Abstract
Adenosine A2A receptor antagonism provides a promising approach to developing nondopaminergic therapy for Parkinson’s disease (PD). Clinical trials of A2A antagonists have targeted PD patients with L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesia (LID) in an effort to improve parkinsonian symptoms. The role of adenosine in the development of LID is little known, especially regarding its actions via A1 receptors. We aimed to examine the effects of genetic deletion and pharmacological blockade of A1 and/or A2A receptors on the development of LID, on the induction of molecular markers of LID including striatal preprodynorphin and preproenkephalin (PPE), and on the integrity of dopaminergic nigrostriatal neurons in hemiparkinsonian mice. Following a unilateral 6-hydroxydopamine lesion A1, A2A and double A1-A2A knockout (KO) and wild-type littermate mice, and mice pretreated with caffeine (an antagonist of both A1 and A2A receptors) or saline were treated daily for 18–21 days with a low dose of L-DOPA. Total abnormal involuntary movements (AIMs, a measure of LID) were significantly attenuated (p<0.05) in A1 and A2A KOs, but not in A1-A2A KOs and caffeine-pretreated mice. An elevation of PPE mRNA ipsilateral to the lesion in WT mice was reduced in all KO mice. In addition, neuronal integrity assessed by striatal dopamine content was similar in all KOs and caffeine-pretreated mice following 6-hydroxydopamine lesioning. Our findings raise the possibility that A1 or A2A receptors blockade might also confer a disease-modifying benefit of reduced risk of disabling LID, whereas the effect of their combined inactivation is less clear.
Keywords: basal ganglia, 6-hydroxydopamine, A1 and A2A knockout, adenosine receptor antagonist, preprodynorphin, preproenkephalin
1. Introduction
Blockade of adenosine A2A receptors is being pursued as a non-dopaminergic alternative or adjunctive treatment of Parkinson’s disease (PD). Several studies have investigated the usefulness of A2A receptor antagonism to treat L-DOPA-induced dyskinesia (LID), a complication from current PD therapy, in both animal models and clinical trials (Chen, 2003; Morelli, et al., 2007). The use of A2A antagonists for symptomatic benefit with reduced risk of adverse effects in PD and LID is based inter alia on the discrete CNS distribution of the A2A receptor and its colocalization with D2 receptors in the ‘indirect’ pathway of the basal ganglia motor circuitry (Ferre, et al., 1997; Kase, 2001; Fredholm, et al., 2003). Elimination or blockade of A2A receptors expressed by forebrain neurons attenuates LID and related behaviors in hemiparkinsonian rodents or parkinsonian non-human primates (Bibbiani, et al., 2003; Xiao, et al., 2006).
Adenosine also activates the adenosine A1 receptor, which – in contrast to discretely expressed A2A receptor – is widely distributed throughout the CNS including the hippocampus and cortex as well as on the striatal neurons of the ‘direct’ and ‘indirect’ pathways of the basal ganglia (Fastbom, et al., 1987; Ferre, et al., 1996; Johansson, et al., 1997; Tohyama, 1998) making a selective action difficult to deduce. It has been proposed that blocking A1 receptors on striatonigral neurons of the direct pathway may facilitate motor activity by disinhibiting the motor stimulant actions of colocalized dopamine D1 receptors on these neurons, whereas blocking A2A receptors on striatopallidal neurons of the indirect pathway may produce a parallel behavioral activation by mimicking the motor stimulant actions of colocalized D2 receptors on these neurons (Ferre et al., 1997). By contrast, presynaptic A1 and A2A receptors (e.g., those colocalized on corticostriatal nerve terminals) can inhibit and activate, respectively, adenylyl cyclase and thus transmitter release (van Calker, et al., 1979; Olah and Stiles, 1995; Dunwiddie and Masino, 2001; Fredholm, et al., 2005; Ciruela, et al., 2006).
Thus adenosine may modulate LID through cooperative or opposing actions on two of its receptors in the CNS. To explore the roles of these receptors alone and in combination in a mouse model of LID in PD, we characterized single A1 and A2A knockout (KO), as well as double A1-A2A receptor KO phenotypes in (6-hydroxydopamine-lesioned) hemiparkinsonian mice treated daily with L-DOPA for three weeks. To avoid genetic background confounds mice were generated from double heterozygote crosses in a congenic C57Bl/6 background. In addition to this genetic approach to addressing adenosine receptor involvement in LID, we investigated the effect of pharmacological blockade of adenosine receptors using the widely consumed non-specific adenosine antagonist caffeine. The investigation of caffeine was prompted by preliminary clinical data that raised the possibility of a link between higher levels of caffeine consumption among PD patients and a reduced risk of subsequent dyskinesia development (Schwarzschild, et al., 2003). We chose both a dose of caffeine, which elicits hyperlocomotion (15 mg/kg), and a lower dose of caffeine (3 mg/kg), which is capable of modifying neuroplasticity (as in that of conditioning preference) without necessarily producing a motor stimulant effect (Fredholm, et al., 1999).
2. Results
2.1. Effect of adenosine receptor knockout on 6-OHDA-induced neurotoxicity
Previous studies showed that inactivation of A2A receptors by either genetic depletion or pharmacological blockade (caffeine and more specific A2A antagonists, but not a specific A1 receptor antagonist) can protect against brain dopaminergic neurotoxicity induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Chen, et al., 2001). Accordingly, we first determined whether the dopaminergic lesion induced by 6-OHDA differs between control mice and KO or caffeine pre-treated mice. We found that genotype (Table 1) or pharmacological (Table 2) blockade did not affect levels of dopamine and its metabolite DOPAC in the 6-OHDA-lesioned and contralateral (non-lesioned) striata.
Table 1.
