Abstract
Our understanding of the pathophysiology of multiple sclerosis (MS) has evolved significantly over the past two decades as the fields of immunology and neurobiology provide new avenues of exploration into the cause and mechanism of the disease. It has been known for decades that T cells have different cytokine phenotypes, yet the cytokine phenotype of pathogenic T cells in MS is still an area of debate. In EAE, it appears that IFNγ and IL-17, produced by Th1 and Th17 cells respectively, are not the critical factor that determines T cell encephalitogenicity. However, there are molecules such as IL-23, T-bet and STAT4, that appear to be critical, yet it is unclear whether all these molecules contribute to a common, yet undefined pathway, or act in a synergistic manner which culminates in encephalitogenicity has still to be determined. Therefore, the focus of research on effector T cells in MS should focus on pathways upstream of the cytokines that define Th1 and Th17 cells, since downstream products, such as IFNγ and IL-17, probably are not critical determinants of whether an effector T cells is capable of trafficking to the CNS and inducing inflammatory demyelination.
Introduction
Our understanding of the pathophysiology of multiple sclerosis (MS) has evolved significantly over the past two decades as the fields of immunology and neurobiology provide new avenues of exploration into the cause and mechanism of the disease. Experimental autoimmune encephalomyelitis (EAE) has been used as a model for multiple sclerosis (MS) for more than forty years and has been a major factor in determining the path of MS research. As the field of immunology advances, many of the fundamental observations in EAE are questioned and revisited to further our understanding of immune-mediated neurodegeneration with the ultimate goal of defining the pathophysiology of MS and development of a cure. Although MS is speculated to be a T cell-mediated autoimmune disease directed against myelin proteins, the cause of the disease is unknown. It has been known for decades that T cells have different cytokine phenotypes, yet the cytokine phenotype of pathogenic T cells in MS is still an area of debate. Understanding the phenotype of the T cells that mediate MS and other autoimmune diseases may be essential to determining the cause of disease and the development of therapies.
CD4 T Cell Cytokine Phenotype in EAE
The EAE model evolved from the observation that a small number of individuals receiving the rabies virus vaccine, a live attenuated strain grown in rabbit CNS, developed encephalomyelitis. The realization that the encephalomyelitis was not caused by the rabies virus, but a hypersensitivity to the CNS debris contaminating the vaccine, initiated the development of EAE as a model for MS. Although EAE was initially induced by immunization with myelin proteins emulsified in Complete Freund’s Adjuvant, it can also be induced by adoptive transfer of myelin-specific CD4+ TH1 cells into naïve recipient mice [1–7]. Since myelin-specific CD4+ TH1 cells were sufficient to induce EAE, MS research focused on these IFNγ-producing T cells in MS patients. Several studies that specifically suppressed IFNγ in the myelin-specific T cells prior to transfer into recipient mice demonstrated that altering the signaling pathway which results in IFNγ production in CD4+ T cells decreases the encephalitogenic capacity of these cells [6,8,9]. In addition, STAT4 and T-bet, which are transcription factors in the TH1 differentiation pathway, have been shown to be essential for EAE induction [6,10–12]. The critical experiment to determine if IFNγ was in fact a viable target for autoimmune encephalomyelitis was the induction of EAE in IFNγ and IFNγ receptor deficient mice [13,14]. Not only were IFNγ-deficient mice susceptible to EAE, the disease appeared to be more severe. This observation was confirmed with systemic administration antibodies to neutralize IFNγ [15,16]. The number of myelin-specific CD4+ T cells was expanded in IFNγ-deficient mice, which may have occurred due to loss of regulatory cells that were dependent on IFNγ [17]. Together, these data suggest that the differentiation pathway that generates TH1 cells may be important in encephalitogenicity, but the downstream production of IFNγ by myelin-specific T cells is not critical.
