Abstract
The posterodorsal preoptic nucleus (PdPN), lateral part of the posterodorsal medial amygdala (MeApd) and medial part of the medial preoptic nucleus (MPNm) are activated at ejaculation in male gerbils as assessed by Fos expression. We sought to immunocytochemically visualize substance P (SP), cholecystokinin (CCK), oxytocin, vasopressin and tyrosine hydroxylase (TH), a catecholaminergic marker, in the mating-activated cells, but the need for colchicine precluded behavioral testing. Instead, we detailed distributions of cells containing these molecules in the medial amygdala, caudal preoptic area and caudal bed nuclei of the stria terminalis (BST) and quantified their densities in the PdPN, MPNm and lateral MeApd for comparison to densities previously assessed for mating-activated efferents from these sites. TH cells were as dense in the PdPN and lateral MeApd as activated efferents to the anteroventral periventricular nucleus. In the lateral MeApd, TH cells were grouped where cells activated at ejaculation are clustered and where CCK cells form a ball. Lateral MeApd CCK cells and PdPN SP cells were as dense as activated efferents to the principal BST. Oxytocinergic PdPN cells and SP cells in the MPNm were as dense as mating-activated efferents to the lateral MeApd. If some oxytocin cells in the PdPN project to the neurohypophysis, as in rats, they could be a source of the oxytocin secreted at ejaculation. Since gerbils are monogamous and biparental, it was also interesting that, unlike monogamous prairie voles, they had few TH cells in the MeApd or dorsal BST, resembling promiscuous rats, hamsters and meadow voles.
Keywords: tyrosine hydroxylase, cholecystokinin, substance P, oxytocin, vasopressin, posterodorsal preoptic nucleus
1. Introduction
Three areas of the gerbil forebrain contain cells activated specifically at ejaculation as assessed by Fos expression: the posterodorsal preoptic nucleus (PdPN), the lateral part of the posterodorsal medial amygdala (MeApd) and the medial part of the medial preoptic nucleus (MPN; Heeb and Yahr, 1996). After ejaculation, male gerbils, like rats (Baum and Everitt, 1992; Coolen et al., 1996), have a cluster of Fos-containing cells in the PdPN and lateral MeApd that is not seen in males that intromit equally often but are separated from the female before ejaculation. In the medial MPN (MPNm), cells activated at ejaculation are intermingled with cells activated when males re-enter the environment in which they gained sexual experience (Heeb and Yahr, 1996), perhaps in anticipation of sexual reward (Balfour et al., 2004). Whether the activation associated with ejaculation reflects sensory processing or the start of a pre-motor program for ejaculation or one of its sequelae, such as oxytocin secretion (Hughes et al., 1987), is not known.
To learn more about PdPN, MPNm and lateral MeApd cells activated with mating, we traced their projections (Heeb and Yahr, 1996; Simmons and Yahr, 2002) and identified some of their transmitters, including γ-aminobutyric acid (GABA), glutamate and nitric oxide (NO; Simmons and Yahr, 2003; Simmons and Yahr, unpublished). Cholecystokinin (CCK), substance P (SP), vasopressin (VP), oxytocin and catecholamines were also of interest. In hamsters, rats and voles, MeApd, PdPN and MPN cells containing CCK, SP and VP are sexually dimorphic and/or sensitive T (Simerly and Swanson, 1987; Micevych et al., 1988; Simerly et al., 1989; Swann and Newman, 1992; Wang, 1995; Wang and De Vries, 1995). In prairie voles, which, like gerbils, are monogamous and biparental, MeApd cells containing tyrosine hydroxylase (TH), a catecholaminergic marker, express Fos with mating, particularly ejaculation (Cavanaugh et al., 2010). In rats, oxytocin is found in cells of the caudal PdPN (Sofroniew, 1985; Yamada et al., 1996), previously thought to be the rostral anterior commissural nucleus (AC; Ju et al., 1989; Paxinos and Watson, 1986, 1997). Many of those cells project to the posterior pituitary (Vanhatalo and Soinila, 1995), and rats secrete oxytocin after ejaculation (Hughes et al., 1987).
In gerbils, colchicine treatment was needed to visualize cell bodies containing oxytocin, VP, SP, CCK and TH in the PdPN, MPNm and lateral MeApd, which precluded testing males for sex behavior. Since we could not study cellular colocalization of mating-induced Fos with these molecules, we detailed distributions of cells containing them in the caudal preoptic area (POA), caudal bed nuclei of the stria terminalis (BST) and medial amygdala (MeA) and quantified their densities in the PdPN, MPNm and lateral MeApd for comparison to densities of mating-activated efferents from these sites that we had identified previously (Heeb and Yahr, 2001; Simmons and Yahr, 2002).
2. Materials and methods
2.1 Subjects
Gerbils were purchased as adults (Harlan Sprague Dawley, Indianapolis, IN), housed in same-sex pairs and exposed to a 14:10-hr light:dark cycle with food and water freely available. All of the males used were sexually experienced, having copulated to ejaculation at least once during 1–3 pairings of 30–40 min with females made sexually receptive by subcutaneous implantation of a 5-mm Silastic capsule (1.57 mm i.d., 3.16 mm o.d.) of estradiol benzoate 5–10 days before use. All gerbils were anesthetized with sodium pentobarbital before surgery (50 mg/kg) or perfusion (10 mg/male). All procedures conformed to the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
2.2 Colchicine treatment
Males were given infusions of colchicine (50–400 μg; Sigma; St. Louis, MO) in 10 μl of distilled water, or 0.9% saline, 1–2 days before perfusion, by stereotaxically directing a pipette (50–80 μm tip) connected to a syringe pump at the right lateral ventricle (1.65 mm anterior and 0.85 mm lateral to Bregma; 2.7 mm below the skull surface; incisor bar set at + 4.0 mm). Infusions lasted 8–12 min. Five min later, the pipette was removed and the wound closed. For CCK, 200–400 μg was needed. For the other peptides and TH, 50 μg proved adequate. Analgesic (0.03 mg/kg Torbutrol; Western Medical Supply, Arcadia, CA) was injected subcutaneously after surgery and then twice daily.