Neurochemical measure of nigrostriatal innervation in wild-type and adenosine receptor KO mice chronically treated with L-DOPA after a unilateral 6-OHDA lesion
| Genotype | DA (pm/mg tissue) | DOPAC (pm/mg tissue) |
|---|---|---|
| WT (n=12) | ||
| Ipsilateral (lesioned) | 4.2 ± 1.4* | 0.0 ± 0.0* |
| Contralateral (intact) | 143.6 ± 7.5 | 11.3 ± 1.7 |
| A2A KO (n=9) | ||
| Ipsilateral (lesioned) | 4.3 ± 3.0* | 0.7 ± 0.5* |
| Contralateral (intact) | 129 ± 10 | 15.9 ± 7.4 |
| A1 KO (n=8) | ||
| Ipsilateral (lesioned) | 6.9 ± 3.0* | 0.4 ± 0.4* |
| Contralateral (intact) | 120 ± 14 | 9.7 ± 0.9 |
| A1-A2A KO (n=7) | ||
| Ipsilateral (lesioned) | 10.4 ± 8.2* | 1.2 ± 0.5* |
| Contralateral (intact) | 118 ± 18 | 9.6 ± 0.9 |
p < 0.05 versus respective contralateral (intact) striatum. The dopamine levels in the intact (right) side were not significantly different between wild-type and each of the three KO genotypes (p>0.05, Student’s t-test).
Table 2.
Neurochemical measure of nigrostriatal innervation in unilateral 6-OHDA-lesioned mice treated daily with L-DOPA following pretreatment with saline or caffeine (3mg/kg)
| Treatment | DA (pm/mg tissue) | DOPAC (pm/mg tissue) |
|---|---|---|
| Saline (n=21) | ||
| Ipsilateral (lesioned) | 1.3 ± 0.6* | 1.2 ± 0.4* |
| Contralateral (intact) | 82.5 ± 8.3 | 13.1 ± 6.5 |
| Caffeine (n=24) | ||
| Ipsilateral (lesioned) | 3.6 ± 1.7* | 0.3 ± 1.3* |
| Contralateral (intact) | 88.8 ± 10.6 | 14.8 ± 7.6 |
p < 0.05 versus respective contralateral striatum. The dopamine levels in the intact (right) side were not significantly different between wild-type and each of the three KO genotypes (p>0.05, Student’s t-test).
2.2. Effect of adenosine receptor knockout on behavioral sensitization
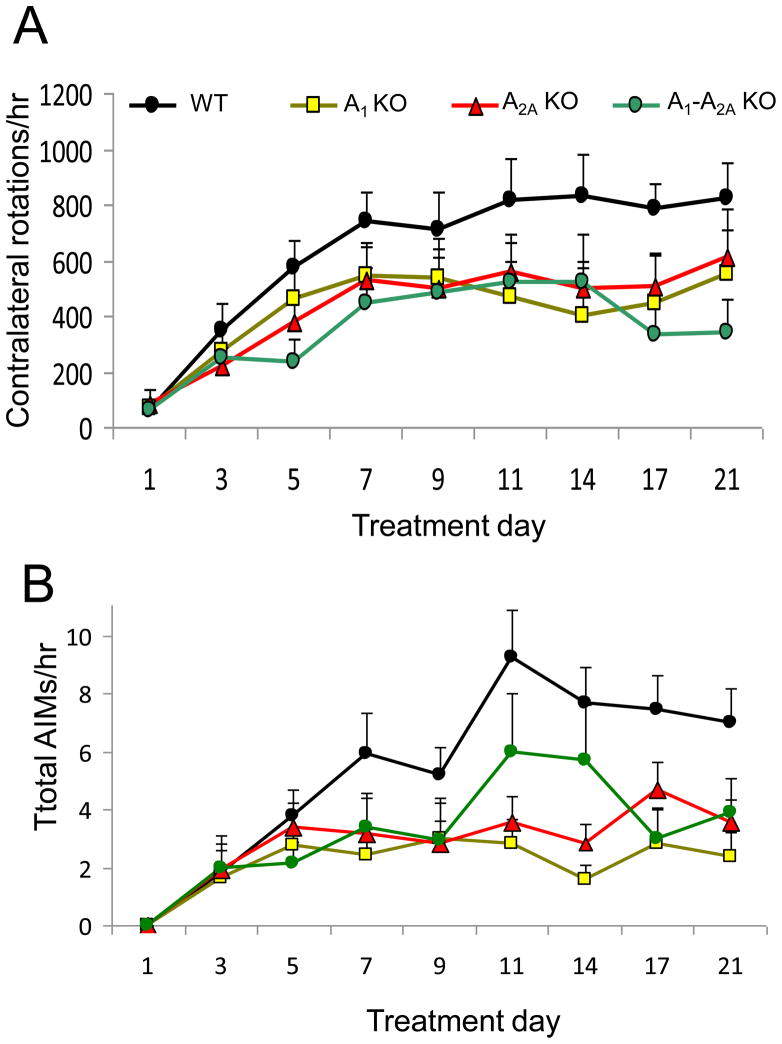
Following daily L-DOPA treatment, the hemiparkinsonian mice developed behavioral sensitization, as recorded by contralateral rotations and dyskinesia, quantified by an abnormal involuntary movements (AIMs) scale (Fig. 1). Acutely (on day 1), responses to L-DOPA were indistinguishable between adenosine receptor genotypes. Chronically, rotational sensitization on the plateau phase (day 11–21) in A1 KO, A2A KO or A1-A2A double KO mice showed a trend of attenuation over the 21 days, but the difference was not statistically significant (p=0.12, repeated measures model) in comparison to WT (Fig. 1A). Total AIMs in A1 KO, or A2A KO were attenuated significantly in comparison to WT (p=0.0003 for A1 KO and p=0.014 for A2A KO, pairwise comparison with Bonferroni correction), but not in A1-A2A double KO mice (p=0.091, pairwise comparison without correction) (Fig. 1B). The attenuation appeared incomplete in all KO genotypes, with responses in KO mice during the plateau phase still significantly increased compared to their initial (day 1) response (p<0.05, Fig. 1). Importantly, the effects of A1 and A2A deletion on total AIMs were non-additive. In fact, for total AIMs, the A1-A2A double KO mice appeared to have higher levels than either the A1 KO or A2A KO mice at time points of days 11 and 14.
Fig. 1.