The realization that CD4+ TH1 cells were sufficient to cause EAE, yet IFNγ was not necessary, caused many investigators to look at other cytokines that may influence pathogenic capacity of T cells or focus on other characteristics of T cells such as signaling and transcription proteins. Since IL-12 was necessary for signaling events that promote the differentiation of TH1 cells, mice deficient in the p40 and p35 proteins which comprise IL-12 were deleted in mice and EAE was evaluated. Although IL-12p40 deficient mice failed to develop EAE, the IL-12p35-deficient mice did develop EAE [18,19]. Since IL-12p40 was also a component of IL-23, the IL-23 specific protein p19 was deleted in mice and these mice were also resistant to EAE [20]. As a result, IL-23 was deemed an essential cytokine in EAE development, whereas IL-12 was not. In 2005, it was found that IL-23 could promote the expansion of myelin-specific IL-17+ T cells derived from MOG immunized mice and these IL-23-driven IL-17+ T cells were capable of transferring EAE [21]. This led to speculation that myelin-specific TH17 cells were the primary encephalitogenic T cell population in EAE, and perhaps MS. Development of IL-17-deficient mice illustrated that although EAE was less severe in the absence of IL-17, IL-17 was also not necessary for the development of EAE [22,23]. Although IL-23 can enhance IL-17 production, the difference in susceptibility to EAE suggests that the essential role of IL-23 in EAE induction is not linked to it ability to promote IL-17 expression. The data from both the IFNγ and IL-17 deficient mice suggested that cytokine production by myelin-specific T cells, although an easy potential therapeutic target, was not the determining factor for CD4 T cell encephalitogenicity.
Several studies found that IL-6 and TGFβ were sufficient to differentiate CD4 T cells into Th17 cells in vitro [24–26]. However, Th17 cells generated by differentiation with IL-6 and TGFβ in vitro are not encephalitogenic when transferred into naïve recipient mice, suggesting that this is not the mechanism that occurs in vivo to generate pathogenic Th17 cells in EAE [27]. Recently, it has been demonstrated that TGFβ is dispensable during Th17 differentiation, and that IL-6 in the absence of cytokines that promote Th1 or Th2 differentiation will generate Th17 cells that are encephalitogenic [27,28]. Thus, it appears that Th1, Th2 and Th17 cells differentiate in distinct cytokine microenvironments which may be influenced by the host interactions with environmental factors such as pathogens.
Transcriptional regulation of CD4 T cell differentiation
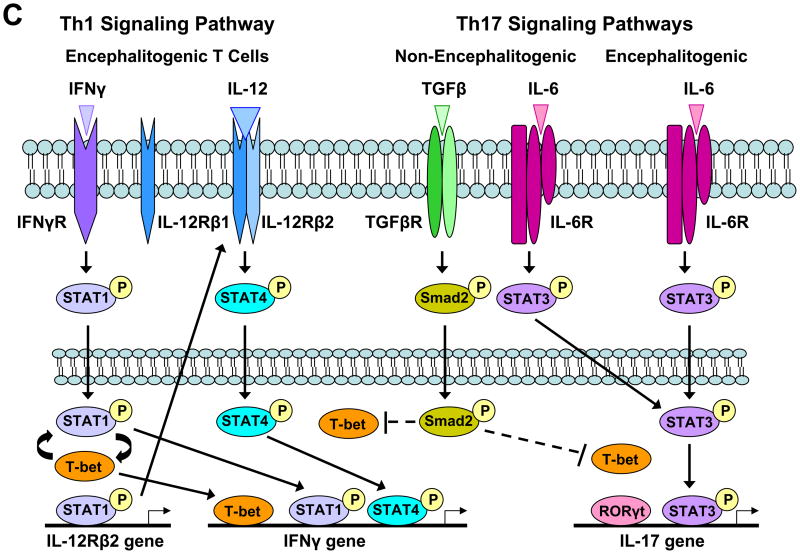
Counter-intuitive data generated from mice deficient in transcription factors that play critical roles in T cell differentiation have also complicated attempts to define the pathogenic T cell population in EAE. Naïve CD4 T cells require both T cell receptor engagement with peptides presented by MHC class II molecules, as well as costimulation mediated by CD28 and B7 molecules. In addition, cytokines in the micro-environment influence the phenotype of CD4 T cells. TH1 cells develop when the environment is rich in IFNγ and IL-12 (Figure 1A). IFNγ binds to the constitutively expressed IFNγ receptor (IFNγR) on the surface of naïve CD4 T cells which results in phosphorylation of STAT1 (pSTAT1) and its translocation (Figure 1C). pSTAT1 contributes to the transcription of T-bet which promotes the transcription of STAT1, and thus a positive feedback loop exists between these two transcription factors that initially drive a CD4 T cell towards a TH1 phenotype. pSTAT1 and possibly T-bet transcriptionally activate the IL-12Rβ2, which is the inducible chain of the IL-12 receptor and forms a heterodimer with the constitutively expressed IL-12Rβ1 chain, forming a complete IL-12R on the cell surface. IL-12 in the micro-environment binds to the IL-12R, resulting in STAT4 phosphorylation, translocation and binding to the IFNγ promoter. It has been demonstrated that pSTAT1, pSTAT4 and T-bet all bind to the IFNγ promoter, as well as other transcription factors, and initiate IFNγ production and contribute to the full differentiation of a TH1 cell (Figure 1C). Mice deficient in STAT4 and T-bet are resistant to EAE induction, suggesting that the Th1 differentiation pathway is necessary for the generation of encephalitogenic CD4 T cells [6,10,11]. However, STAT1 mice are susceptible to EAE indicating the IFNγ receptor signaling is not essential for disease induction [11].
