Drug and Alcohol Dependence. 2008;98:203–209. doi: 10.1016/j.drugalcdep.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100:159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- Marrone GF, Paulpillai M, Evans RJ, Singleton EG, Heishman SJ. Breath carbon monoxide and semiquantitative salive cotinine as biomarkers for smoking. Human Psychopharmacology. 2010;25:80–83. doi: 10.1002/hup.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez ME, Reid M, Jiang MS, Einspahr J, Alberts DS. Accuracy of self-reported smoking status among participants in a chemoprevention trial. Preventive Medicine. 2004;38:492–497. doi: 10.1016/j.ypmed.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Noland MP, Kryscio RJ, Riggs RS, Linville LH, Perritt LJ, Tucker TC. Saliva cotinine and thiocyanate: chemical indicators of smokeless tobacco and cigarette use in adolescents. Behavioral Medicine. 1988;11:423–433. doi: 10.1007/BF00844836. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Dwyer T, DiGiusto E, Carpenter T, Hannam C, Amin A, Yong C, Sarfaty G, Shaw J, Burke N. Cotinine validation of self-reported smoking in commercially run community surveys. Journal of Chronic Disease. 1987;40:689–695. doi: 10.1016/0021-9681(87)90105-6. [DOI] [PubMed] [Google Scholar]