2.3 General immunocytochemistry (ICC) procedures
Unless otherwise stated in a specific protocol, the following procedures were followed. Males were perfused with saline followed by fixative in phosphate-buffered saline (PBS). Brains were removed and postfixed for 1.5–2 hr at 4 °C in the same fixative and cryoprotected by storage for 15–36 hr at 4 °C in the same PBS containing 20–30% sucrose. They were then frozen and cut coronally at 30 μm. Every section through the MPN, PdPN and MeA was collected in PBS. Some were collected as alternates or sets of three for processing with different antibodies or antibody dilutions.
Procedures were done at room temperature. Sections were rinsed initially, and after each incubation, except the preliminary blocking step. Rinses involved three changes over 15 min of the buffer from the previous incubation. Rinses and incubations involved gentle agitation. If acrolein was used for perfusion, residual aldehydes were removed by incubating sections for 15 min before the preliminary blocking step in PBS containing 1% NaBH4.
To block non-specific binding sites, sections were incubated for 1–1.5 hr in PBS containing 0.3% Triton X-100 and 20% normal serum (NS) from horse (TH) or goat (peptides). Then they were incubated for 48–72 hr at 4 °C in PBS containing 0.3% Triton X-100, 5% NS and either mouse monoclonal (TH) or rabbit polyclonal (peptides) IgG. Next, they were incubated for 1–1.5 hr in PBS containing 0.3% Triton X-100, 5% NS and a 1:200 dilution of biotinylated horse anti-mouse (TH) or goat anti-rabbit (peptides) IgG (Vector Labs; Burlingame, CA). Antibodies were visualized with Vectastain Elite ABC kits (Vector Labs), following kit instructions. The chromogen, 3,3’-diaminobenzidine (DAB), was used at 0.05% in Tris-buffered saline (TBS; pH 7.6) and developed with increasing concentrations (0.006–0.01%) of H2O2. Development was stopped with PBS. Sections were rinsed and stored in PBS until they were mounted onto slides coated with gelatin and chrom-alum. After air drying, the sections were dehydrated in graded alcohols, delipidized with xylene and coverslipped using DePeX (BDH Laboratory Supplies, Poole, UK). For TH and each peptide, some sections were processed without the primary antibody, a procedure that never produced any staining.
2.4 Oxytocin and Fos ICC
The fixative (150 ml over 10 min) was 4% paraformaldehyde (PFA) in NaPBS (pH 7.4). Postfixing and cryoprotection were combined by overnight storage at 4°C in PFA with 20% sucrose. NaPBS was used for ICC. Anti-oxytocin (Chemicon; Temecula, CA) was used at 1:5,000–1:7,000 and anti-c-Fos (Ab-5; Oncogene; Cambridge, MA) at 1:50,000. Males used for Fos ICC were not given colchicine and were retested for mating on the day of perfusion. They were perfused 1–1.5 hr after ejaculation [see Heeb and Yahr (1996) for details].
2.5 SP and CCK ICC
These antigens were often studied in alternate sections. Fixation was done over 12 min with either 200 ml of PFA + 2.5% acrolein (pH 6.8) or 50 ml of PFA + acrolein followed by 150 ml of PFA (pH 7.4) alone [all prepared in KPBS (0.05M; pH 7.4)]. PFA was used for postfixation. Either KPBS or NaPBS was used for ICC. KPBS gave better results for CCK. Anti-CCK (Diasorin; Stillwater, MN) was used at 1:5,000 – 1:10,000 and anti-SP (Diasorin) at 1:8,000 – 1:20,000.
CCK staining was sometimes amplified with biotinylated tyramine. For this, fixation was done with PFA and acrolein followed by PFA alone. KPBS was used for ICC. After incubation with the secondary antibody (1:5,000), sections were incubated (30 min) in a 1:8 dilution of ABC solution containing 0.3% Triton X-100, then 20 min in a 1:1,000 dilution of biotinylated tyramine (NEN Life Sciences; Boston, MA) in KPBS containing 0.005% H2O2 and then 1 hr at 37 °C in a 1:16 dilution of ABC solution with 0.3% Triton X-100. Visualization involved DAB as above.
2.6 VP ICC
Fixation was done with 5% acrolein in NaPBS (150 ml/10 min). No postfixing was done. The ICC buffer was TBS. Preliminary blocking (15 min) used 2% NS and 0.3% Triton X-100. Incubation with anti-VP (1:1,000; ICN; Costa Mesa, CA) was done at 37 °C for 1–1.5 hr.
2.7 TH ICC
Fixation was done with PFA and acrolein followed by PFA alone and PFA postfixing (see 2.5 SP and CCK). The ICC buffer was TBS. Before exposure to NaBH4, sections were incubated for 15 min in 3% H2O2 to exhaust endogenous peroxidase. Preliminary blocking used 10% NS. Anti-TH (Diasorin) was used at 1:10,000.
2.8 Histological analyses
Distributions of immunoreactive (IR) cells in the MeA, caudal POA and caudal BST were assessed at 40–125X with brightfield illumination. Cell groups were identified by landmarks using rat brain maps and atlases (Paxinos and Watson, 1997; Swanson, 1992) and a description of the gerbil POA (Commins and Yahr, 1984). At a caudal level of the MPN, cells IR for TH, CCK, SP and VP were charted. Using a drawing tube, they were traced as they appeared at 78X on one side of one section from one male per antigen. Landmarks were also traced, and outlines of the MPNm, central MPN (MPNc) and ventral BST from an archived, thionin-stained section were superimposed. Cells were considered to be IR if they had outlines consistent with neuronal soma that were distinct from the surround due to reaction product in the cytoplasm. A blue filter (Wratten 47A; Kodak, New York, NY) was sometimes used to make the brown reaction product appear darker. Oxytocin-IR cells, which were the least common in the MPNm, were not charted.
