Abstract
Reversible inactivation of the basolateral amygdala (BLA) disrupts the acquisition and expression of conditioned defeat (CD), an ethological model of conditioned fear, suggesting that the BLA may be a critical component of the neural circuit mediating behavioral plasticity associated with the experience of social defeat. We have also shown that this effect is N-methyl-D-aspartic acid (NMDA) receptor-dependent, because infusion of D,L-2-amino-5-phosphovalerate (APV) into the BLA also impairs the acquisition of CD. APV is a non-selective NMDA antagonist, however, thus it disrupts the entire heteromeric receptor complex, making it difficult to distinguish the relative contributions of either the NR2A or NR2B receptor subtypes on the acquisition of CD. There is ample evidence, however, that the NR2B subunit of the NMDA receptor in the amygdala is critical for mediating long-term potentiation and plasticity related to fear learning. The purpose of the present experiment was to determine whether infusion of ifenprodil, a selective antagonist of the NR2B subunit, into the BLA would block the acquisition (but not expression) of CD. In Experiment 1, infusion of ifenprodil immediately before defeat training significantly decreased submissive behaviors and restored territorial aggression when hamsters were later paired with a non-aggressive intruder (NAI). Conversely, infusion of ifenprodil immediately before CD testing failed to inhibit the expression of submissive behaviors in previously defeated hamsters. These results support the hypothesis that the BLA is a critical site for the plasticity underlying social defeat-induced changes in behavior.
Keywords: Social defeat, conditioned fear, stress, anxiety, defensive behaviors
1. Introduction
Over the past several years our lab has utilized an ethologically-based model of conditioned fear called conditioned defeat (CD), wherein Syrian hamsters (Mesocricetus auratus) are first defeated by a larger, more aggressive opponent (the resident aggressor, or RA) in the resident aggressor's cage. Upon subsequent exposure to a smaller, non-aggressive intruder (NAI), the defeated hamster will display an array of submissive and defensive behaviors instead of their normal territorial aggression [14]. Strikingly, this learned response is elicited even when the defeated animal is tested in its own home cage, and it persists for at least one month post-defeat in the majority of animals [5]. We maintain that developing an understanding of how the brain changes in response to social defeat in hamsters will offer important insights into the mechanisms of experience-induced behavioral plasticity and perhaps into the mechanisms whereby social stress increases social avoidance and other depressive-like symptoms.
Previous studies in our lab have suggested that the basolateral amygdala (BLA) is a critical component of the neural circuit mediating the acquisition and expression of CD and that neural activity underlying the defeat experience is NMDA receptor-dependent. Thus, we have demonstrated that reversible inactivation of the BLA using muscimol significantly impairs both the acquisition and expression of CD [6], results that are consistent with studies using more traditional models of fear learning such as Pavlovian fear conditioning and fear-potentiated startle (FPS) [8, 3, 17]. Next, we have shown that infusion of APV, an NMDA receptor antagonist, into the BLA also blocks the acquisition and expression of CD [7]. This is also consistent with a wide body of evidence suggesting that the acquisition of fear learning is mediated via NMDA receptors [16, 1, 2, 21].
NMDA receptors contain of two families of receptor subunits, NR1 and NR2 [15], and within the amygdala two subtypes of the NR2 subunit have been identified, NR2A and NR2B [12]. Because APV is a non-selective NMDA antagonist, it blocks the entire receptor complex, making it impossible to distinguish the relative contributions of each subunit to constitutive neural transmission in the BLA versus a role in the actual synaptic plasticity surrounding the defeat experience. Several studies, however, now suggest that the NR2B subunit plays a more critical role in the plasticity mediating acquisition of conditioned fear. For example, Rodrigues and colleagues [16] have shown that infusion of ifenprodil, a selective antagonist of the NR2B receptor subtype, into the basolateral complex of the amygdala causes a significant impairment in the acquisition of auditory and contextual fear conditioning. More recently, Walker and Davis [21] have shown that blockade of the NR2B subunit via infusion of CP101,606 into the amygdala inhibits the acquisition (but not expression) of FPS. Together, these findings provide strong evidence that the NR2B subunit in the amygdala plays a critical role in fear memory formation. The previously discussed fear conditioning studies, however, use models that are comparatively simple in terms of the causative stimuli as well as the motor outputs involved. CD, on the other hand, is a complex, ethologically relevant social behavior with relatively undefined antecedents and a complex and variable outcome.