Contralateral rotations and abnormal involuntary movements (AIMs) in A1, A2A and A1-A2A double KO mice compared to WT. Mice were treated daily with L-DOPA (2 mg/kg, i.p.) in combination with benserazide (2 mg/kg, i.p.) for 3 weeks. A, Contralateral turns in mice treated with L-DOPA. B, Total AIMs in mice treated with L-DOPA. Significant differences in the total AIMs in the plateau phase were observed between the WT (n=12) and the A1KO (n=9), or A2AKO (n=8) groups (p<0.05 for each comparison, Bonferroni correction).
2.3. Effect of caffeine on L-DOPA-induced behavioral sensitization
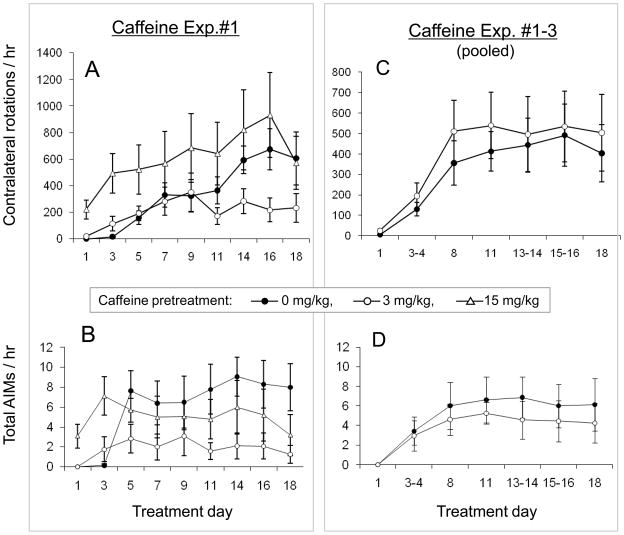
Given the possible link between caffeine consumption among PD patients and a reduced risk of dyskinesia development (Schwarzschild et al., 2003), we tested the effect of caffeine on LID. In an initial experiment a low dose of caffeine (3 mg/kg, ip) significantly attenuated total AIMs (Fig. 2). By contrast, a higher caffeine dose (15 mg/kg, ip) did not significantly reduce the extent of AIMs that developed, though its chronic effects may have been confounded by its acute (day 1) stimulation of AIMs (as well as contralateral rotations) (Fig. 2A–B) The attenuation observed with low dose caffeine warranted efforts at replication and comparison to saline pretreatment. Despite the initial observation the pooled results of all three experiments with low dose of caffeine (Fig. 2C–D) showed no significant effect on AIMs that developed in response to repeated L-DOPA administration (p>0.05, repeated measures model), and similarly no effect on sensitized rotational responses (p>0.05, repeated measures model, Fig. 2C–D). The basis for this variability is not clear. However, an initial motor stimulant or potentiating effect, which was particularly prominent at the higher dose of 15 mg/kg caffeine, may confound any attenuating effect of caffeine on chronic contralateral rotations. On day 1, contralateral rotations in the caffeine group (17 ±6.0 per hour) were in fact significantly higher than that in saline group (0.4 ±0.2 per hour, Student’s t-test, p=0.017; Fig. 2A). Not surprisingly and consistent with our previous finding (Yu, et al., 2006), the higher dose of caffeine (15 mg/kg) produced an even greater contralateral rotation on day 1 (221 ±70 per hour) versus control (0.4 ±0.3 per hour; p=0.019), and did not significantly attenuate either L-DOPA-induced rotations or AIMs (p>0.05, Fig. 2).
Fig. 2.
L-DOPA-induced contralateral rotations and AIMs in caffeine-treated mice compared to saline controls. Mice were treated daily for 18 days with L-DOPA (2 mg/kg, i.p. in combination with benserazide 2 mg/kg, i.p.) 10 mins after i.p. adminstration of saline or caffeine. (A,B) Rotational responses and total AIMs in an initial experiment with three treatment groups, 0 (n=7), 3 (n=6) or 15 (n=7) mg/kg caffeine (p<0.05, comparing 3 mg/kg caffeine to saline). (C,D) A composite of rotational responses and total AIMs from the initial and two subsequent experiments that compared only the low dose 3 mg/kg caffeine (n=24) with saline (n=21) pretreatments (p>0.05, comparing saline to caffeine).
2.4. Modulation of striatal gene expression
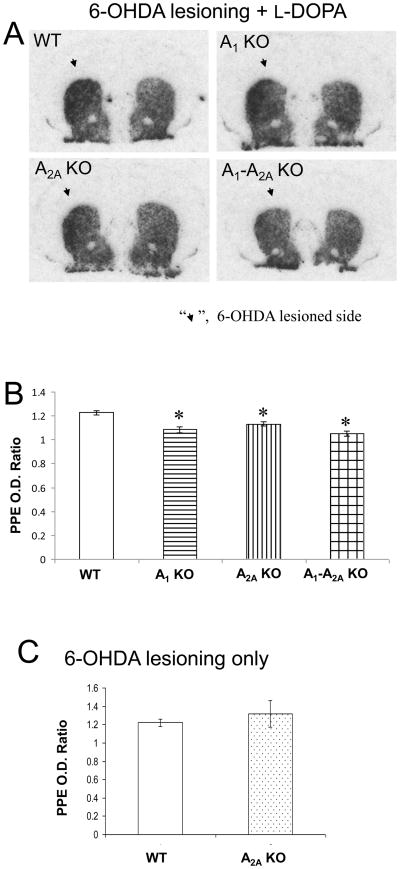
In WT mice striatal preproenkephalin (PPE) mRNA levels were significantly elevated on the 6-OHDA-lesioned side and showed a similar increase after lesioning with subsequent chronic L-DOPA treatment (Fig. 3), consistent with prior observations of the activation of PPE-expressing striatopallidal neurons with the development of LID in parkinsonian rodents and primates (Bedard, et al., 1999; Zeng, et al., 2000; Winkler, et al., 2002; Morissette, et al., 2006). Elevation of PPE following 6-OHDA lesioning in otherwise untreated WT mice was undiminished in A2A KO, but absent in all adenosine receptor KOs after chronic L-DOPA treatment. They showed significantly lower striatal PPE mRNA ipsilateral to 6-OHDA lesioning after chronic L-DOPA treatment compared to their WT littermates (Fig. 3). The PPD on the lesioned side tended to be reduced in comparison to the non-lesioned side (Student’s t-test, p=0.07) in A2A KO mice, consistent with our previous finding (Fredduzzi, et al., 2002), in A1 KO (p=0.09) and A1-A2A KO (p=0.09) (data not shown). Comparison of these markers in the contralateral (non-lesioned) striatum showed no difference in PPE and PPD (p>0.05 for all KOs mice, Student’s t-test) in comparison with WT control, suggesting that L-DOPA treatment had no differential effect on gene expression in unlesioned striata across genotypes.
