Abstract
Magnetic resonance spectroscopy (MRS) is sensitive to movements, in part due to motion-induced phase and frequency variations that lead to incoherent averaging. For in vivo proton MRS, the unsuppressed or under-suppressed water signal can be used to restore coherent averaging, but this approach results in baseline distortions due to the large water peak. Therefore, a novel water-suppression cycling scheme was developed that alternates between positive and negative residual water signal. Using the residual water signal, the method allows for shot-to-shot phase and frequency correction of individual FIDs and restoration of signal losses due to incoherent averaging, yet near-complete elimination of residual water. It is demonstrated that residual (cycled) water signal can be used to restore metabolite peaks in a brain spectrum from a subject who performed intentional head movements. The ability to correct phase and frequency fluctuations during subject motion is vital for use with adaptive motion correction approaches that ensure proper voxel positioning during head movements.
INTRODUCTION
Proton magnetic resonance spectroscopy (1H MRS) makes it possible to measure the concentration of several metabolites in the human brain in vivo. MRS scans also are non-invasive and repeatable, and may be performed in children and infants. However, subject motion continues to be a substantial problem, and subjects are required to lie still over extended time periods. Subject motion during MRS acquisitions can cause scan-to-scan variations in phase and frequency of 1H MRS signals, resulting in incoherent averaging and reduced signal-to-noise ratio (SNR) (1). The undersuppressed residual water signal can be used to restore coherence of the averaging process (2), by correcting phase and frequency errors from shot to shot. However, a large residual water peak due to incomplete water suppression is generally undesirable since it may cause problems during spectral fitting.
The goal of this work was to develop a phase navigator approach that alternates between a positive and negative residual water signal. The residual water in each individual acquisition can be used to perform shot-to-shot phase and frequency correction. However, when the individual FIDs are added during final signal averaging, the opposing water signals are cancelled, yielding a spectrum with minimal residual water. The new sequence is also insensitive to variations in longitudinal relaxation time (T1) and radiofrequency strength (B1).
METHODS AND THEORY
Studies were performed at 3 Tesla field strength (Siemens TIM Trio, version VB13), using a 12-channel receive-only head coil and the body coil for transmission. The new sequence was evaluated in a phantom containing typical concentration of major brain metabolites, as well as in 3 human volunteers. Prior to scanning, each human subject provided oral and written consent, using a form approved by our local Institutional Review Board.
Cycling of water suppression
As noted above, our navigator approach alternates between a positive and a negative residual water signal from one excitation to the next. This cycling scheme involves four chemical shift-selective RF pulses, as shown in Figure 1A:
90° - τ1 - 90° - τ2 - 180° - τ3 - 180° - τ4 for positive residual water
90° - τ1 - 90° - τ2 - 0° - τ3 - 180° - τ4 for negative residual water
The water-suppression pulses had a Gaussian envelope, and had 25.6ms duration with 35 Hz bandwidth. Each of the four intervals τi was 50ms, resulting in recovery r of longitudinal magnetization (Mz) by about 5% between pulses (since r ~ 50ms/T1 with T1 ~ 1,000ms for brain tissue at 3T). Since each τi interval contains a strong crusher gradient, transverse magnetization is ignored in the remainder of this section.
Figure 1.
A) Pulse sequence for cycling of water suppression cycling. The basic sequence (yielding positive residual water) consists of two 90° and two 180° RF pulses, with a fixed inter-pulse delay and crusher gradients. The first of the two 180° RF pulses is omitted to obtain negative residual water signal. B) and C) show the evolution of the z-magnetization for both cases.
Here, we will perform a first-order analysis of the evolution of longitudinal magnetization for the two conditions (Figure 1B and C). The first two 90° pulses ensure that the longitudinal magnetization Mz2− = Δ at the start of τ2 is close to zero. Therefore, Mz2+ at the end of τ2 is approximately:
| [1] |
The second half of the sequence differs between the two conditions. For positive residual water (Figure 1B), two additional 180° pulses are played out. The first of these inverts z-magnetization:
| [2] |
During the subsequent τ3 interval, Mz recovers by approximately r, yielding Mzpos3+ ≈ −Δ. The second 180° pulse leads to another inversion (Mzpos4− ≈ Δ), resulting in the final amplitude of the positive residual water:
| [3] |
For the second condition of negative residual water (Figure 1C), the first 180° pulse is not played out, leading to
| [4] |
During the subsequent τ3 interval, Mz continues to recover by approximately r, yielding Mzneg3+ ≈ Mzneg3− + r = ≈ Δ + 2 r. The 180° pulse inverts this magnetization, resulting in the final amplitude of the negative residual water:
| [5] |
Therefore, in first order approximation, the positive residual water signal (Eqn. 3) cancels out the negative residual water signal (Eqn. 5). This is true even when small offsets Δ in Mz after the second 90° pulse are present, suggesting that the sequence is insensitive to B1-variations, which tend to alter Mz after the two initial 90° pulses. A numerical simulation was performed to further evaluate these issues (see Results). Also, the dependence of the residual water signal in the human brain on B1-variations was determined for both conditions (positive and negative residual water), by intentionally varying the flip-angle of the 4 water-suppression pulses by a common factor f, with 0.7 ≤ f ≤ 1.3 in steps of 0.05. These measurements were performed in 3 volunteers, using the scanning protocol detailed below.