The gerbil MPNm and ventral BST were initially identified as parts of a sexually dimorphic area (SDA) in the hypothalamus (Commins and Yahr, 1984). Based on similarities between the lateral SDA and parts of the rat BST as discussed in Finn and Yahr (2005), we now refer to the lateral SDA as the ventral BST because it corresponds to the magnocellular subnucleus of the rat BST (BSTmg) plus the parts of the ventral subnucleus lying ventral and lateral to the BSTmg as described by Ju and Swanson (1989). It includes the dorsal parts of the preoptic BST subnucleus as described by Moga et al. (1989) and may correspond to the magnocellular medial preoptic nucleus (MPN mag) in hamsters (Wang and Swann, 2006). Based on homologies discussed in Finn et al. (1993), the medial SDA, the area lateral to it and the SDA pars compacta correspond to the MPNm, lateral MPN (MPNl) and MPNc of rats, respectively.
2.9 Quantification of IR cell densities
IR cells in the PdPN, MPNm and lateral MeApd were counted at 125X, 312X and 500X, respectively, in areas defined by boxes superimposed on the tissue with the drawing tube. The PdPN box (135 × 135 μm subtended tissue) was positioned so that its lateral edge was just lateral to the fornix and its top edge was a third of the way down the third ventricle (below the anterior commissure), at or just caudal to the level where the fornix abuts the anterior commissure. The lateral MeApd box (160 × 160 μm) was placed at the edge of the MeApd, as revealed by the stria terminalis, where the edge is farthest from the optic tract and most concave. This was 30–150 μm rostral to the lateral ventricle. For the MPNm, the third ventricle, optic chiasm and anterior commissure were used to position the box (120 × 240 μm), vertically, in the caudal MPNm, ventromedial to the MPNc.
Using the drawing tube, each IR cell body inside the box or on its edges was drawn. From the drawings, cells in the box or overlapping its top or right edge were counted. Those overlapping the left or bottom edge were counted if at least half of the cell body was inside the box. When possible, counts were done at two rostrocaudal levels per area, always using nonadjacent sections. The sides selected were those that appeared to have the most stained cells, which was usually the side of the colchicine infusion. If more than one hemisection was counted for an area, the total count for that male was divided by the number of hemisections used before the group mean was computed.
2.10 Image preparation
Digitized images were acquired with a Zeiss AxioImager M2 light microscope using a 10X objective and AxioVision software v4.7. To better reproduce the appearance of the tissue at microscopy, images were adjusted for tonal and/or color qualities using Adobe Photoshop CS3 (levels, curves and color balance tools). Figures were assembled in Adobe Illustrator CS3.
3. Results and Discussion
3.1 Catecholaminergic neurons
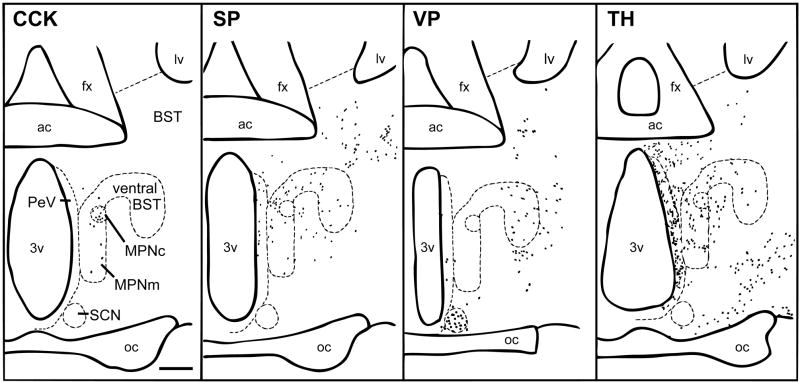
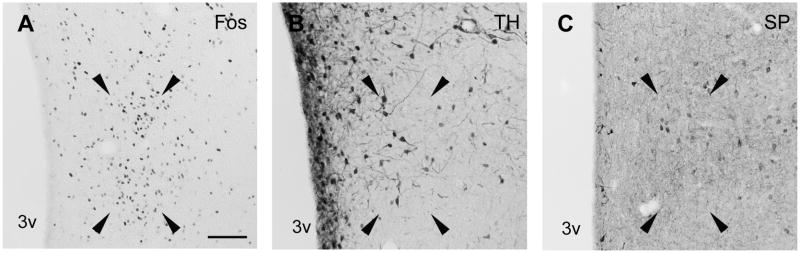
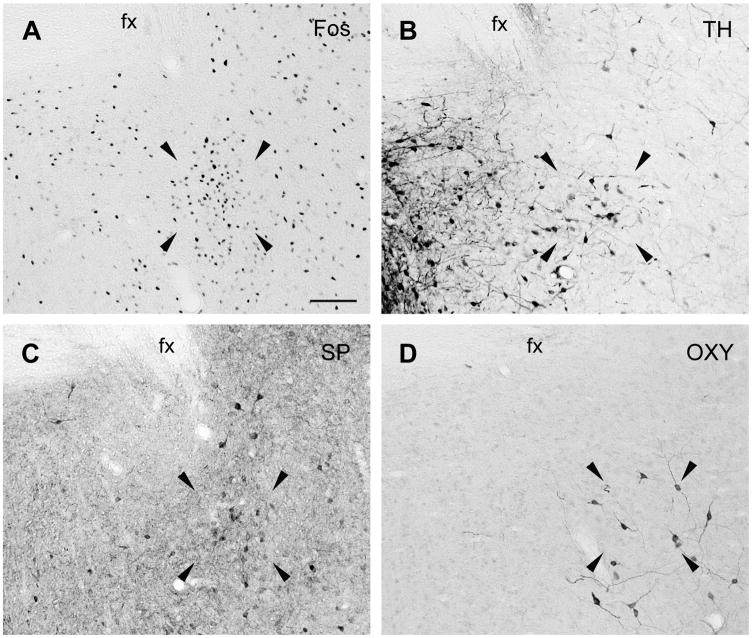
In the caudal POA, many dark TH-IR cells corresponding to the dorsal A14 and A15 dopaminergic cells of rats (Hökfelt et al., 1984) were seen in the PeV and anterodorsal preoptic nucleus as shown in Figure 1. The A14 group formed a band below the ventral BST that extended diagonally from the SCN along the optic chiasm and through the lateral POA. TH-IR cells were also found in the MPNm (see Figure 2B), where they seemed denser than in rats (Simerly et al., 1986), and in the MPNl and MPNc. The PdPN had TH-IR cells (see Figure 3B) that were as dense as mating-activated efferents to the anteroventral periventricular nucleus (AVPv; Simmons and Yahr, 2002). The distributions of Fos-IR cells in the MPNm and PdPN of a mated male are shown in Figures 2A and 3A for comparison to the distributions of TH-IR cells at those sites. For TH and every peptide studied here, the density of MPNm, PdPN and lateral MeApd cells containing them is given in Table 1.