In the present study, we tested the hypothesis that NMDA receptors in the BLA play a critical role in the synaptic plasticity related to the defeat experience by selectively blocking the NR2B subtype of the NMDA receptor. We infused ifenprodil into the BLA either before initial defeat training (acquisition) or before testing with the NAI (expression). If the NR2B subunit is a critical for the formation of fear memories related to social defeat, the infusion of ifenprodil should impair the acquisition, but not expression, of CD.
2. Materials and Methods
2.1 Animals and housing conditions
Male Syrian hamsters, weighing 120-130 g at the beginning of the experiment, were obtained from Charles River Laboratories (New York, NY) and group housed for one week prior to surgery. Hamsters serving as RA's were individually housed and weighed between 150-180 g, while non-aggressive intruders (NAI) were group housed and weighed between 100-110 g at the start of the experiment. All animals were housed in a temperature and humidity-controlled room on a 14:10 h light/dark cycle with lights off at 11:00 h and kept in clear polycarbonate cages (20 × 40 × 20 cm) with wire tops and food and water available ad libitum. All testing occurred in the first three hours of the dark phase of the daily light/dark cycle to minimize circadian variation of the behaviors. All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with National Institutes of Health and United States Department of Agriculture guidelines.
2.2 Surgical Procedures
Hamsters were anesthetized with sodium pentobarbital (90 mg/kg, i.p.) and placed into a Kopf stereotaxic frame (David Kopf Instruments, Tujunga, CA). Guide cannula (26 gauge stainless steel; Plastics One, Roanoke, VA) were bilaterally implanted and aimed at the BLA (0.4 mm posterior and 3.9 mm lateral to bregma, and 2.1 mm below dura). The guide cannula was secured to the skull using cyanoacrylate ester gel, wound clips and dental acrylic. A removable obturator needle was kept in place to maintain patency of the cannula. Following surgery, all animals were handled daily and their obturators removed and replaced into the cannula to habituate each animal to the infusion procedure. During the injection procedure, a 33-gauge needle with a 4.2 mm projection from the base of the cannula guide was lowered into the BLA to give a final dorsal-ventral depth of 6.3mm. This was done in order to minimize tissue damage in and around the amygdala.
2.3 Social defeat and behavioral testing
Hamsters were matched by weight and randomly assigned to experimental or control groups. Animals were transported from the colony room to the behavioral testing room on the day of defeat training. Training sessions consisted of one 15-min exposure to the RA in the aggressor's home cage, upon which the subject was reliably attacked by the RA within 60 seconds. This training duration is based on previous studies from our lab showing that a single 15 minute defeat results in reliable levels of conditioned defeat during testing with the NAI. The testing session occurred 24 hours later. Subjects were again transported to the same testing room and were exposed for 5 min to a NAI in the experimental animals’ own home cage. All training and testing sessions were recorded on VHS tape, transferred to CD-ROM and scored by observers blind to the experimental condition using Noldus Observer (version 3; Noldus Information Technology, Wageningen, Netherlands). The total duration of four classes of behaviors were scored during the test session: (a) social behavior (stretch, approach, sniff, nose touching and flank marking), (b) non-social behavior (locomotion, exploration, grooming, nesting, feeding and sleeping), (c) submissive/defensive behaviors (flight, avoidance, tail up, upright, side defense, full submissive posture, stretch attend, head flag, attempted escape from cage, and (d) aggressive behaviors (upright and side offense, chase and attack, including bite).