MR protocol
Three structural MRI scans were obtained in each subject: (1) 3-plane localizer (repetition time (TR) / echo time (TE) = 20/5 ms; field of view(FOV) = 28 × 28 cm2), (2) sagittal high-resolution 3D magnetization-prepared rapid gradient echo (MP-RAGE) (TR/TE/inversion time (TI) = 2500/4.5/1000 ms, 208×256×160 matrix, 1mm3 isotropic resolution), and (3) transversal fluid-attenuated inversion recovery (FLAIR) (TR/TE/TI = 9100/83/2500 ms, FOV=23 × 23 cm2, 44 slices of 3mm thickness, no gap).
1H MRS was performed in the frontal gray matter (voxel size 20×20×20 mm3) using a Point RESolved Spectroscopy (PRESS) sequence (3). The data acquisition parameters were: TE 30ms, TR 3sec, 1024 data-points and 1200 Hz spectral bandwidth with 32 averages. The original pulse sequence was modified to allow saving of individual FIDs, and water suppression was alternated between negative and positive residual water from shot to shot, as described above. Spectra were acquired without intentional head movement, and while rapidly oscillating the head up and down at small amplitude (nodding, simulated tremor).
Data processing
Data were processed in Matlab. First, the phase of each individual FID was adjusted such that the phase of the first time point in each FID (i.e. echo maximum) was 0° (for positive residual water) or 180° (for negative residual FIDs). Next, FIDs were multiplied with a decaying exponential (time constant 512ms), zero-filled to 16K, and Fourier transformed. The frequency of the residual water signals was then determined (from peak maximum) for each resulting spectrum, and the water signals centered at 4.7 ppm (= fH2O). The individual spectra were then summed, yielding the final phase and frequency-corrected spectrum with attenuated residual water. Spectra were analyzed using the spectral analysis package, LCModel (4). Metabolite peak areas were quantified with LCModel, using a 3T basis data set that included the resonances of N-acetyl-aspartate (NAA), creatine plus phosphocreatine (Cr), total choline (Cho), myo-inositol (mI), glutamate, glutamine, and GABA. The effects of motion correction were evaluated by calculating the percent differences in NAA, Cho, and Cr concentrations, as well as the Cho/Cr ratios, between scans with motion and the baseline scan (without motion) for each subject. Significance of differences between data acquired with and without motion was then determined using paired t-tests.
Spectra with positive and negative residual water [denoted S+(f) and S−(f)] were also averaged using a weighting factor w, as follows:
| [6] |
The weight was chosen such that the summed residual water resonance was minimal, using a least-squares fitting algorithm. Specifically, maximum of the magnitude spectrum | S(f) | was minimized in a range of ±0.25 ppm around the water resonance. The effect of weighting on the signal-to-noise ratio (SNR) on the combined spectrum was calculated based on the assumption that the noise in the two conditions (positive and negative residual water) is Gaussian. It is then easy to demonstrate that the SNR(w) of the weighted average is:
| [7] |
where SNR(w=1.0) reflects the SNR of the non-weighted spectrum.
RESULTS
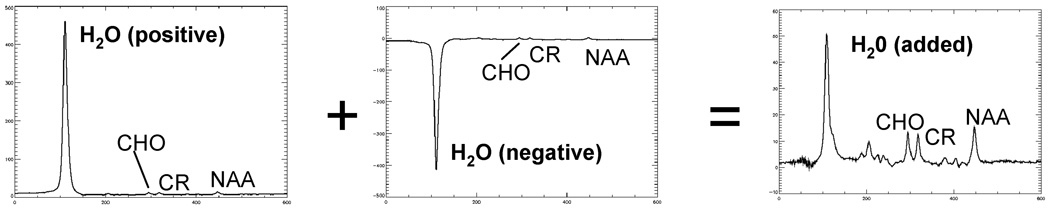
Figure 2 shows the result of a scan in a subject without head motion. A substantial amount of residual water signal is visible for the scans with positive (left) and negative (center) water condition. However, when the two spectra are added, very little water signal remains, yielding a flat baseline across the entire spectrum. Across 3 subjects, the residual water signal varied between 5.1 and 5.8% of the unsuppressed water amplitude, and between 0.33 and 0.52% (average 0.45%) for the summed spectra. Therefore, the final averaging step reduced the residual water approximately 10-fold compared to the initial (positive or negative) conditions. Using an optimized average as per Eq. [6], the residual water amplitude was between 0.06 and 0.24% of the unsuppressed water, for an additional 2.5-fold improvement over simple averaging.