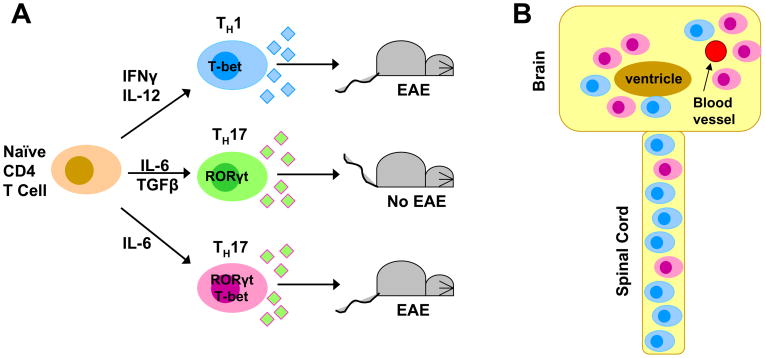
Figure 1. Th1 and Th17 cell differentiation and trafficking to the CNS.
(A) Naïve CD4 T cells can differentiate into Th1 or Th17 cells. During Th17 cell differentiation, TGFβ can enhance IL-17 production, but this diminishes the capacity of these cells to induce EAE. (B) The distribution of Th1 and Th17 cells within the CNS various in EAE. Th1 cells home preferentially to the spinal cord, while Th17 cells appear to preferentially traffic to the brain. The number of Th1 cells in the CNS significantly outnumbers Th17 cells in myelin-immunized mice. (C) Transcriptional regulation of Th1 and Th17 differentiation lends insight into signaling pathways that may be important in the development of encephalitogenic T cells. T-bet is found encephalitogenic Th1 and Th17 cells. The presence of TGFβ during Th17 cell differentiation contributes to the inhibition of T-bet, resulting in non-encephalitogenic Th17 cells if primed with IL-6+TGFβ.
RORγt has been identified as the essential transcriptional regulator of Th17 cells. Mice deficient in RORγt have attenuated EAE [29]; however, these mice do not form lymph nodes [30]. Thus, the resistance to EAE may be due to the inability of CD4 T cells to be activated and differentiate in the periphery, and may be unrelated to a failure to generate Th17 cells. In fact, T-bet deficient mice generate a high frequency of myelin-specific Th17 cells upon EAE induction [27], yet fail to develop disease [11]; further indicating that myelin-specific Th17 cells are not sufficient to cause EAE. Ex vivo analysis of myelin-specific T cells from mice immunized with MOG35-55 peptide indicates that T-bet is found in both Th1 and Th17 cells, suggesting that T-bet plays a role in T cell encephalitogenicity that is unrelated to the cytokine profile of the autoreactive T cells [27]. Generation of Th17 cells with IL-6 in the absence of exogenous TGFβ generates Th17 cells that express T-bet and can induce EAE when transferred into mice [27]. Although it is unclear what critical gene T-bet is regulating in encephalitogenic T cells, the IL-23 receptor and osteopontin genes have been shown to be regulated by T-bet [7,31]. Although osteopontin deficient mice have reduced EAE severity [32,33], IL-23 deficient mice are not susceptible to EAE [20], suggesting the IL-23 receptor gene may be the essential gene regulated by T-bet.