2.4 Drug Infusions and site verification
Ifenprodil (Sigma, 200ng/200μl and 400ng/200μl) dissolved in a 10% solution of dimethyl sulfoxide (DMSO) or vehicle control (200μl 10% DMSO in saline) was infused bilaterally into the BLA over a 1 min period using a Hamilton syringe connected to a 33-gauge needle via polyethylene tubing. The needle was kept in place for an additional 1-min before being removed to ensure complete diffusion of the drug after which the dummy was replaced. Training or testing began 20 min after infusion. At the conclusion of the experiment, hamsters were given a lethal dose of sodium pentobarbital and infused with 200nl of India ink in order to verify the location of the cannula placement. The brains were removed and flash frozen in dry ice and placed in a -80° C freezer. The brains were then blocked and sectioned on a cryostat at 30μm and stained with neutral red. Sections were cover slipped with DPX mountant and examined under light microscopy for placement of injection. Only animals with injection sites within 0.5 mm of the BLA were included in the statistical analysis.
2.5 Experiment 1a: Acquisition of conditioned defeat
The goal of Experiment 1 was to determine whether blockade of the NMDA receptor subunit NR2B in the BLA would inhibit the acquisition of CD. Animals (n=40) were matched by weight and randomly assigned to one of three conditions. Hamsters received ifenprodil (200 or 400ng in 200μl vehicle) or vehicle alone 20 min before being placed in the home cage of the RA for 15 min. One the following day, animals were tested drug-free in their own home cage against a NAI for 5 min.
2.6 Experiment 1b: No Defeat Control
Considering the high levels of aggression observed in ifenprodil-treated animals in Experiment 1, we performed an additional study wherein ifenprodil or vehicle was infused in undefeated animals to ensure that the heightened levels of aggression were not a by-product of the ifenprodil infusion, itself. Animals (n=20) with guide cannula aimed at the BLA were infused with ifenprodil (400ng/200μl vehicle) or vehicle alone (200μl) 20 min prior to being placed in the cage of a resident aggressor for 15 min without the aggressor being present. On the following day, animals were tested for 5 min in their own home cage in the presence of a NAI, as described above.
2.7 Experiment 2a: Expression of conditioned defeat
The purpose of Experiment 2 was to determine whether blockade of the NMDA receptor subtype NR2B in the BLA would inhibit the expression of CD. Animals (n=32) were matched by weight and defeated by a RA for 15 min. On the following day, animals were randomly assigned to one of two conditions and infused with either ifenprodil (400ng in 200μl vehicle) or vehicle alone 20 min prior to being tested in their own home cage for 5 min with a NAI. In this experiment, we used only the most effective dose of ifenprodil based on the results from Experiment 1.
2.8 Experiment 3: State-dependency controls
The reduction in submissive behaviors observed in Experiment 1 may have been the result of a difference in the drug state of subjects receiving only pre-training or pre-testing infusions of ifenprodil. In order to assess this possibility, we infused ifenprodil (400ng/200μl) or vehicle 20 minutes before defeat training and then again 20 minutes before testing with the NAI (n=10) to ensure that CD was not restored when animals were trained and tested in the same drug state.
2.9 Statistical analysis
For all experiments, data was analyzed using a one-way between-subjects analysis of variance (ANOVA) with dose as the between-subjects factor. In cases where variance between groups were not homogenous, data was analyzed using Mann-Whitney U test when comparing only two groups and Kruskal-Wallis test when comparing three groups. Significant differences for all analyses were ascribed at p < 0.05. Statistically significant differences were analyzed using a LSD post-hoc test to compare all pairwise differences. Exact probabilities and test values have been omitted for simplification and clarity of the presentation of these results.
3. Results
3.1 Histology
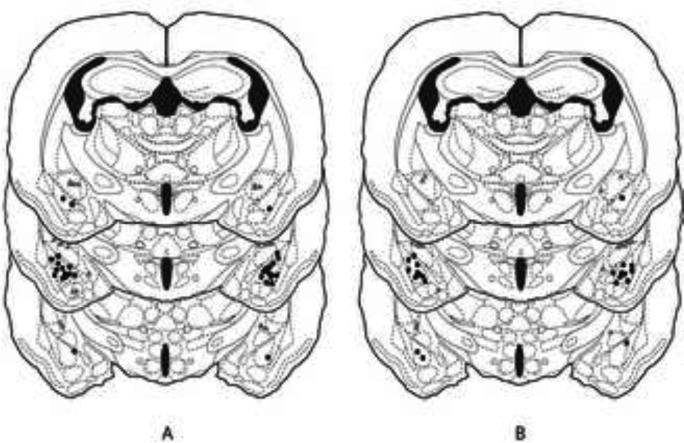
Figures 1a and 1b shows the injections sites for animals in Experiments 1 and 2, respectively. Only bilateral placements of the injection needles within 500μm of the BLA were included in the analysis.