Figure 2.
In vivo spectra from frontal gray matter acquired with positive (left) and negative residual water signal (TE/TR = 30/3000ms, 16 averages per condition). Summation of the two spectra results in a spectrum with little residual water and a well-defined baseline (right).
B1 and T1-sensitivity
To assess the B1-sensitivity of the sequence, the flip angle of the water suppression RF pulses was modified by a common scaling factor during the Bloch simulation. This simulates imperfections in the RF amplitudes on the scanner, or changes in B1 due to head movement. Parameters were typical for in vivo MRS of the brain (τ = 50ms, T1 = 900ms). Figure 3A demonstrates that the residual Mz after averaging the two conditions is within 1% of the original M0 for a variation in flip angles as large as ±25% (±2dB). Therefore, the proposed sequence is insensitive to variations in B1 even without additional scaling the positive versus negative water signals during final averaging.
Figure 3.
Effect of varying the B1-field strength of water suppression pulses on the positive and negative residual water signal. The black line shows the calculated effect based on evolution of z-magnetization. The colored lines represent in vivo measurements in the frontal cortex of 3 volunteers. While the amplitude of the residual water signals shows some dependency on the RF power, the summed water signal is attenuated over 100-fold over a range of ± 25% (± 2dB) relative to the unsuppressed water.
The result of these simulations was confirmed in vivo, by varying the flip angles of the water suppression pulses by a common factor on the scanner (Figure 3). The measurements are in general agreement with the simulations, both in terms of the B1-dependency of the positive and negative residual water, and the near perfect cancellation of the summed water signal over a relatively wide range of RF power.
Furthermore, we simulated the effect of T1 variations on the water suppression sequence (Figure 4). The sum of positive plus negative residual water signals is below 1% for T1-values >700ms. Therefore, the sequence is also insensitive to T1 for typical brain T1-values at 1.5 or 3T (800–1000ms). Of note, the residual CSF signal (with its long T1) is attenuated more than the residual water signal from brain tissue.
Figure 4.
Calculated effect of the T1-value on the positive and negative residual water signals, based on evolution of z-magnetization. While the amplitude of the residual water signals shows some dependency on the T1 value, the summed water signal is attenuated over 100-fold for T1-values greater than 700ms.
Phase correction during intentional head movement
The efficacy of the water suppression cycling scheme was evaluated in a human volunteer who intentionally moved his head as described above. Figure 5 shows the frontal gray matter spectra when the subject held still (left) and intentionally oscillated his head by a small amount (simulated tremor). The phase fluctuation was ±5° without motion and ±102° with motion, resulting in almost complete signal loss with simple averaging (Fig. 5, center). Phase-correction of individual FIDs, using the residual (cycled) water signal, almost restored the original spectrum and resulted in a flat baseline (Fig. 5, right). Quantitatively, the Cr signal-to-noise ratio (SNR) of the spectrum without motion was 35. With head motion, the SNR decreased to 4 without shot-to-shot correction, whereas phase and frequency of individual FIDs all but restored the SNR (32). Likewise, metabolite peak areas were less than 10% of the initial value when no shot-to-shot correction was applied, but were restored to within 85–90% with correction.
Figure 5.
Frontal gray matter spectra without subject motion (left), motion (simulated tremor) without phase correction (center), and motion with phase correction (right). Each spectrum was acquired at short echo time (TE/TR 30/3000ms, 32 averages).
Furthermore, to evaluate whether CSF flow might impair phase navigators, a 20mm voxel was placed in pure white matter (CSF content 1.5%) and also across the left and right lateral ventricles (CSF content 45.7%). However, inclusion of the ventricles did not increase the standard deviation of the water phase (±3.2° with large CSF percentage versus ±4.6° in pure white matter, across 32 FIDs).