Role of Cytokine Phenotype on T Cell Trafficking and Cell Recruitment
One potential role of the cytokine phenotype is to mediate trafficking of myelin-specific T cells to distinct sites within the CNS which yield different functional deficits. Several studies have noted unique behavioral changes associated with myelin-specific Th17 cells in EAE. In contrast to classical EAE induced by Th1 cells in which mice develop an ascending paralysis, EAE induced by Th17 cells is often characterized by spinning (dysequilibrium), ataxia, spasticity and proprioceptive defects [34]. Analysis of the distribution of Th1 and Th17 cells within the CNS revealed that infiltration of the brain parenchyma occurs when the number of Th17 cells exceeds the number of Th1 cells [34] (Figure 1B). However, a wide range of Th1:Th17 ratios permitted spinal cord parenchymal inflammation. This suggests that Th17 cells may preferentially migrate to the brain, generating plaques at distinct sites with different functional losses. This was confirmed by administering an IL-17 neutralizing antibody to EAE-affected mice which resulted in a decrease in atypical EAE and an increase in classical EAE [34]. It should be noted that the absolute number of Th1 cells infiltrating the CNS in EAE induced by immunization far exceeds the number of Th17 cells, and lesions in the spinal cord are more prevalent early in the disease.
Adhesion molecules clearly contribute to EAE and MS has evidenced by the success of natalizumab, the anti-VLA4 antibody used to treat MS. In 1992, VLA4 was identified as the primary adhesion molecule mediating the attachment of lymphocytes to the CNS vascular endothelium [35]. It was subsequently observed that myelin-specific Th1 cells that expressed VLA4 were highly encephalitogenic, while myelin-specific Th1 cells that had minimal VLA4 expression were not encephalitogenic [36]. These two studies prompted the development of a humanized VLA4 antibody for the treatment of MS. Although natalizumab therapy has been complicated by rare cases of Progressive Multifocal Leukoencephalopathy [37], it has proven very effective in controlling CNS inflammation in MS patients [38]. Ex vivo analysis of VLA4 expression on CSF lymphocytes from MS patients illustrates that both Th1 and Th17 cells express high levels of VLA4 relative to Th1 and Th17 cells derived from the blood [39], suggesting that VLA4 expression plays a critical role in effector T cell entry into the CNS, regardless of cytokine phenotype.
Lesions in the spinal cords of mice immunized to induce EAE have high numbers of macrophages and activated microglia, a classic indicator of a Th1-driven inflammatory response. Consistent with this observation is the upregulation of ELR- CXC chemokines, CXCL9, CXCL10 and CXCL11, which are upregulated in the CNS of EAE-affected mice induced by transfer of Th1 cells [40]. In contrast, lesions induced by transfer of IL-23 driven Th17 cells are characterized by massive neutrophil recruitment and upregulation of the neutrophil-attracting ELR+ CXC chemokines, CXCL1 and CXCL2 [40]. The critical role of IL-23 in the development of CNS inflammation was originally thought to be due to it role in enhancing IL-17 production, but more recently it has been found that IL-23 plays a critical role in homing to the CNS and survival of myelin-specific T cells in the CNS microenvironment [41]. This reconciles the data that IL-23 deficiency results in no disease, while IL-17 deficiency results in less severe EAE. Since loss of IL-23 prevents CNS inflammation there is no disease onset, while loss of IL-17 reduces neutrophil recruitment and secondary damage mediated by matrix metalloproteases. There has been speculation that IL-23 may influence T cell trafficking by modulating a chemokine receptor, such as CCR6 which has been shown to be highly expressed on human Th17 [39,42]. However, there is contradictory data in CCR6 mice with regard to their EAE susceptibility [43–46], making it difficult to determine whether CCR6 is in fact a relevant player in the recruitment of Th17 cells to the brain. Interestingly, IL-23 driven Th17 cells also cause severe inflammation of the optic nerve, forming lesions reminiscent of neuromyelilitis optica [40]. Together, these data suggest that the effector T cell cytokine phenotype may dictate the trafficking of the T cells to specific regions within the CNS, resulting in differential recruitment of innate immune cells and distinct functional losses.
Effector T cells in MS
Historically, myelin-specific T cells have been found in both MS patients and healthy individuals, which raised questions as to the relevance of these cells in MS patients. However, the observation that myelin-specific T cells from MS patients were more likely to have a TH1 phenotype gave validity to the hypothesis that these cells were potentially pathogenic in MS patients [47–50] Subsequently, several studies demonstrated that although healthy individuals had myelin-specific T cells, these cells were naïve; whereas MS patients had activated and memory myelin-specific T cells, indicating that these cells had been previously activated in vivo [51–53]. In addition, a clinical trial with an altered peptide ligand from myelin basic protein (MBP), which was intended to down-regulate myelin-specific T cells, actually exacerbated disease in several MS patients which was associated with increased frequency of MBP-specific T cells that produced IFNγ, suggesting that MS is mediated by myelin-specific TH1 cells [54].