Figure 1.
Histological reconstructions of injection sites for animals receiving infusions of ifenprodil or vehicle into the BLA in Experiments 1 (A) and 2 (B). Black dots represent the site of one or more injections into the BLA and the gray squares are the misplaced injection sites. Drawings are adapted from Morin and Wood [12].
In Experiment 1, 24 of 40 animals met this criterion. Of the remaining 16 animals, 7 had unilateral placement of the injection needle while 2 subjects had injection sites that missed the BLA bilaterally. Further, subjects that had unilateral placement of the cannula in the BLA were grouped into an ‘anatomical control group’ for comparison purposes. Of the remaining 7 subjects, 3 were excluded because the cap fell off before training/testing could begin, 3 were excluded due to occluded cannula at the time of drug/ink injection, and 1 because of video malfunction.
In Experiment 2, 15 of 32 animals met the criteria outlined above. Of the remaining 17 animals, 5 had unilateral placement of the injection needle while 10 subjects had injection sites that missed the BLA bilaterally. The remaining 2 subjects were excluded because of clogged cannula at the time of drug/ink injection.
3.2 Experiment 1: Effect of ifenprodil on acquisition of CD
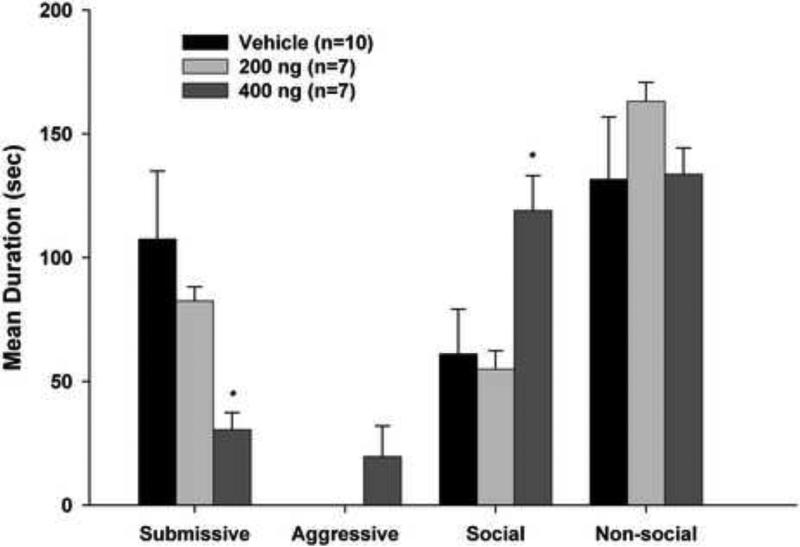
As shown in Figure 2, infusion of ifenprodil significantly and dose-dependently decreased submissive behaviors compared to vehicle infusions. Subjects receiving the high dose of ifenprodil exhibited a significant reduction in submissive behaviors compared with both the low dose and vehicle animals (p < 0.05 for both comparisons). Infusion of ifenprodil also reinstated aggressive behaviors, while no aggression was observed in animals receiving vehicle. Social behaviors were also significantly greater in hamsters infused with high dose ifenprodil compared with animals in the control group (p < 0.05). No statistical differences were observed in nonsocial behaviors. Additionally, analysis of the unilateral BLA group, (i.e., subjects with injection sites that were localized in either the right or left BLA only), show that the mean duration of submissive behaviors during testing with the NAI was 55.8 seconds, compared to 107.4 seconds for the vehicle control group and 30.5 seconds for the high dose ifenprodil group. While approaching statistical significance, the difference between the unilateral BLA and vehicle groups was not significant.