DISCUSSION
The new water-suppression cycling scheme presented allows phase and frequency correction of individual FIDs using the residual water signal and restoration of signal losses due to incoherent averaging. One prior publication proposed cycling between inverted and non-inverted metabolites (5); however, it is difficult to obtain consistent inversion across the entire spectrum. Conversely, our new method only manipulates the water resonance, leaving metabolite signals intact. While the amplitude of the residual water signal in individual FIDs is approximately ±5% relative to the unsuppressed water, yielding sufficient SNR to perform phase and frequency correction, the water signal is attenuated another 10-fold in the final averaged spectrum. Therefore, the final spectra show minimal residual water, which tends to minimize baseline distortions.
One of the advantages of using the residual water signal to correct the phase and frequency of individual FIDs is that the (residual) water spins simultaneously undergo the exact same coherence pathway as the metabolite spins. Therefore, any effects of subject motion on MR signals will be identical for metabolite and water signals. In contrast, methods that rely on separate measurements for water and metabolite signals, such as having a separate water (navigator) acquisition that precedes the metabolite acquisition (6), may result in discrepancies between the 2 acquisitions, and possibly phase and frequency errors. One issue that might affect all existing methods for phase navigation is that of phase-errors induced by CSF flow in voxels with large CSF content. However, an experiment demonstrated that even inclusion of nearly 50% CSF in the MRS voxel did not affect the shot-to-shot reproducibility of the navigator phase (no intentional motion).
The new method presented is insensitive to changes in T1 and B1, similar to existing, conventional techniques (7,8). This insensitivity is important to ensure proper water suppression for a variety of brain tissues, such as gray matter and white matter, imperfect adjustment of RF power, or application at different field strengths. Of note, this B1 and T1-insensitivity is present in the summed spectra, whereas the individual positive and negative FIDs show some dependence on B1 and T1. However, changing the weighting factor w during final spectrum summation (Eq. 6) makes it possible to achieve near-perfect water suppression even when there is an asymmetry between the positive and negative residual water conditions, with typically minimal loss of SNR.
In vivo studies demonstrate that the water cycling scheme is very efficient at correcting phase fluctuations due to gross head movements. In scans with motion, the phases of individual FIDs were sufficiently variable to markedly attenuate metabolite peaks when signals were simply averaged, whereas correcting the phase of individual FIDs almost restored the original spectra. Likewise, the residual water signal may be used to correct frequency changes due to head motion, by shifting the water peak of each individual FID to the correct position in the spectrum. The ability to correct phase and frequency fluctuations even during substantial movements will be vital for use in conjunction with adaptive motion correction schemes that ensure proper voxel positioning during head movements.
ACKNOWLEDGEMENTS
We would like to thank Drs. Linda Chang and Steven Buchthal for assistance in some of the data collection and technical assistance. This work was partially supported by the National Institute on Drug Abuse (K02-DA16991 and 1R01 DA021146 to T.E.), core resources from the National Center for Research Resources (G12-RR003061), and the National Institute on Neurological Disorders and Strokes (U54-NS56883).
REFERENCES
- 1.Felblinger J, Kreis R, Boesch C. Effects of physiologic motion of the human brain upon quantitative 1H-MRS: analysis and correction by retro-gating. NMR Biomed. 1998;11(3):107–114. doi: 10.1002/(sici)1099-1492(199805)11:3<107::aid-nbm525>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Helms G, Piringer A. Restoration of motion-related signal loss and line-shape deterioration of proton MR spectra using the residual water as intrinsic reference. Magnetic Resonance in Medicine. 2001;46(2):395–400. doi: 10.1002/mrm.1203. [DOI] [PubMed] [Google Scholar]
- 3.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Annals of the New York Academy of Sciences. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 4.Provencher S. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001 Jun;14(4) doi: 10.1002/nbm.698. 2001;14(4):260-264. [DOI] [PubMed] [Google Scholar]
- 5.Dreher W, Leibfritz D. New method for the simultaneous detection of metabolites and water in localized in vivo 1H nuclear magnetic resonance spectroscopy. Magn Reson Med. 2005;54(1):190–195. doi: 10.1002/mrm.20549. [DOI] [PubMed] [Google Scholar]
- 6.Zaitsev M, Speck O, Hennig J, Buechert M. Single-voxel MRS with prospective motion correction and retrospective frequency correction. NMR Biomed. 2010;23(3):325–332. doi: 10.1002/nbm.1469. [DOI] [PubMed] [Google Scholar]
- 7.Ogg RJ, Kingsley PB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B. 1994;104(1):1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]
- 8.Ernst T, Hennig J. Improved water suppression for localized in vivo 1H spectroscopy. J Magn Reson B. 1995;106(2):181–186. doi: 10.1006/jmrb.1995.1030. [DOI] [PubMed] [Google Scholar]