A decade ago it was found that IL-17 mRNA was augmented in the blood and CSF of MS patients, with IL-17 transcript levels higher in blood than CSF, as well as higher levels in blood during clinical exacerbations increased [55]. Subsequently, DNA microarray analysis of MS tissues indicated that IL-17 was present in MS lesions [56]. In 2005, a comprehensive analysis of cytokines expressed in the CSF of conventional MS and opticospinal MS was performed [57]. The Th1 cytokines, IFNγ and TNFα, were both higher in MS patients, but only the TNFα levels were statistically significant. However, comparison of number of CD4 T cells that expressed IFNγ was significantly higher the CSF lymphocytes relative to blood lymphocytes of MS patients, regardless of subtype of disease. In addition, the IFNγ/IL-4 ratio of CD4 T cells was significantly increased in conventional MS patients. IL-17 expression in the CSF of opticospinal MS patients was also significantly increased relative to controls and conventional MS. The spatial distribution of lymphocytes within the CNS of mice with EAE induced by IL-23 driven Th17 cells found extensive lymphocyte infiltration in the spinal cord from the subpial surface to deep within the white matter parenchyma, as well as extensive inflammation of the optic nerve, both characteristic of opticospinal MS [40],
An extensive comparison of Th1 and Th17 cells in the peripheral blood and CSF of MS patients found that the absolute number of Th1 cells both in the blood and CSF is about 10-fold higher than the number of Th17 cells [39], indicating that Th1 effector cells are significantly more prevalent both in the periphery and target organ in MS patients. However, there was a statistically significant increase in the number Th17 cells in the CSF during an exacerbation in MS patients that was not observed in the Th1 cells. This may be due to a role of Th17 cells in the exacerbation, but may also be due to the fact that the number of Th1 cells is already so much higher that changes may be more difficult to discern. IL-23 has been found to induce IL-17 expression in human memory CD4 T cells, with a significant proportion of these cells co-expressing IFNγ [58]. Although both Th1 and Th17 cells have been found capable of crossing the blood-brain barrier, the IFNγ+IL-17+ T cells in MS patients appear to cross the blood-brain barrier more efficiently, suggesting that this unique population of effector T cells may have greater encephalitogenic capacity [58].
Several clinical trials have been performed to determine if targeting effector T cells may be beneficial for MS patients. As early as the mid 1980’s, the potential contribution of cytokines in regulating aberant immune responses was recognized, prompting a clinical trial using IFNγ to treat MS patients which resulted in an increased number of exacerbations[59]. It was subsequently found that IFNγ induces apoptosis in human oligodendrocytes, and in MS lesions IFNγ expression colocalizes with apoptotic oligodendrocytes [60,61], indicating that IFNγ contributes to the pathology observed in MS lesions. These observations led to a clinical trial in which a neutralizing antibody specific for IFNγ was administered to MS patients, demonstrating a therapeutic benefit [62]. This trial was conducted at the same time as the EAE experiments in IFNγ-deficient mice and the anti-IFNγ treatment studies in EAE, which resulted in exacerbated disease [15,16]. The contradictory data between the human and mouse treatment studies with anti-IFNγ generated concern that inhibiting IFNγ may have unforeseen negative consequences. The role of IL-12/IL-23 pathways in mediating MS was recently tested in a phase II clinical trial in which MS patients were given a neutralizing antibody to the p40 subunit common to IL-12 and IL-23 [63]. In theory, this antibody should prevent the differentiation of Th1 cells mediated by IL-12 and prevent the production of IL-17 mediated by IL-23. No significant changes in new gadolinium-enhancing lesions or clinical findings were observed. The negative outcome of this study does not necessarily mean that the IL-12/IL-23 is an inappropriate therapeutic target. The MS patient population was quite diverse in disease severity and duration, and since the IL-12/IL-23 pathway is critical during T cell differentiation, many of the patients may have established effector T cell populations that may mediate pathology independent of the IL-12/IL-23 pathway. In addition, it is unclear whether the antibody can and must access the CNS to neutralize T cells in the target organ.