Figure 1.
Camera lucida drawings of coronal sections through the caudal POA of male gerbils showing the distributions of cells IR for CCK, SP, VP and TH. Each dot represents one cell. Scale bar = 100 μm. ac = anterior commissure; fx = fornix; lv = lateral ventricle; 3v = third ventricle; oc = optic chiasm.
Figure 2.
Brightfield photomicrographs of coronal sections through the MPN of male gerbils showing the distributions of cells IR for Fos after ejaculation (A), TH (B) and SP (C). Arrowheads point to the corners of the area in which MPNm cells were counted. Scale bar = 100 μm. 3v = third ventricle.
Figure 3.
Brightfield photomicrographs of coronal sections through the PdPN of male gerbils showing the cluster of Fos-IR cells seen after ejaculation (A) in relation to the distributions of cells IR for TH (B), SP (C) and oxytocin (OXY; D). Arrowheads point to the corners of the area in which PdPN cells were counted. Scale bar = 100 μm. fx = fornix.
Table 1.
Mean (± SEM) Number of MPNm, PdPN and lateral1 Immunoreactive for TH, CCK, SP, Vasopressin or Oxytocin
| N | MPNm | PdPN | Lateral MeApd | |
|---|---|---|---|---|
| TH | 7 | 12 ± 1 | 9 ± 1 | 6 ± 12 |
| CCK | 11 | 4 ± 13 | 0 | 17 ± 2 |
| SP | 8 | 9 ± 1 | 6 ± 1 | 11 ± 2 |
| Vasopressin | 5 | 0 | 3 ± 1 | 0 |
| Oxytocin | 6 | 1 ± 02 | 2 ± 0 | 0 |
Per 0.029 (MPNm), 0.018 (PdPN) and 0.026 (lateral MeApd) mm2.
N = 6;
N = 5
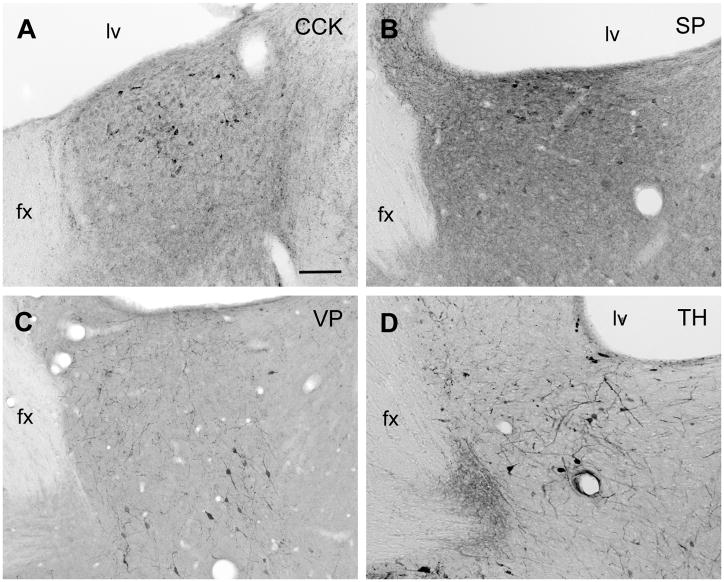
TH-IR cells were less common in the caudal BST than in the caudal POA, but a few were seen in the ventral BST (see Figure 1). Slightly more were noted in the BSTpr, where they were densest rostrally. This is shown in Figure 4D.
Figure 4.
Brightfield photomicrographs of coronal sections through the caudodorsal BSTpr (top row) or rostral BSTpr (bottom row) of male gerbils showing the distributions of cells IR for CCK (A), SP (B), VP (C), or TH (D). Scale bar = 100 μm. fx = fornix; lv = lateral ventricle.