Fig. 3.
Reduction of PPE mRNA in lesioned striata of adenosine receptor KO mice following chronic L-DOPA treatment. mRNA levels in the 6-OHDA-lesioned striata (arrow) were quantified as optical density (OD) at the mid-striatum level and expressed as a ratio to OD of the contralateral (unlesioned) striatum. (A,B) Chronic treatment with L-DOPA significantly reduced striatal PPE levels in 6-OHDA lesioned A1 KO (n=4), A2A KO (n=5) and A1-A2A KO (n=4) mice (*p<0.05; Student’s t-test compared with the WT group, n=5). (C) 6-OHDA lesioning itself increased striatal PPE on the lesioned side in WT (n=6). This increase was not altered in A2A KO (n=6)
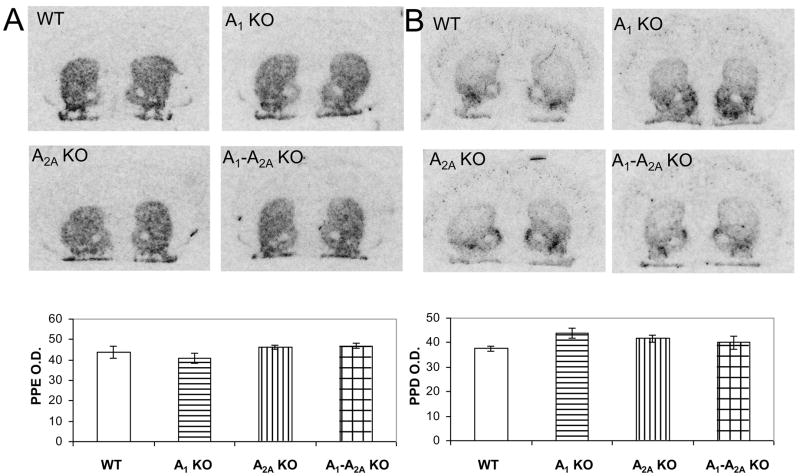
To address the possibility of a change in prepeptides gene expression due to the gene KO itself, we also studied the baseline changes (without L-DOPA treatment or 6-OHDA lesioning), if any, of PPE and PPD in the colony of A1, A2A and A1-A2A double KO mice. We found that there was no difference of the striatal levels of peptide gene expression among the different genotypes (p>0.05, one-way ANOVA and Student’s t-test, Fig. 4), consistent with previous findings (Chen, et al., 1999).
Fig. 4.
Basal mRNA expression levels of PPE (A) and PPD (B) in WT, A1 KO, A2A KO and A1-A2A double KO mice. No differences in levels for either mRNA were observed among the different genotypes, WT (n=6), A1 KO (n=3), A2A KO (n=5) and A1-A2A KO (n=4) (p>0.05, one-way ANOVA).
Consistent with adenosine A1-A2A receptor KO phenotype, low dose of caffeine (3 mg/kg) pretreatment tended to reduce the expression of PPE on the 6-OHDA lesioned side (n=6, p=0.08, Student’s t-test), corresponding to caffeine’s non-significant apparent effect on L-DOPA-induced dyskinesia. However, a higher dose of caffeine (15 mg/kg) had no effect on LID or PPE expression in comparison to the saline treated group (p=0.71, Student t-test, data not shown).
3. Discussion
Genetic elimination of either adenosine receptor – either A1 or A2A – attenuated the development of AIMs in response to L-DOPA in a 6-OHDA lesion model of PD. However, a double KO of both adenosine receptor subtypes and caffeine had no clear effect on rotational sensitization and AIMs. In this 6-OHDA lesion model of PD and LID, striatal dopamine levels were unaffected in KO or caffeine-treated mice, suggesting that A1 and A2A receptors facilitate its maladaptive neuroplasticity rather than the nigrostriatal lesion that is required for its full manifestation. In addition, induction of PPE, the activation of which in striatopallidal neurons links to the development of LID in parkinsonian rodents and primates (Bedard et al., 1999; Zeng et al., 2000; Winkler et al., 2002; Morissette et al., 2006), was attenuated in adenosine receptor KO striata.
3.1. The role of A1 and A2A receptors in L-DOPA-induced dyskinesia
Earlier studies have shown that blockade of A2A receptors can improve symptoms of PD and decrease neurodegenerative changes in PD models (Chen, 2003; Chen, et al., 2007). Here we show that LID can be reduced by A2A receptor depletion, which produced no apparent protective phenotype in a 6-OHDA model in keeping with our previous findings (Fredduzzi et al., 2002; Xiao et al., 2006). A2A depletion has consistently shown no effect on the neurochemical (dopamine content) or neuroanatomical (dopamine transporter ligand binding) indicators of dopaminergic neuron injury in the unilateral 6-OHDA-leison model used here [(Fredduzzi et al., 2002; Xiao et al., 2006); Tables 1 and 2]. This lack of neuroprotection by A2A elimination in this relatively progressive model of PD contrasts the neuroprotective phenotype of the A2A receptor blockade seen with the more acutely acting neurotoxin MPTP (Chen, et al., 2001; Ikeda, et al., 2002; Pierri, et al., 2005; Carta, et al., 2009), and may reflect a greater opportunity for KO adaptation in the setting of a more gradual injury. In any event, the lack of neuroprotection against 6-OHDA in the A2A KO reduces the likelihood of a confounding attenuation in the nigrostriatal lesion in these mice. Thus the reductions in LID reported here suggest a role for adenosine receptors in the long-term maladaptive neuroplasticity underlying LID rather than in cell death.