Figure 2.
Total duration (mean ± SEM) of behaviors exhibited during the 5-min test with a non-aggressive intruder (NAI). Animals received bilateral infusions of ifenprodil or saline into the BLA prior to being defeated for 15 min. * P < 0.05 compared to vehicle controls
Results from the no defeat control group showed no statistical differences in aggression between the ifenprodil (Mean duration/SEM: 50.8 sec ± 15.0) and vehicle control (Mean duration/SEM: 43.2 sec ± 15.3) groups, indicating that ifenprodil, alone, was unlikely to explain the increase in aggressive behaviors we observed in the acquisition study. Additionally, neither group showed any submissive behaviors when tested with the NAI.
3.3 Experiment 2: Effects of ifenprodil on expression of CD
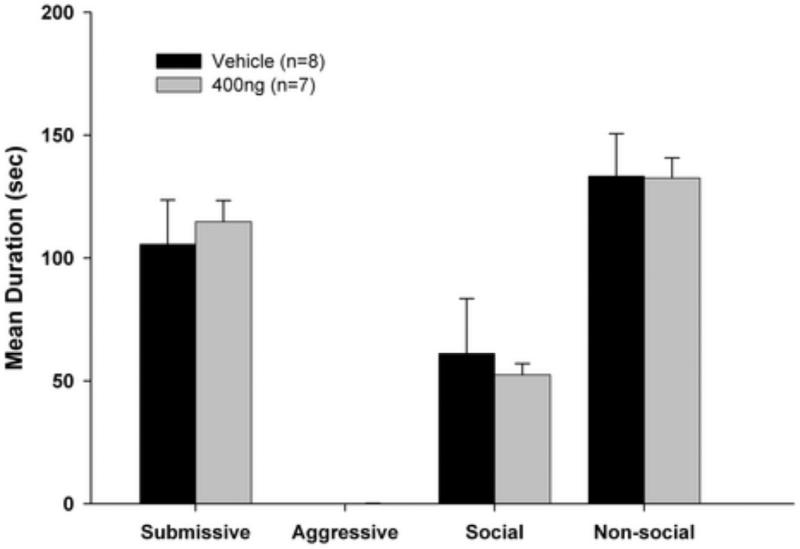
As shown in Figure 3, infusion of ifenprodil into the BLA did not alter the expression of CD, as no significant differences were found between the ifenprodil and control groups in levels of submissive, aggressive, social or nonsocial behaviors.
Figure 3.
Total duration (mean ± SEM) of behaviors exhibited by defeated animals during the 5-min test with a non-aggressive intruder (NAI). Animals received bilateral infusions of ifenprodil or saline into the BLA prior to being tested with the NAI.
3.4 Experiment 3: State-dependency control
Results from the state-dependency control group showed that the ifenprodil group (Mean duration/SEM: 0.82 ± 0.39) displayed significantly lower levels of submissive behaviors (p < 0.05) compared to the vehicle control group (Mean duration/SEM: 37.3 ± 9.0), indicating that the inhibition of submissive behaviors observed in Experiment 1 was not due to the drug state of the subjects.
4. Discussion
There is now substantial evidence that plasticity related to fear memory formation is dependent on NMDA receptors in the amygdala [11, 3, 19, 16, 20, 7]. In the present study, we examined the role of the NR2B subunit of the NMDA receptor complex within the BLA in the development of defeat-induced behavioral changes by determining whether selective blockade of this subunit would interfere with the acquisition of CD. Animals that received the high dose of ifenprodil (400ng), a selective antagonist of the NR2B subunit, before defeat training (Experiment 1) displayed significantly lower levels of submissive and defensive behaviors compared to controls when paired with a NAI 24 hour later. Importantly, there is some suggestion that unilateral inhibition of the NR2B receptors, as analysis of the anatomical control group (i.e., animals with cannula placements in either the right or left BLA) show an intermediate level of submissiveness (55.8 sec) compared to the vehicle and high dose ifenprodil groups (107.4 sec and 30.5 sec, respectively). In contrast, infusion of ifenprodil in defeated animals prior to testing with the NAI failed to inhibit submissive behaviors compared to controls (Experiment 2). This would be consistent with our earlier finding that unilateral inactivation of the BLA with muscimol is sufficient to block the acquisition of CD [10].