IFNβ, the most commonly prescribed immunomodulatory therapy for relapsing-remitting MS, has already been shown to mitigate effector T cells. IFNβ therapies have been shown to dampen Th1 responses [64], but the ability of IFNβ to attenuate Th17 responses is controversial. In vitro studies of human CD4 T cells found that IFNβ inhibited Th17 cell differentiation and could induce apoptosis in Th17 cells [65,66]. However, an ex vivo analysis of IFNγ and IL-17 mRNA, as well as their respective transcription factors, T-bet and RORC, demonstrated that IFNβ therapy for one year resulted in decreased IFNγ and T-bet levels but no significant changes in IL-17 or RORC expression [67]. Interestingly, decreases in T-bet mRNA levels were only associated with patients who had a clinical benefit from IFNβ therapy, suggesting that T-bet expression may be a prognostic indicator of IFNβ responsiveness in MS patients. This supports the hypothesis that T-bet is critical for T cell encephalitogenicity, irrespective of T cell cytokine phenotype.
Conclusion
Although the exact roles of Th1 and Th17 cells in the development of MS lesions are not established, it appears that both of these effector T cell populations can cause CNS inflammation and demyelinating lesions. The data in mice deficient in various cytokines, trafficking molecules and transcription factors indicate that encephalitogenic T cells have multiple critical molecules that give them the capacity to induce CNS inflammation. The observation that VLA4 high expressing Th1 cells are encephalitogenic, while VLA4 low expressing Th1 cells are not encephalitogenic illustrates that all Th1 cells are not equal in their pathogenic capacity [36]. Similarly, the observation that T-bet positive Th17 cells are encephalitogenic, while T-bet negative Th17 cells are not encephalitogenic demonstrates that Th17 cells differ in their pathogenic potential because of a transcription factor previously known for its role in Th1 cells [27]. The data in EAE does demonstrate that there are pathways mediated by T-bet and Stat 4 that are clearly important in encephalitogenicity [6,7,10,11,27]. In addition, IL-23, IL-1β and IL-6 appear to contribute significantly to disease onset [20,21,27,68–70]. Whether these various transcription factors and cytokines all contribute to a common, yet undefined pathway, or act in a synergistic manner which culminates in encephalitogenicity has still to be determined. Analysis of the various molecules, previously shown to play a vital role in EAE, in MS patients has proven to be complicated and sometimes contrary to what has been observed in EAE. In particular, anti-IFNγ therapy was not beneficial in EAE, but showed promising results in a small clinical trial in MS [62]. However, VLA4 and T-bet expression appear to be involved in effector T cells in MS patients, similar to what has been shown in EAE. Therefore, the focus of research on effector T cells in MS should focus on pathways upstream of the cytokines that define Th1 and Th17 cells, since downstream products, such as IFNγ and IL-17, probably are not critical determinants of whether an effector T cell is capable of trafficking to the CNS and inducing inflammatory demyelination.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pettinelli CB, McFarlin DE. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt1+2- T lymphocytes. J Immunol. 1981;127:1420–1423. [PubMed] [Google Scholar]
- 2.McDonald AH, Swanborg RH. Antigen-specific inhibition of immune interferon production by suppressor cells of autoimmune encephalomyelitis. J Immunol. 1988;140:1132–1138. [PubMed] [Google Scholar]
- 3.Ando DG, Clayton J, Kono D, Urban JL, Sercarz EE. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989;124:132–143. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 4.Waldburger KE, Hastings RC, Schaub RG, Goldman SL, Leonard JP. Adoptive transfer of experimental allergic encephalomyelitis after in vitro treatment with recombinant murine interleukin-12. Preferential expansion of interferon-gamma-producing cells and increased expression of macrophage-associated inducible nitric oxide synthase as immunomodulatory mechanisms. Am J Pathol. 1996;148:375–382. [PMC free article] [PubMed] [Google Scholar]
- 5.Yura M, Takahashi I, Serada M, Koshio T, Nakagami K, Yuki Y, Kiyono H. Role of MOG-stimulated Th1 type “light up” (GFP+) CD4+ T cells for the development of experimental autoimmune encephalomyelitis (EAE) J Autoimmun. 2001;17:17–25. doi: 10.1006/jaut.2001.0520. [DOI] [PubMed] [Google Scholar]
- 6.Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, Hussain RZ, Ratts RB, Sikder D, Racke MK. Silencing T-bet defines critical role in the differentiation of autoreactive T lymphocytes. Immunity . 2004;21:719–731. doi: 10.1016/j.immuni.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Gocke AR, Cravens PD, Ben L, Hussain RZ, Northrop SC, Racke MK, Lovett-Racke AE. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178:1341–1348. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 8.Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Rocken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Racke MK, Burnett D, Pak SH, Albert PS, Cannella B, Raine CS, McFarlin DE, Scott DE. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J Immunol. 1995;154:450–458. [PubMed] [Google Scholar]