The facilitative role of A2A in LID neuroplasticity may include both pre- and post-synaptic mechanisms, interacting with dopaminergic neurotransmission at a network level, as previously discussed (Fredduzzi et al., 2002; Xiao et al., 2006). The effects of blocking A1 receptor in LID are less well understood, as there is clear evidence for multiple sites of presynaptic, as well as postsynaptic expression and actions of A1 receptors in the striatum. For example, presynaptic striatal A1 receptors inhibiting glutamate (Ambrosio, et al., 1996; Ciruela et al., 2006), dopamine or acetylcholine release in the striatum and elsewhere might contribute to this complexity (Fredholm and Dunwiddie, 1988; Jin, et al., 1993; Borycz, et al., 2007). In addition, it is not known whether the absence of A1 receptors alters the dopamine receptor function that contributes to LID.
Finally, the activation of PPE-expressing striatopallidal neurons is linked with the development of LID in parkinsonian rodents and primates (Bedard et al., 1999; Zeng et al., 2000; Winkler et al., 2002; Morissette et al., 2006). Several studies have described that 6-OHDA lesion itself produces an increase in PPE as well (Henry, et al., 1999; Carta, et al., 2002; Tel, et al., 2002; Ravenscroft, et al., 2004); for a review see (Xu, et al., 2005). The increased PPE expression was attenuated in adenosine A1 or A2A KOs compared to WT mice after chronic L-DOPA treatment, not after 6-OHDA lesioning alone in A2A KOs, suggesting an interaction of dopaminergic and adenosinergic systems and possibly accounting for the attenuation of LID, as discussed previously (Xiao et al., 2006). The activation of PPD-expressing striatonigral neurons is also linked with the development of LID in parkinsonian rodents (Cenci, et al., 1998; Winkler et al., 2002). Surprisingly, A2A or A1 receptor deletion had indistinguishable effects on striatal PPE and PPD expression despite the widely divergent distributions of these receptors and their effects on motor activity.
3.2. A1 and A2A receptor interactions in L-DOPA-induced dyskinesia
Although the attenuation of LID was partial in both the A1 and A2A KO mice, there was no clear additivity or synergy of their attenuating effects on LID in the double KO. In seeming contrast, the combination of A1 and A2A antagonists (Karcz-Kubicha, et al., 2003) produced an additivity of their individual motor-activating effects. Adenosine receptor mechanisms and hence their interactions in acute motor activation likely differ from those in LID. Similarly, less than additive effects of D1 and D2 dopamine receptor antagonists have been reported on complex adaptive behaviors, whereas acute effects of these drugs in combination are typically at least additive on motor activity (Schneider, et al., 1991). In LID A1 and A2A receptors may have sufficiently redundant effects in parallel to preclude an additional effect of blocking one receptor after the other has been inactivated. Alternatively, A1 and A2A receptor roles in LID may occur sequentially–either when colocalized to a single cell or when interacting in different cells at a network level [(e.g. review by (Lopes, et al., 2002; Xu et al., 2005)].
The results of this study suggest that both A1 as well as A2A receptor antagonists could be useful therapeutically to lower the risk of L-DOPA-induced dyskinesia in PD patients. Our demonstration of a similar dependence on A1 receptors prompted a complementary pharmacological test of the mixed A1-A2A caffeine in this model of LID. We found that caffeine with the doses of 3 and 15 mg/kg showed no significant attenuation on L-DOPA-induced dyskinesia or rotational sensitization, possibly due to its general motor stimulant actions even at a low dose of 3 mg/kg considering significantly higher contralateral rotations on day 1 in mice pretreated with caffeine (Fig. 2). The initial motor stimulating effect may confound a chronic attenuation effect of caffeine on L-DOPA-induced behavioral sensitization. The results also suggest that blocking both A1 and A2A receptors simultaneously, as occurs with caffeine use, may not provide a better prophylaxis against dyskinesia development than that of a specific A1 or A2A antagonist alone.
4. Experimental procedures
4.1. Generation of adenosine receptor KO mice
Wild-type (WT) control (A1+/+, A2A+/+), A1 KO (A1−/−, A2A+/+), A2A KO (A1+/+, A2A−/−) and A1-A2A double KO (A1−/−, A2A−/−) mice were generated by double heterozygous mating (A1+/−, A2A+/− × A1+/−, A2A+/−) and genotyped by PCR analysis of tail DNA as described previously (Bastia, et al., 2005; Kachroo, et al., 2005; Xiao et al., 2006). The double heterozygotes were the offspring of crosses between homozygous A1 KO and homozygous A2A KO mice from lines that we rendered congenic for the C57Bl/6 strain background (BanburyConference, 1997).
All experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with an approval from the animal subjects review board of Massachusetts General Hospital. We have made all efforts to minimize the number of animals used and their suffering (along with giving the mice the best care).
4.2. 6-hydroxydopamine lesion of striatum in mice
All mice were maintained in home cages with a 12 h light/dark cycle. The dopaminergic nigrostriatal pathway on the left side of each mouse was lesioned by a stereotactic intrastriatal infusion of 10 ug 6-hydroxydopamine (6-OHDA, hydrobromide, Sigma, St.Louis,MO) as described previously (Fredduzzi et al., 2002; Xiao et al., 2006). On the day of surgery, mice were anesthetized with avertin-HCl (2% 2,2,2-tribromoethanol and 1% amyl alcohol; 10–15 ml/kg, i.p.). Two microliters of freshly prepared 6-OHDA bromide salt (5 μg/μl in 0.05% ascorbic acid and shielded from light) were delivered by a microinfusion pump (0.5 μl/min) into the left striatum at the following coordinates (from bregma: 0.5 mm anterior, 2 mm lateral, and 2.8 mm ventral) (Franklin and Paxinos, 1997). To minimize damage to noradrenergic neurons, the mice were pretreated with desipramine hydrochloride (25 mg/kg, i.p.).