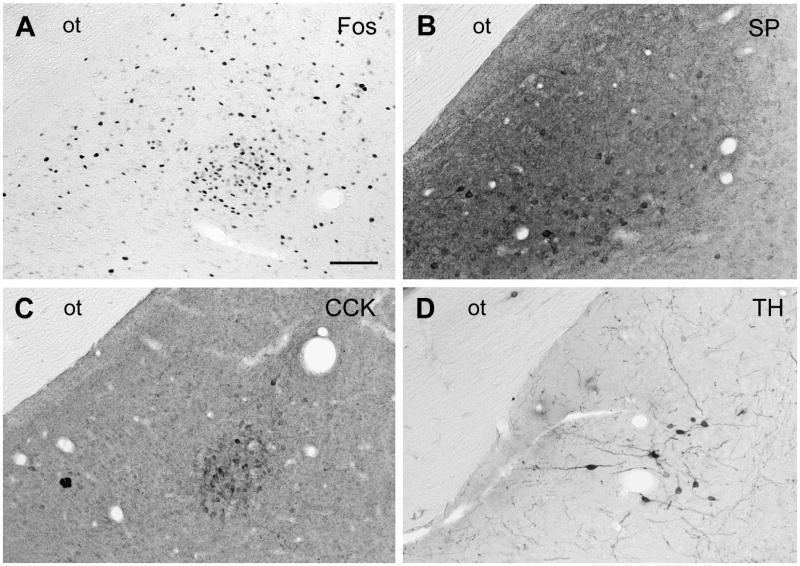
In the MeA, a few TH-IR cells were seen in the medial MeApd and anterodorsal MeA, but the posteroventral MeA had none. More were noted in the caudal MeApd, where most were scattered. However, in the lateral MeApd, TH-IR cells were clustered where the Fos-cell cluster appears at ejaculation, as shown in Figure 5A and D, and their density (see Table 1) matched that of mating-activated efferents to the AVPv (Simmons and Yahr, 2002). Like TH cells at midrostral MeA levels in hamsters (Asmus et al., 1992), some TH cells in the lateral MeApd had prominent dendrites extending into the medial MeApd, where cells express Fos in response to odors (Kollack-Walker and Newman, 1997) and/or anticipation of sexual reward elicited by exposure to an environment associated with mating (Balfour et al., 200; Heeb and Yahr, 1996).
Figure 5.
Brightfield photomicrographs of coronal sections through the MeApd of male gerbils showing the cluster of Fos-IR cells seen after ejaculation (A) in relation to the distributions of cells IR for SP (B), CCK (C) and TH (D). Scale bar = 100 μm. ot = optic tract.
In rats, TH cells were not seen in the MeApd with in situ hybridization for TH mRNA or TH ICC using colchicine (Kiyama et al., 1990; Kosaka et al., 1987). A few TH-IR cells were detected without colchicine in the MeApd of meadow voles and hamsters (Northcutt et al., 2007; Vincent, 1988), but those in hamsters did not express Fos with mating (Asmus and Newman, 1994). In contrast, many TH-IR cells were seen without colchicine in the MeApd and dorsal BST of prairie voles (Northcutt et al., 2007), and they expressed Fos and another immediate-early gene, erg-1, with mating, particularly ejaculation (Cavanaugh and Lonstein, 2010).
Because the MeApd and dorsal BST are sites where chemosensory and hormonal inputs converge, they are foci for research on affiliative behaviors, such as pair bonding and biparental care of young, as well as male sex behavior. Prairie voles are a model for research on affiliative behaviors because they are monogamous and biparental and closely related to meadow voles, which are promiscuous, like hamsters and rats. Thus, it is interesting that gerbils, which are monogamous and biparental, resemble rats, hamsters and meadow voles, not prairie voles, in TH-cell density in the MeApd and dorsal BST. Gerbils also differ from many monogamous rodents in that T levels and aggressiveness toward male nest intruders do not decline when males become paternal (Juana et al., 2010). Thus, just as gene-behavior correlations among selected lines are prone to confounds if either direction of selection is represented by only one line (DeFries et al., 1974), brain-behavior correlations among rodents may be prone to confounds if selection for an adaptation such as monogamy and biparental care of young is represented by only one species. Comparing prairie voles to gerbils could be useful in this regard.
3.2 SP neurons
The distribution of SP-IR cells in the caudal POA and BST of gerbils resembled that in rats and hamsters (Ju et al., 1989; Neal and Newman, 1991; Simerly et al., 1986; Swann and Newman, 1992). In the caudal POA, SP-IR cells were seen primarily in the PeV but also in the MPNm (see Figure 2C), where they were mostly in its caudal aspect. Their density in the MPNm (see Table 1) matched that of mating-activated efferents to the lateral MeApd (Heeb and Yahr, 2001). SP-IR cells were also seen dorsal to the MPNm, in the cell bridge connecting the MPNm to the ventral BST, in the MPNl and in the suprachiasmatic nucleus (SCN), as shown in Figure 1. They were not seen in the MPNc, which stood out because it lacked both SP fibers and cells, whereas its surround had both. SP-IR cells were found in the PdPN, as shown in Figure 2C, and their density there (see Table 1) matched that of mating-activated efferents to the BSTpr (Simmons and Yahr, 2002).
In the caudal BST, SP-IR cells were seen in and dorsal to the ventral BST as well as in the rostral BSTpr, near the fornix, and, further caudally, lateral to the BSTpr, next to the internal capsule. They were densest, though, in the dorsal BSTpr, just below the lateral ventricle. This distribution is shown in Figure 4B.
SP-IR cells were seen throughout the MeApd but were densest caudally. Their densities were similar in the medial MeApd, anteroventral and posteroventral MeA, and lateral MeApd, where they were densest ventrally (see Figure 5B).
3.3 VP neurons
Many darkly stained VP-IR cells were seen in the SCN and supraoptic nucleus. There were a few VP-IR cells in the PeV but none in the MPNm, the bridge connecting it to the ventral BST, the MPNc (see Figure 1) or PdPN. VP has not been studied in PdPN cells of other species. VP-IR cells were moderately dense ventral and lateral to the ventral BST.
In the caudal BST, VP-IR cells were moderately dense in the ventral BST and BSTpr. In the BSTpr, they were particularly dense rostrally (see Figure 4C), as they are in voles, jerboas and Siberian, but not Syrian, hamsters (De Vries et al., 1985; Sofroniew, 1985).