Our findings are consistent with studies demonstrating the involvement of NR2B receptors in the amygdala as the primary factor associated with the formation of fear based memories. A recent study by Walker and Davis [21] compared the role of NR2A and NR2B receptors in the acquisition of FPS. The infusion of an NR1/NR2B receptor antagonist into the amygdala was shown to selectively block the acquisition (but not expression) of FPS. In contrast, infusion of an NR1/NR2A receptor antagonist into the same region blocked both the acquisition and expression of FPS. The results from our study confirm these findings, because the infusion of ifenprodil, a specific NR2B antagonist, only impaired the acquisition but not expression of CD. Thus although our experiment did not specifically investigate the contribution of the NR2A subunit, it nonetheless implicates the NR2B subunit as the primary mechanism through with fear memories are formed in the amygdala. Additionally, it suggests the possibility that our previous results [7], in which we showed that the infusion of APV blocked both the acquisition and expression of CD, may also have been due to a more general disruption in synaptic transmission in the amygdala.
While ifenprodil significantly impaired submissive and defensive behaviors in Experiment 1, there are several possible confounding factors that may have contributed to this finding. First, it is possible that ifenprodil infusion in Experiment 1 could have caused a non-specific alteration in the behavior that may have interfered with the ability of the subject to properly express normal levels of submission during subsequent testing with the NAI. This is not likely however, as there were no differences in nonsocial behaviors (including general locomotion, grooming and rearing) in the ifenprodil group compared to vehicle-treated animals. Additionally, animals infused with ifenprodil actually showed significantly higher levels of aggression compared to controls during testing with the NAI, a finding that argues strongly against ifenprodil having a non-specific effect on behavior. We also analyzed the training sessions in order to ensure that ifenprodil did not interfere with the expression of normal agonistic behaviors during defeat, which could, in turn, lead to a change in the attack pattern of the RA. Analysis of the training sessions revealed that both the ifenprodil and control groups exhibited comparable levels of submissiveness during defeat and that the RAs attacked both groups with equal intensity (Table 1).
Table 1.
Total attack and submission duration, in seconds (mean ± SEM) of resident aggressors and experimental animals, respectively, during defeat training.
| Experimental Condition | Attack duration of RAs | Submission duration of subjects |
|---|---|---|
| Saline Control (0ng) | 88.1 (±30.6) | 362.8 (±58.1) |
| Ifenprodil (200ng + 400ng) | 67.8 (±30.7) | 348.4 (±52.7) |
A third possibility is that the heightened level of aggression may have been a result of the ifenprodil infusions, themselves. We examined this possibility by adding a ‘no defeat’ group to Experiment 1, wherein undefeated subjects were infused with ifenprodil (or vehicle) and were simply placed in the empty cage of a RA in lieu of defeat training. The following day, subjects were exposed to a NAI. Results show that there were no statistical differences in levels of aggression between the vehicle and ifenprodil groups when they were paired with a NAI, indicating that the increase in aggression we observed in Experiment 1 was not a by-product of the ifenprodil infusions. Additionally, comparable levels of aggression were found between the ifenprodil/defeat and ifenprodil/no defeat groups. Finally, a fourth possible explanation for the present finding is that a difference in the drug state between acquisition and testing may account for the decreased submissiveness observed during testing with the NAI. We examined this possibility in a group of animals that were infused with ifenprodil before acquisition and again before testing. These animals displayed low levels of submissive behaviors similar to those observed in animals receiving ifenprodil only before training. Additionally, there were no differences in any behavioral measures between the ifenprodil and control groups in Experiment 2, indicating that ifenprodil infusions alone did not alter the subjects’ behavior. It therefore appears that our finding in Experiment 1 was not a state-dependent effect.