The mice were fed with both veterinary Nutri-Cal (Frenchtown, NJ) and human infant nutritional supplements (Enfamil) immediately after surgery for several days, followed by soft food (Bio-Serv Nutra-Gel Diet) to help ensure survival. Twelve days following surgery each mouse underwent a cylinder test (Cenci and Lundblad, 2007), in which asymmetry of forepaw placement on the inner wall of a plexiglass cylinder was used as an indirect assessment of lesion extent. Mice with <90% of dopamine loss (~15% of the lesioned mice) in the lesioned striatum (compared to the contralateral, non-lesioned striatum) were excluded from all data analysis (except for that of dopamine content).
The mice included in the final analyses of the KO experiment comprised 29 male and 9 female mice (3–14 months old with average age 7.5 months) and were balanced for age, weight and gender across genotypes (n=8–12 in each genotype group). Unlesioned WT and A2A KO mice were used for a control study of lesioning effects on neuropeptides, and comprised 7 male and 5 female mice (with an average age of 8 months) and were balanced for age, weight and gender across genotypes (n=6 in each genotype group). The mice included in the caffeine experiment comprised 52 males (3 months old, n=21 for saline, n=24 for low dose 3 mg/kg of caffeine, n=7 for high dose 15 mg/kg caffeine), and were balanced for home cage residence, cylinder test results and weight loss after lesion across treatment groups.
4.3. Pharmacological treatment and behavioral testing
Two weeks after 6-OHDA lesioning, mice were treated daily with L-DOPA (L-3,4-Dihydroxyphenylalanine, Sigma, St.Louis,MO, freshly prepared, 2 mg/kg ip) for 18–21 days. In the adenosine receptor KO experiment, benserazide (Sigma, St.Louis,MO, 2 mg/kg ip in saline), as previously used (Lundblad, et al., 2004; Xiao et al., 2006), was administered 20 min prior to each dose of L-DOPA. In an initial caffeine experiment, 20 mice were divided into three groups: 0 (n=7), 3 (n=6) and 15 (n=7) mg/kg caffeine (Sigma, St.Louis,MO) ip in saline, which preceded by 10 min each daily ip dose of L-DOPA (2 mg/kg, mixed with benserazide 2 mg/kg in saline, to reduce the number of injections). Additional two caffeine experiments were performed at the lower 3 mg/kg dose with saline control (n=14) and caffeine (n=18, in saline) treatment groups. Pooled data from the three caffeine (low dose) experiments are presented (Fig. 2C–D).
Behaviors – total AIMs (including axial, limb and orolingual AIMs) (Cenci and Lundblad, 2005) and rotations – were recorded every 2–3 days for one hour. Dyskinetic behaviors were assessed and scored by an observer blind to the genotype or treatment, and based on each of the following subscale: 1, axial AIMs (i.e., twisted posture of the neck or the upper body toward the contralateral side); 2, forelimb AIMs (i.e. jerky movements or purposeless fluttering movement of the fore paw); 3, orolingual AIMs (i.e., twitching of orofacial muscles, empty jaw movemnts, and tongue protrusion), as previously established and validated (Lundblad et al., 2004). The AIM subscales were evaluated together 15, 30, 45 and 60 min after L-DOPA injection, and each mouse was observed for 1 min.
Both contralateral and ipsilateral turns were recorded immediately after L-DOPA administration for 60 min in 10 min time bins, using an automated rotometry system (San Diego Instruments, San Diego, CA), as described previously (Xiao et al., 2006). Briefly, each mouse was placed at the center of 1 of 12 opaque glass flat-bottom bowls and connected to the lower end of a cable tether by a rubber band snugly fitted around the chest. The upper end of the cable is attached to a swivel box linked to a computer interface.
4.4. Molecular and neurochemical assessments
One to three days after the last L-DOPA injection for both KO and caffeine experiments, whole brains were dissected out, frozen on dry ice and stored at −80 °C. The brains through the striata were sectioned with a cryostat (Shandon) at thickness of 12 μm as described before (Benn, et al., 2004; Xiao et al., 2006). The rostral half of the striata was used for mRNA expression quantification of preproenkephalin (PPE) and preprodynorphin (PPD) by in situ hybridization histochemistry using radiolabeled oligonucleotide 35S, as described previously (Benn et al., 2004; Xiao et al., 2006). The probe sequence (5′ to 3′) for PPE mRNA was as follows: ATC TGC ATC CTT CTT CAT GAA ACC GCC ATA CCT CTT GGC AAT GAT CTC. The probe sequence (5′ to 3′) for PPD mRNA was as follows: ATG GGG GCT TCC TGC GGC GCA TTC GCC CCA AGC TGA AGT GGG ACA. Optical densities (O.D.) of the mRNA transcripts were quantified for the striatum using Image J 1.40g software (National Institutes of Health, USA). Approximately three sections through the striatum, at rostral and middle levels, were analyzed for each mouse.
The remaining frozen brains containing the caudal half of the striata were sectioned to a thickness of 400 μm at −17° C, and the striata were micropunched (Stoelting, 1.25 mm diameter). The cores from the left and right striata were analyzed by HPLC with electrochemical detection for dopamine (DA) and DOPAC content (Chen et al., 2001; Xu, et al., 2006).
4.5. Statistical analysis
All data are expressed as group average ± SEM. The difference between treatments (WT versus KO; caffeine versus saline) during the plateau phase of LID (from day 11 on for each analysis) was evaluated using a repeated measures model with a compound symmetry covariance structure. No significant time by treatment interaction was observed in any model, so only the main effect of group is reported. If a global difference between groups was observed, post-tests were performed with Bonferroni correction to account for multiple comparisons. A Wilcoxon signed rank test was used to compare each of the plateau measurements to the day 1 measurements for each genotype. The analysis was generated using SAS software, Version 9.1 of the SAS System for Windows (SAS Institute Inc., Cary, NC) and GraphPad. Student’s t-test was used for the remaining statistical analyses of molecular correlates and dopamine loss (Tables).
Acknowledgments
This work was supported by NIH grants NS054978 and NS060991, DoD grant W81XWH-04-1-0881, and the Michael J. Fox Foundation. We thank Cody Desjardins and Yuehang Xu for excellent technical assistance.