- 10.Chitnis T, Najafian N, Benou C, Salama AD, Grusby MJ, Sayegh MH, Khoury SJ. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J Clin Invest. 2001;108:739–747. doi: 10.1172/JCI12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nath N, Prasad R, Giri S, Singh AK, Singh I. T-bet is essential for the progression of experimental autoimmune encephalomyelitis. Immunology . 2006;118:384–391. doi: 10.1111/j.1365-2567.2006.02385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferber IA, Brock S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 14.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 15.Lublin FD, Knobler RL, Kalman B, Goldhaber M, Marini J, Perrault M, D’Imperio C, Joseph J, Alkan SS, Korngold R. Monoclonal anti-gamma interferon antibodies enhance experimental allergic encephalomyelitis. Autoimmunity . 1993;16:267–274. doi: 10.3109/08916939309014645. [DOI] [PubMed] [Google Scholar]
- 16.Heremans H, Dillen C, Groenen M, Martens E, Billiau A. Chronic relapsing experimental autoimmune encephalomyelitis (CREAE) in mice: enhancement by monoclonal antibodies against interferon-gamma. Eur J Immunol. 1996;26:2393–2398. doi: 10.1002/eji.1830261019. [DOI] [PubMed] [Google Scholar]
- 17.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 20.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature . 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 21.Langrish CL, Chen Y, Blumenschein M, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komiyama Y, Nakae S, Matsuki T, Nambu A, Kakuta HS, Suko K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 23.Haak S, Croxford AL, Dreymborg K, Heppner FL, Pouly S, Becher B, Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine nilieu supports de novo differentiation of IL-17-producing T cells. Immunity . 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Bettelli E, Carrier Y, Gao W, Kom T, Strom TB, Oukka M, Weiner HL, Kuchroo VJ. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature . 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 26.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the Th17 lineage. Nature . 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Weiner J, Liu Y, Smith AJ, Huss DJ, Winger R, Peng H, Cravens PD, Racke MK, Lovett-Racke AE. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, Van Kaer L, Shi Y, Das G. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med. 2009;206:2407–2416. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell . 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 30.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science . 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 31.Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci U S A. 2005;102:17101–106. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science . 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 33.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168:2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 34.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature . 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 36.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 38.Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O’Connor PW International Natalizumab Multiple Sclerosis Trial Group. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- 39.Brucklacher-Waldert V, Sturner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain . 2009;132:3329–3341. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 40.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gyulveszi G, Haak S, Becher B. IL-23-driven encephalo-tropism and Th17 polarization during CNS-inflammation in vivo. Eur J Immunol. 2009;39:1864–1869. doi: 10.1002/eji.200939305. [DOI] [PubMed] [Google Scholar]
- 42.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liston A, Kohler RE, Townley S, Haylock-Jacobs S, Comerford I, Caon AC, Webster J, Harrison JM, Swann J, Clark-Lewis I, Korner H, McColl SR. Inhibition of CCR6 function reduces the severity of experimental autoimmune encephalomyelitis via effects on the priming phase of the immune response. J Immunol. 2009;182:3121–3130. doi: 10.4049/jimmunol.0713169. [DOI] [PubMed] [Google Scholar]
- 44.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choriod plexus is required for the intiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 45.Villares R, Cadenas V, Lozano M, Almonacid L, Zaballos A, Martinez C, Varona R. CCR6 regulates EAE pathogenesis by controlling regulatory CD4+ T-cell recruitment to target tissues. Eur J Immunol. 2009;39:1671–1681. doi: 10.1002/eji.200839123. [DOI] [PubMed] [Google Scholar]