The findings from the present experiment, together with our earlier studies, now provide substantial evidence that the BLA is the key structure mediating the formation of fear memory based on a defeat experience. We have also shown that the infusion of anisomycin, a protein synthesis inhibitor, in the BLA selectively blocks the acquisition of CD [9]. This last finding is especially significant because the same treatment in other brain regions with connections to the BLA (hippocampus, medial amygdala) fails to impair the acquisition of CD [9, 10], suggesting that plasticity based on the defeat experience is mediated primarily in the BLA.
In summary, these results provide support that the NR2B subunit of the NMDA receptor complex plays a key role in the mechanisms underlying fear learning in an ethologically relevant model of conditioned fear. We believe that an examination of the neural mechanisms of CD is particularly important because behavioral plasticity in response to a defeat experience requires the activation of multiple sensory and effector systems and is additionally sensitive to the effects of gonadal hormones [4,18].
Acknowledgements
This study was supported by MH62044 to KLH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–49. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair HT, Sotres-Bayon F, Moita MA, LeDoux JE. The lateral amygdala processes the value of conditioned and unconditioned aversive stimuli. Neuroscience. 2005;133:561–9. doi: 10.1016/j.neuroscience.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 3.Campeau S, Miserendino MJ, Davis M. Intra-amygdala infusion of the N-methyl-D-aspartate receptor antagonist AP5 blocks acquisition but not expression of fear-potentiated startle to an auditory conditioned stimulus. Behav Neurosci. 1992;106:569–74. doi: 10.1037//0735-7044.106.3.569. [DOI] [PubMed] [Google Scholar]
- 4.Faruzzi AN, Solomon MB, Demas GE, Huhman KL. Gonadal hormones modulate the display of submissive behaviors in socially defeated female Syrian hamsters. Horm Behav. 2005;47:569–75. doi: 10.1016/j.yhbeh.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–9. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Jasnow AM, Huhman KL. Activation of GABA(A) receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res. 2001;920:142–50. doi: 10.1016/s0006-8993(01)03054-2. [DOI] [PubMed] [Google Scholar]
- 7.Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotrophin-releasing factor in behavioral responses to social defeat. Behav Neurosci. 2004;118:1052–61. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- 8.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn Mem. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markham CM, Taylor SL, Huhman KL. Role of the amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn Mem. 2010;17:109–16. doi: 10.1101/lm.1633710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonist in the amygdala. Nature. 1990;345:716–8. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- 12.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lemeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–21. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 13.Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. Academic Press; San Diego, CA: 2001. [Google Scholar]
- 14.Potegal M, Huhman K, Moore T, Meyerhoff J. Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus). Behav Neural Biol. 1993;60:93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- 15.Prybylowski K, Wenthold RJ. N-methyl-D-aspartate receptors: subunit assembly and trafficking to the synapse. J Biol Chem. 2004;279:9673–6. doi: 10.1074/jbc.R300029200. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–96. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sananes CB, Davis M. N-methyl-D-aspartate lesions of the lateral and basolateral nuclei of the amygdala block fear-potentiated startle and shock sensitization of startle. Behav Neurosci. 1992;106:72–80. doi: 10.1037//0735-7044.106.1.72. [DOI] [PubMed] [Google Scholar]
- 18.Solomon MB, Karom MC, Norvelle A, Markham CM, Erwin WD, Huhman KL. Gonadal hormones modulate the display of conditioned defeat in male Syrian hamsters. Horm Behav. 2009;56:423–8. doi: 10.1016/j.yhbeh.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker DL, Davis M. Involvement of NMDA receptors within the amygdala in short-versus long-term memory for fear conditioning as assessed with fear-potentiated startle. Behav Neurosci. 2000;114:1019–33. [PubMed] [Google Scholar]
- 20.Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71:379–92. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- 21.Walker DL, Davis M. Amygdala infusions of an NR2B-selective or an NR2A-preferring NMDA receptor antagonist differentially influence fear conditioning and expression in the fear-potentiated startle test. Learn Mem. 2008;15:67–74. doi: 10.1101/lm.798908. [DOI] [PMC free article] [PubMed] [Google Scholar]