VP-IR cells were not seen in the MeA, but a few were interspersed with fibers of the stria terminalis between the MeApd and the central nucleus of the amygdala. In lacking VP cells in the MeA, gerbils resemble Syrian hamsters (Ferris et al., 1995) and differ from rats (Caffe and van Leeuwen, 1983; De Vries et al., 1985; Sofroniew, 1985), mice (Rood et al., 2008), voles (Wang, 1995), jerboas (Lakhdar-Ghazal et al., 1995) and Siberian hamsters (Dubois-Dauphin et al., 1994). In having VP cells interspersed with fibers of the stria terminalis just outside the MeApd, gerbils resemble voles, jerboas and Siberian hamsters, and differ from Syrian hamsters.
3.4 CCK neurons
In the caudal POA, caudal BST and MeA, CCK-IR cells were distributed as they are in rats (Ju et al., 1989; Micevych et al., 1987, 1988; Roberts et al., 1982; Simerly et al., 1986; Simerly et al., 1989; Simerly and Swanson, 1987). They were dense in the MPNc, sparse in the MPNm, absent from the PdPN (see Table 1 and Figure 1) and common in the dorsal BSTpr, especially just below the lateral ventricle (see Figure 4A).
In the MeA, CCK-IR cells were densest in the MeApd caudal to the ejaculation-related site but seemed less dense than in the MeApd of rats. Some were seen in the medial MeApd and in the anteroventral and posteroventral MeA. In the lateral MeApd, their density (see Table 1) matched that of mating-activated efferents to the BSTpr (Simmons and Yahr, 2002). Moreover, at the rostrocaudal level of the MeApd where a cluster of Fos cells appears after ejaculation, CCK-IR cells were seen only at the site of that cluster, where they formed a distinct ball. This is shown in Figure 5A and C.
3.5 Oxytocin neurons
In the caudal POA, oxytocin-IR cells were densest in the PeV, SON and PdPN. They were very sparse in the MPNm and ventral and lateral to the ventral BST. The few seen in the MPNl were part of a group that continued into the area below the caudal PdPN. In the PdPN (see Figure 3D), oxytocin-IR cells had a density (see Table 1) matching that of mating-activated efferents to the lateral MeApd (Heeb and Yahr, 2001).
Oxytocin cells are also seen in the rat PdPN (Sofroniew, 1985), from which many project to the posterior pituitary (Vanhatalo and Soinila, 1995). When tracing PdPN efferents in gerbils (Simmons and Yahr, 2002), we did not study the pituitary. We did, however, note short, continuing, vertically oriented fibers along the top of the optic chiasm in the POA and rostral hypothalamus. Those fibers may have been en route to the posterior pituitary. If oxytocin cells in the PdPN of rats, and possibly gerbils, that project to the posterior pituitary are among the PdPN cells activated at ejaculation, they could be a source of the oxytocin released into the circulation after ejaculation (Hughes et al., 1987).
The caudal BST had fewer oxytocin-IR cells than the caudal POA. Those seen were in the ventral BST and BSTpr. The latter are shown in Figure 5C. No oxytocin-IR cells were seen in the MeA.
3.6 Summary and conclusions
TH, SP, CCK, VP and oxytocin were each found in at least one forebrain area activated at ejaculation, i.e., the PdPN, lateral MeApd or MPNm, and each of these areas had cells containing three or more of these molecules. At all three sites, cells containing TH, SP, CCK, VP or oxytocin were less abundant than cells that make GABA and/or NO (Simmons and Yahr, 2003; Simmons and Yahr, unpublished). Except in the PdPN, they are also less abundant than putatively glutamatergic neurons. Of course, the peptide-containing cells visualized here could be cells that also produce GABA, glutamate or NO. Similarly, TH could coexist with NO. Expressing more than one molecule for intercellular communication is a common way for neurons to be subdivided into subsets serving different functions. Alternatively, or in addition, TH- or peptide-containing cells may be interspersed with the more abundant types. In either case, catecholaminergic cells, and cells containing CCK, SP, VP or oxytocin, may play a role in integrating the activities or outputs of the PdPN, MPNm and lateral MeApd. This includes the possibility that they could be among, or interact with, cells activated at ejaculation. Similarities in some of their densities to the densities of mating-activated efferents of the PdPN, MPNm and lateral MeApd are consistent with this possibility.
Research Highlights.
The posterodorsal preoptic nucleus (PdPN) contains TH, SP and oxytocin cells.
The lateral posterodorsal medial amygdala (MeApd) contains TH, SP and CCK cells.
The medial MPN has more TH and SP cells than CCK or oxytocin cells.
TH, CCK, ejaculation-related cells are grouped at the same site in the lateral MeApd.
The PdPN may be a source of the oxytocin that males secrete at ejaculation.
Acknowledgments
Supported by NIMH Research Grant MH26481 and NIMH Training Grant MH14599 and submitted by DAS in partial satisfaction of the requirements for the Ph.D. in Biological Sciences.