- 46.Elhofy A, Depaolo RW, Lira SA, Lukacs NW, Karpus WJ. Mice deficient for CCR6 fail to control chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;213:91–99. doi: 10.1016/j.jneuroim.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olsson T, Zhi WW, Höjeberg B, Kostulas V, Jiang YP, Anderson G, Ekre HP, Link H. Autoreactive T lymphocytes in multiple sclerosis determined by antigen-induced secretion of interferon-gamma. J Clin Invest. 1990;86:981–985. doi: 10.1172/JCI114800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun JB, Olsson T, Wang WZ, Xiao BG, Kostulas V, Fredrikson S, Ekre HP, Link H. Autoreactive T and B cells responding to myelin proteolipid protein in multiple sclerosis and controls. Eur J Immunol. 1991;21:1461–1468. doi: 10.1002/eji.1830210620. [DOI] [PubMed] [Google Scholar]
- 49.Voskuhl RR, Martin R, Bergman C, Dalal M, Ruddle NH, McFarland HF. T helper 1 (Th1) functional phenotype of human myelin basic protein-specific T lymphocytes. Autoimmunity . 1993;15:137–143. doi: 10.3109/08916939309043888. [DOI] [PubMed] [Google Scholar]
- 50.Pelfrey CM, Rudick RA, Cotleur AC, Lee JC, Tary-Lehmann M, Lehmann PV. Quantification of self-recognition in multiple sclerosis by single-cell analysis of cytokine production. J Immunol. 2000;165:1641–1651. doi: 10.4049/jimmunol.165.3.1641. [DOI] [PubMed] [Google Scholar]
- 51.Allegretta M, Nicklas JA, Sriram S, Albertini RJ. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science . 1990;247:718–721. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- 52.Lovett-Racke AE, Trotter JL, Lauber J, Perrin PJ, June CH, Racke MK. Decreased dependence of myelin basic protein-reactive T cells on CD28-mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells. J Clin Invest. 1998;101:725–730. doi: 10.1172/JCI1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burns J, Bartholomew B, Lobo S. Isolation of myelin basic protein-specific T cells predominantly from the memory T-cell compartment in multiple sclerosis. Ann Neurol. 1999;45:33–39. [PubMed] [Google Scholar]
- 54.Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, Martin R. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 55.Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 56.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 57.Ishizu T, Osoeawa M, Mei FJ, Kikuchi H, Tanaka M, Takakura Y, Minohara M, Murai H, Mihara F, Taniwaki T, Kira J. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain . 2005;128:988–1002. doi: 10.1093/brain/awh453. [DOI] [PubMed] [Google Scholar]
- 58.Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, Duquette P, Prat A. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann Neurol. 2009;66:390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- 59.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet . 1987;1:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 60.Pouly S, Becher B, Blain M, Antel JP. Interferon-gamma modulates human oligodendrocyte susceptibility to Fas-mediated apoptosis. J Neuropathol Exp Neurol. 2000;59:280–6. doi: 10.1093/jnen/59.4.280. [DOI] [PubMed] [Google Scholar]
- 61.Vartanian T, Li Y, Zhao M, Stefansson K. Interferon-gamma-induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol Med. 1995;1:732–43. [PMC free article] [PubMed] [Google Scholar]
- 62.Skurkovich S, et al. (2001) Randomized study of antibodies to IFN-gamma and TNF-alpha in secondary progressive multiple sclerosis. Mult Scler. 2001;7:277–84. doi: 10.1177/135245850100700502. [DOI] [PubMed] [Google Scholar]
- 63.Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH Ustekinumab MS Investigators. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomized, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 64.Revel M, Chebath J, Mangelus M, Harroch S, Moviglia GA. Antagonism of interferon beta on interferon gamma: inhibition of signal transduction in vitro and reduction of serum levels in multiple sclerosis patients. Mult Scler. 1995;1(Suppl 1):S5–S11. [PubMed] [Google Scholar]
- 65.Durelli L, Conti L, Clerico M, Boselli D, Contessa G, Ripellina P, Ferrero B, Eid P, Novelli F. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol. 2009;65:499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- 66.Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-beta inhibits human Th17 cell differentiation. J Immunol. 2009;183:5418–5427. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 67.Drulovic J, Savic E, Pekmezovic T, Mesaros S, Stojsavljevic N, Dujmovic-Basuroski I, Kostic J, Vasic V, Mostarica Stojkovic M, Popadic D. Expression of Th1 and Th17 cytokines and transcription factors in multiple sclerosis patients: does baseline T-bet mRNA predict the response to interferon-beta treatment? J Neuroimmunol. 2009;215:90–95. doi: 10.1016/j.jneuroim.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 68.Sutton C, Brereton C, Keogh B, Mills KM, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mendel I, Katz A, Kozak N, Ben-Nun A, Revel M. Interleukin-6 functions in autoimmune encephalomyelitis: a study in gene-targeted mice. Eur J Immunol. 1998;28:1727–1737. doi: 10.1002/(SICI)1521-4141(199805)28:05<1727::AID-IMMU1727>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 70.Okuda Y, Sakoda S, Bernard CC, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int Immunol. 1998;10:703–708. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]