Abbreviations
- AIM
abnormal involuntary movement
- KO
knockout
- L-DOPA
L-3,4-dihydroxyphenylalanine
- 6-OHDA
6-hydroxydopamine
- LID
L-DOPA-induced dyskinesia
- PD
Parkinson’s disease
- PPD
preprodynorphin
- PPE
preproenkephalin
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrosio AF, Malva JO, Carvalho AP, Carvalho CM. Modulation of Ca2+ channels by activation of adenosine A1 receptors in rat striatal glutamatergic nerve terminals. Neurosci Lett. 1996;220:163–6. doi: 10.1016/s0304-3940(96)13252-3. [DOI] [PubMed] [Google Scholar]
- BanburyConference. Mutant mice and neuroscience: recommendations concerning genetic background. Banbury Conference on genetic background in mice. Neuron. 1997;19:755–9. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- Bastia E, Xu YH, Scibelli AC, Day YJ, Linden J, Chen JF, Schwarzschild MA. A crucial role for forebrain adenosine A(2A) receptors in amphetamine sensitization. Neuropsychopharmacology. 2005;30:891–900. doi: 10.1038/sj.npp.1300630. [DOI] [PubMed] [Google Scholar]
- Bedard PJ, Blanchet PJ, Levesque D, Soghomonian JJ, Grondin R, Morissette M, Goulet M, Calon F, Falardeau P, Gomez-Mancilla B, Doucet JP, Robertson GS, DiPaolo T. Pathophysiology of L-dopa-induced dyskinesias. Mov Disord. 1999;14(Suppl 1):4–8. [PubMed] [Google Scholar]
- Benn CL, Farrell LA, Cha JH. Neurotransmitter receptor analysis in transgenic mouse models. Methods Mol Biol. 2004;277:231–60. doi: 10.1385/1-59259-804-8:231. [DOI] [PubMed] [Google Scholar]
- Bibbiani F, Oh JD, Petzer JP, Castagnoli N, Jr, Chen JF, Schwarzschild MA, Chase TN. A2A antagonist prevents dopamine agonist-induced motor complications in animal models of Parkinson’s disease. Exp Neurol. 2003;184:285–94. doi: 10.1016/s0014-4886(03)00250-4. [DOI] [PubMed] [Google Scholar]
- Borycz J, Pereira MF, Melani A, Rodrigues RJ, Kofalvi A, Panlilio L, Pedata F, Goldberg SR, Cunha RA, Ferre S. Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J Neurochem. 2007;101:355–63. doi: 10.1111/j.1471-4159.2006.04386.x. [DOI] [PubMed] [Google Scholar]
- Carta AR, Kachroo A, Schintu N, Xu K, Schwarzschild MA, Wardas J, Morelli M. Inactivation of neuronal forebrain A receptors protects dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurochem. 2009;111:1478–89. doi: 10.1111/j.1471-4159.2009.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta AR, Pinna A, Cauli O, Morelli M. Differential regulation of GAD67, enkephalin and dynorphin mRNAs by chronic-intermittent L-dopa and A2A receptor blockade plus L-dopa in dopamine-denervated rats. Synapse. 2002;44:166–74. doi: 10.1002/syn.10066. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–706. [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. Utility of 6-hydroxydopamine lesioned rats in the preclinical screening of novel treatments for Parkinson disease. In: LeDoux M, editor. Animal models of movement disorders. Chapter B7. Elsevier Inc; Boston, MA: 2005. pp. 193–208. [Google Scholar]
- Cenci MA, Lundblad M. Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson’s disease in rats and mice. Curr Protoc Neurosci. 2007;Chapter 9(Unit 9):25. doi: 10.1002/0471142301.ns0925s41. [DOI] [PubMed] [Google Scholar]
- Chen JF. The adenosine A(2A) receptor as an attractive target for Parkinson’s disease treatment. Drug News Perspect. 2003;16:597–604. doi: 10.1358/dnp.2003.16.9.829342. [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. Journal of Neuroscience. 1999;19:9192–200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, Beilstein MA, Hackett E, Fink JS, Low MJ, Ongini E, Schwarzschild MA. The role of the D(2) dopamine receptor (D(2)R) in A(2A) adenosine receptor (A(2A)R)-mediated behavioral and cellular responses as revealed by A(2A) and D(2) receptor knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1970–5. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, Geiger J, Lopes LV, de Mendonca A. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol. 2007;83:310–31. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Jr, Schwarzschild MA. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J Neurosci. 2001;21:RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26:2080–7. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annual Review of Neuroscience. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Fastbom J, Pazos A, Probst A, Palacios JM. Adenosine A1 receptors in the human brain: a quantitative autoradiographic study. Neuroscience. 1987;22:827–39. doi: 10.1016/0306-4522(87)92962-9. [DOI] [PubMed] [Google Scholar]
- Ferre S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends in Neurosciences. 1997;20:482–7. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferre S, O’Connor WT, Svenningsson P, Bjorklund L, Lindberg J, Tinner B, Stromberg I, Goldstein M, Ogren SO, Ungerstedt U, Fredholm BB, Fuxe K. Dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission in the rat strioentopenduncular pathway and its modulation by adenosine A1 receptor-mediated mechanisms. Eur J Neurosci. 1996;8:1545–53. doi: 10.1111/j.1460-9568.1996.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates. Academic press; San Diego: 1997. [Google Scholar]
- Fredduzzi S, Moratalla R, Monopoli A, Cuellar B, Xu K, Ongini E, Impagnatiello F, Schwarzschild MA, Chen JF. Persistent behavioral sensitization to chronic L-DOPA requires A2A adenosine receptors. Journal of Neuroscience. 2002;22:1054–62. doi: 10.1523/JNEUROSCI.22-03-01054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacological Reviews. 1999;51:83–133. [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Cunha RA, Svenningsson P. Pharmacology of adenosine A2A receptors and therapeutic applications. Current Topics in Medicinal Chemistry. 2003;3:413–26. doi: 10.2174/1568026033392200. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release? Trends Pharmacol Sci. 1988;9:130–4. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Henry B, Crossman AR, Brotchie JM. Effect of repeated L-DOPA, bromocriptine, or lisuride administration on preproenkephalin-A and preproenkephalin-B mRNA levels in the striatum of the 6-hydroxydopamine-lesioned rat. Exp Neurol. 1999;155:204–20. doi: 10.1006/exnr.1998.6996. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kurokawa M, Aoyama S, Kuwana Y. Neuroprotection by adenosine A2A receptor blockade in experimental models of Parkinson’s disease. Journal of Neurochemistry. 2002;80:262–70. doi: 10.1046/j.0022-3042.2001.00694.x. [DOI] [PubMed] [Google Scholar]