Abbreviations
- 3v
third ventricle
- ac
anterior commissure
- fx
fornix
- lv
lateral ventricle
- oc
optic chiasm
- ot
optic tract
- sm
stria medullaris
- AC
anterior commissural nucleus
- AVPv
anteroventral periventricular nucleus
- BST, mg, pr
bed nuclei of the stria terminalis, magnocellular subnucleus, principal subnucleus
- MeA, pd
medial nucleus of the amygdala, posterodorsal
- MPN, l,m,c,
medial preoptic nucleus, lateral, medial, central
- MPN mag
magnocellular medial preoptic nucleus (hamsters)
- PdPN
posterodorsal preoptic nucleus
- PeV
periventricular nucleus
- POA
preoptic area
- SCN
suprachiasmatic nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asmus S, Kincaid A, Newman SW. A species-specific population of tyrosine hydroxylase-immunoreactive neurons in the medial amygdaloid nucleus of the Syrian hamster. Brain Res. 1992;575:199–207. doi: 10.1016/0006-8993(92)90080-s. [DOI] [PubMed] [Google Scholar]
- Asmus G, Newman S. Colocalization of tyrosine hydroxylase and Fos in the male Syrian hamster brain following different states of arousal. J Neurobiol. 1994;25:156–168. doi: 10.1002/neu.480250207. [DOI] [PubMed] [Google Scholar]
- Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacol. 2004;29:718–730. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Everitt BJ. Increased expression of c-fos in the medial preoptic area after mating in male rats: Role of afferent inputs from the medial amygdala and midbrain central tegmental field. Neuroscience. 1992;50:627–646. doi: 10.1016/0306-4522(92)90452-8. [DOI] [PubMed] [Google Scholar]
- Caffe A, van Leeuwen F. Vasopressin-immunoreactive cells in the dorsomedial hypothalamic region, medial amygdaloid nucleus and locus coeruleus of the rat. Cell Tissue Res. 1983;233:23–33. doi: 10.1007/BF00222229. [DOI] [PubMed] [Google Scholar]
- Cavanaugh BL, Lonstein JS. Androgenic and oestrogenic influences on tyrosine hydroxylase-immunoreactive cells of the prairie vole medial amygdala and bed nucleus of the stria terminalis. J Neuroendocrinol. 2010;22:217–225. doi: 10.1111/j.1365-2826.2010.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins D, Yahr P. Adult testosterone levels influence the morphology of a sexually dimorphic area in the Mongolian gerbil brain. J Comp Neurol. 1984;224:132–140. doi: 10.1002/cne.902240112. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJPW, Veening JG. Fos-immunoreactivity in the rat brain following consummatory elements of sexual behavior: A sex comparison. Brain Res. 1996;738:67–82. doi: 10.1016/0006-8993(96)00763-9. [DOI] [PubMed] [Google Scholar]
- DeFries JC, Hegmann JP, Halcomb RA. Response to 20 generations of selection for open-field activity in mice. Behav Biol. 1974;11:481–495. doi: 10.1016/s0091-6773(74)90800-1. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs R, van Leeuwen F, Caffe A, Swaab D. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y, Miller MM, Dorsa DM, De Vries GJ. Distribution of small vasopressinergic neurons in golden hamsters. J Comp Neurol. 1995;360:589–598. doi: 10.1002/cne.903600404. [DOI] [PubMed] [Google Scholar]
- Finn PD, De Vries GJ, Yahr P. Efferent projections of the sexually dimorphic area of the gerbil hypothalamus: Anterograde identification and retrograde verification in males and females. J Comp Neurol. 1993;338:491–520. doi: 10.1002/cne.903380403. [DOI] [PubMed] [Google Scholar]
- Finn PD, Yahr P. Projection from the ventral bed nucleus of the stria terminalis to the retrorubral field in rats and the effects of cells in these areas on mating in male rats versus gerbils. Horm Behav. 2005;47:123–138. doi: 10.1016/j.yhbeh.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. C-fos immunoreactivity in the sexually dimorphic area of the hypothalamus and related brain regions of male gerbils after exposure to sex-related stimuli or performance of specific sexual behaviors. Neuroscience. 1996;72:1049–1071. doi: 10.1016/0306-4522(95)00602-8. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. Anatomical and functional connections among cell groups in the gerbil brain that are activated with ejaculation. J Comp Neurol. 2001;439:248–258. doi: 10.1002/cne.1346. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Martensson R, Björklund A, Kleinau S, Goldstein M. Handbook of Chemical Neuroanatomy, Vol. 2, Classical Neurotransmitters in the CNS. part I. Elsevier; Amsterdam: 1984. Distributional maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain; pp. 277–379. [Google Scholar]
- Hughes AM, Everitt BJ, Lightman SL, Todd K. Oxytocin in the central nervous system and sexual behavior in male rats. Brain Res. 1987;414:133–137. doi: 10.1016/0006-8993(87)91333-3. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW. Studies on the cellular architecture of the bed nucleus of the stria terminalis in the rat: I. Cytoarchitecture. J Comp Neurol. 1989;280:587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW, Simerly RB. Studies on the cellular architecture of the bed nucleus of the stria terminalis in the rat: II. Chemoarchitecture. J Comp Neurol. 1989;280:603–621. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- Juana L, Vazquez-Gaytan B, Martinez-Torres M, Agustin C, Ramos-Blancas G, Guadalupe O. Neither testosterone levels nor aggression decrease when the male Mongolian gerbil (Meriones unguiculatus) displays paternal behavior. Horm Behav. 2010;57:271–275. doi: 10.1016/j.yhbeh.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Kitahama K, Geffard M, Okamura H, Nagatsu I, Mons N, Jouvet M. Dopamine- and DOPA-immunoreactive neurons in the cat forebrain with reference to tyrosine hydroxylase-immunohistochemistry. Brain Res. 1990;518:83–94. doi: 10.1016/0006-8993(90)90957-d. [DOI] [PubMed] [Google Scholar]
- Kiyama H, Emson PC, Ruth J. Distribution of tyrosine hydroxylase mRNA in the rat central nervous system visualized by alkaline phosphatase in situ hybridization histochemistry. Eur J Neurosci. 1990;2:512–524. doi: 10.1111/j.1460-9568.1990.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker SS, Newman SW. Mating-induced expression of c-fos in the male Syrian hamster brain: Role of experience, pheromones and ejaculation. J Neurobiol. 1997;32:481–501. doi: 10.1002/(sici)1097-4695(199705)32:5<481::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Hama K, Nagatsu I. Tyrosine hydroxylase-immunoreactive intrinsic neurons in the rat cerebral cortex. Exper Brain Res. 1987;68:3939–405. doi: 10.1007/BF00248804. [DOI] [PubMed] [Google Scholar]
- Lakhdar-Ghazal L, Dubois-Dauphin M, Hermes M, Buijs R, Bengelloun W, Pevet P. Vasopressin in the brain of a desert hibernator, the jerboa (Jaculus orientalis): presence of sexual dimorphism and seasonal variation. J Comp Neurol. 1995;358:499–517. doi: 10.1002/cne.903580404. [DOI] [PubMed] [Google Scholar]
- Micevych P, Akesson T, Elde R. Distribution of cholecystokinin-immunoreactive cell bodies in the male and female rat: II. Bed nucleus of the stria terminalis and amygdala. J Comp Neurol. 1988;269:381–391. doi: 10.1002/cne.902690306. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Park SS, Akesson TR, Elde R. Distribution of cholecystokinin- immunoreactive cell bodies in the male and female rat: I. Hypothalamus. J Comp Neurol. 1987;255:124–136. doi: 10.1002/cne.902550110. [DOI] [PubMed] [Google Scholar]
- Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunocytochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989;283:315–332. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- Neal CR, Newman SW. Prodynorphin- and substance P-containing neurons project to the medial preoptic area in the male Syrian hamster. Brain Res. 1991;546:119–131. doi: 10.1016/0006-8993(91)91166-x. [DOI] [PubMed] [Google Scholar]
- Northcutt KV, Wang Z, Lonstein JS. Sex and species differences in tyrosine- hydroxylase synthesizing cells of the rodent olfactory extended amygdala. J Comp Neurol. 2007;500:103–115. doi: 10.1002/cne.21148. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1997. [Google Scholar]
- Roberts GW, Woodhams PL, Pollack JM, Crow TJ. Distribution of neuropeptides in the limbic system of the rat: Amygdaloid complex. Neuroscience. 1982;7:99–131. doi: 10.1016/0306-4522(82)90156-7. [DOI] [PubMed] [Google Scholar]
- Rood BD, Murray EK, Laroche J, Yang MK, Blaustein JD, De Vries GJ. Absence of progestin receptors alters distribution of vasopressin fibers but not sexual differentiation of vasopressin system in mice. Neuroscience. 2008;154:911–921. doi: 10.1016/j.neuroscience.2008.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Gorski RA, Swanson LW. Neurotransmitter specificity of cells and fibers in the medial preoptic nucleus: An immunohistochemical study in the rat. J Comp Neurol. 1986;246:343–363. doi: 10.1002/cne.902460305. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson L. Castration reversibly alters levels of cholecystokinin immunoreactivity within cells of three interconnected sexually dimorphic forebrain nuclei of the rat. Proc Natl Acad Sci USA. 1987;84:2087–2091. doi: 10.1073/pnas.84.7.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Young BJ, Capozza MA, Swanson LW. Estrogen differentially regulates neuropeptide gene expression in a sexually dimorphic olfactory pathway. Proc Natl Acad Sci USA. 1989;86:4766–4770. doi: 10.1073/pnas.86.12.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Yahr P. Projections of the posterodorsal preoptic nucleus and the lateral part of the posterodorsal medial amygdala in male gerbils, with emphasis on cells activated with ejaculation. J Comp Neurol. 2002;444:75–94. doi: 10.1002/cne.10128. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Yahr P. GABA and glutamate in mating-activated cells in the preoptic area and medial amygdala of male gerbils. J Comp Neurol. 2003;459:290–300. doi: 10.1002/cne.10605. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. Vasopressin- and neurophysin-immunoreactive neurons in the septal region, medial amygdala and locus coeruleus in colchicine-treated rats. Neuroscience. 1985;15:347–358. doi: 10.1016/0306-4522(85)90217-9. [DOI] [PubMed] [Google Scholar]
- Swann J, Newman SW. Testosterone regulates substance P within neurons of the medial nucleus of the amygdala, the bed nucleus of the stria terminalis and the medial preoptic area of the male golden hamster. Brain Res. 1992;590:18–28. doi: 10.1016/0006-8993(92)91077-r. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- Vanhatalo S, Soinila S. Nitric oxide synthase in the hypothalamo-pituitary pathways. J Chem Neuroanat. 1995;8:165–173. doi: 10.1016/0891-0618(94)00043-s. [DOI] [PubMed] [Google Scholar]
- Vincent SR. Distributions of tyrosine-, dopamine-β-hydroxylase-, and phenylethanolamine-N-methyltransferase-immunoreactive neurons in the brain of the hamster (Mesocricetus auratus) J Comp Neurol. 1988;268:584–599. doi: 10.1002/cne.902680408. [DOI] [PubMed] [Google Scholar]
- Wang Z. Species differences in the vasopressin-immunoreactive pathways in the bed nucleus of the stria terminalis and medial amygdaloid nucleus in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Behav Neurosci. 1995;109:305–311. doi: 10.1037//0735-7044.109.2.305. [DOI] [PubMed] [Google Scholar]
- Wang Z, De Vries GJ. Androgen and estrogen effects on vasopressin messenger RNA expression in the medial amygdaloid nucleus in male and female rats. J Neuroendocrinol. 1995;7:827–831. doi: 10.1111/j.1365-2826.1995.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Swann JM. The magnocellular medial preoptic nucleus I. Sources of afferent input. Neuroscience. 2006;141:1437–1456. doi: 10.1016/j.neuroscience.2006.04.079. [DOI] [PubMed] [Google Scholar]
- Yamada K, Emson P, Hökfelt T. Immunohistochemical mapping of nitric oxide synthase in the rat hypothalamus and colocalization with neuropeptides. J Chem Neuroanat. 1996;10:295–316. doi: 10.1016/0891-0618(96)00133-0. [DOI] [PubMed] [Google Scholar]