- Jin S, Johansson B, Fredholm BB. Effects of adenosine A1 and A2 receptor activation on electrically evoked dopamine and acetylcholine release from rat striatal slices. Journal of Pharmacology & Experimental Therapeutics. 1993;267:801–8. [PubMed] [Google Scholar]
- Johansson B, Georgiev V, Lindstrom K, Fredholm BB. A1 and A2A adenosine receptors and A1 mRNA in mouse brain: effect of long-term caffeine treatment. Brain Research. 1997;762:153–64. doi: 10.1016/s0006-8993(97)00378-8. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Orlando LR, Grandy DK, Chen JF, Young AB, Schwarzschild MA. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J Neurosci. 2005;25:10414–9. doi: 10.1523/JNEUROSCI.3660-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Muller CE, Fuxe K, Goldberg SR, Popoli P, Ferre S. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28:1281–91. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Kase H. New aspects of physiological and pathophysiological functions of adenosine A2A receptor in basal ganglia. Bioscience, Biotechnology & Biochemistry. 2001;65:1447–57. doi: 10.1271/bbb.65.1447. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JA. Adenosine A(2A) receptor facilitation of hippocampal synaptic transmission is dependent on tonic A(1) receptor inhibition. Neuroscience. 2002;112:319–29. doi: 10.1016/s0306-4522(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16:110–23. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Morelli M, Di Paolo T, Wardas J, Calon F, Xiao D, Schwarzschild MA. Role of adenosine A2A receptors in parkinsonian motor impairment and l-DOPA-induced motor complications. Prog Neurobiol. 2007;83:293–309. doi: 10.1016/j.pneurobio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Morissette M, Dridi M, Calon F, Hadj Tahar A, Meltzer LT, Bedard PJ, Di Paolo T. Prevention of levodopa-induced dyskinesias by a selective NR1A/2B N-methyl-D-aspartate receptor antagonist in parkinsonian monkeys: implication of preproenkephalin. Mov Disord. 2006;21:9–17. doi: 10.1002/mds.20654. [DOI] [PubMed] [Google Scholar]
- Olah ME, Stiles GL. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu Rev Pharmacol Toxicol. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- Pierri M, Vaudano E, Sager T, Englund U. KW-6002 protects from MPTP induced dopaminergic toxicity in the mouse. Neuropharmacology. 2005;48:517–24. doi: 10.1016/j.neuropharm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Ravenscroft P, Chalon S, Brotchie JM, Crossman AR. Ropinirole versus L-DOPA effects on striatal opioid peptide precursors in a rodent model of Parkinson’s disease: implications for dyskinesia. Exp Neurol. 2004;185:36–46. doi: 10.1016/j.expneurol.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Schneider LH, Watson CA, Gibbs J, Smith GP. Infra-additivity of combined treatments with selective D1 and D2 receptor antagonists for inhibiting sucrose reinforcement. Brain Res. 1991;550:122–4. doi: 10.1016/0006-8993(91)90413-p. [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA, Chen JF, Tennis M, Messing S, Kamp C, Ascherio A, Holloway RG, Marek K, Tanner CM, McDermott M, Lang AE the Parkinson Study Group. Relating caffeine consumption to Parkinson’s disease progression and dyskinesias development. Mov Disord. 2003;18:1682–1683. [Google Scholar]
- Tel BC, Zeng BY, Cannizzaro C, Pearce RK, Rose S, Jenner P. Alterations in striatal neuropeptide mRNA produced by repeated administration of L-DOPA, ropinirole or bromocriptine correlate with dyskinesia induction in MPTP-treated common marmosets. Neuroscience. 2002;115:1047–58. doi: 10.1016/s0306-4522(02)00535-3. [DOI] [PubMed] [Google Scholar]
- Tohyama M, Takatsuji K. Atlas of neuroactive substances and their receptors in the rat. Oxford University Press; New York: 1998. [Google Scholar]
- van Calker D, Muller M, Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Bjorklund A, Cenci MA. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson’s disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2002;10:165–86. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]
- Xiao D, Bastia E, Xu YH, Benn CL, Cha JH, Peterson TS, Chen JF, Schwarzschild MA. Forebrain adenosine A2A receptors contribute to L-3,4-dihydroxyphenylalanine-induced dyskinesia in hemiparkinsonian mice. J Neurosci. 2006;26:13548–55. doi: 10.1523/JNEUROSCI.3554-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Bastia E, Schwarzschild M. Therapeutic potential of adenosine A(2A) receptor antagonists in Parkinson’s disease. Pharmacol Ther. 2005;105:267–310. doi: 10.1016/j.pharmthera.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Xu K, Xu Y, Brown-Jermyn D, Chen JF, Ascherio A, Dluzen DE, Schwarzschild MA. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J Neurosci. 2006;26:535–41. doi: 10.1523/JNEUROSCI.3008-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Schwarzschild MA, Chen JF. Cross-sensitization between caffeine- and L-dopa-induced behaviors in hemiparkinsonian mice. Neurosci Lett. 2006;393:31–5. doi: 10.1016/j.neulet.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Zeng BY, Pearce RK, MacKenzie GM, Jenner P. Alterations in preproenkephalin and adenosine-2a receptor mRNA, but not preprotachykinin mRNA correlate with occurrence of dyskinesia in normal monkeys chronically treated with L-DOPA. European Journal of Neuroscience. 2000:12–104. doi: 10.1046/j.1460-9568.2000.00988.x. [DOI] [PubMed] [Google Scholar]