Abstract
Hypoxia is a common condition found in a wide range of solid tumors and is often associated with poor prognosis. Hypoxia increases tumor glycolysis, angiogenesis and other survival response as well as invasion and metastasis by activating relevant gene expressions through hypoxia-inducible factors (HIFs). HIF-1α and HIF-2α undergo oxygen-dependent regulation and their overexpression is frequently associated with metastasis and poor clinical outcomes. Recent studies show that each step of the metastasis process, from the initial epithelial-mesenchymal transition to the ultimate organotropic colonization, can potentially be regulated by hypoxia, suggesting a master regulator role of hypoxia and HIFs in metastasis. Furthermore, modulation of cancer stem cell self-renewal by HIFs may also contribute to the hypoxia-regulated metastasis program. Hypoxia-induced metastatic phenotype may be one of the reasons for the modest efficacy of antiangiogenic therapies and may well explain the recent provocative findings that antiangiogenic therapy increased metastasis in preclinical models. Multiple approaches to targeting hypoxia and HIFs, including HIF inhibitors, hypoxia-activated bioreductive prodrugs and gene therapies may become effective treatments to prevent or reduce metastasis.
Background
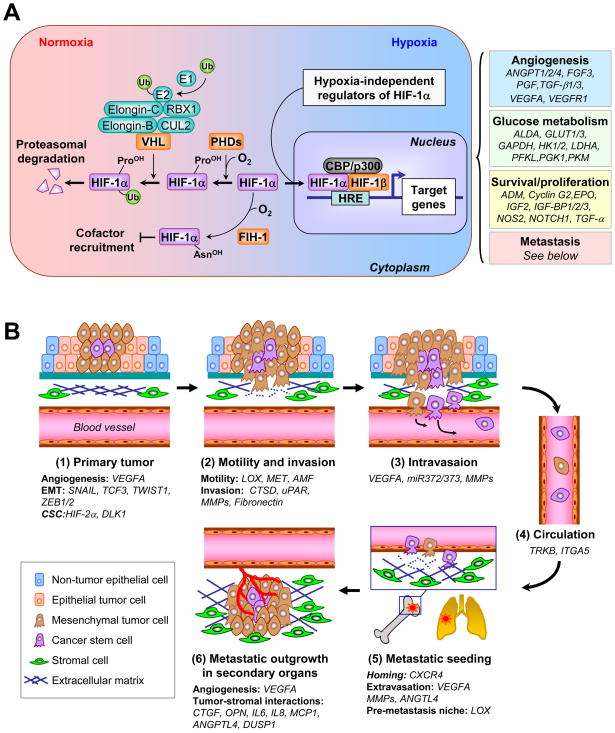
The occurrence of metastasis in solid tumors signals the breakdown of normal tissue homeostasis and dramatic rearrangement of tumor-stromal interactions. It has been increasingly recognized that hypoxia, which commonly refers to a condition in tissues where the oxygen pressure is less than 5–10mmHg, is a powerful driving force for such transitions in tumor progression (1). The best characterized hypoxia response pathway is mediated by hypoxia-inducible factor-1 (HIF-1) (2). HIF-1 is a heterodimer with the HIF-1β subunit constitutively expressed and the HIF-1α subunit regulated in an oxygen-dependent manner (Figure 1A). Under normoxia, prolyl hydroxylases (PHDs) modify Pro-402 and -564 of HIF-1α in a reaction that uses O2 as a substrate. Hydroxylated HIF-1α interacts with von Hippel-Lindau (VHL), which is part of an E3 ubiquitin ligase complex targeting HIF-1α for 26S proteasomal degradation. Under hypoxia, HIF-1α is stabilized because of the lack of O2 and dimerizes with HIF-1β to bind to the hypoxia response element (HRE, 5′-G/ACGTG-35′). By interacting with the co-activator CBP/p300, HIF-1 activates transcription of target genes that fall into four major categories: glucose transporters and glycolysis, angiogenesis, survival and proliferation, as well as invasion and metastasis (2) (Figure 1A). Gene expression profiling analysis in distinct cell types revealed tissue- and cell-specific variations in hypoxia response, although a consensus hypoxia response signature was shown to be a general poor-prognosis indicator for diverse cancer types (3–5). Binding of HIF-1 to CBP/p300 is also regulated in an oxygen-dependent manner, since O2 is the substrate for HIF-1α hydroxylation at Asn-803 by factor inhibiting HIF-1 (FIH-1) and this modification prevents binding to CBP/p300 (1). Two HIF-1α homologs, HIF-2α and HIF-3α, have been identified (1, 2). Similar to HIF-1α, HIF-2α is also regulated by oxygen-dependent hydroxylation. HIF-1α and HIF-2α are structurally similar in DNA binding and dimerization domains but differ in their transactivation domains. Consistently, they share overlapping target genes while each also regulates a set of unique targets. HIF-3α lacks the transactivation domain and may function as an inhibitor of HIF-1α and HIF-2α.
Figure 1. Hypoxia regulation of the metastasis cascade.
(A) In the presence of oxygen (O2), PHDs hydroxylate proline residues of HIF-1α, a reaction that can be inhibited by iron chelation. Hydroxylated HIF-1α interacts with VHL, which is part of an E3 ubiquitin ligase complex that mediates the ubiquitination (Ub) of HIF-1α and targets HIF-1α for degradation in the proteasome. Under hypoxia, HIF-1α is not hydroxylated and is released from VHL-mediated degradation. Stabilized HIF-1 moves into the nucleus, dimerizes with HIF-1β and binds to the hypoxia response element (HRE). By interacting with cofactors such as CBP/p300 and DNA polymerase II, HIF-1 activates transcription of target genes. Binding to CBP/p300 is blocked when HIF-1α is hydroxylated by FIH-1. HIF-1α is additionally regulated by oncogenic pathways (e.g., ERBB2, SRC, ET-1, RAS/MARK pathway, PI3K-Akt-mTOR pathway), mutations of tumor suppressor genes (e.g., PTEN, VHL, SDH and FH), and reactive oxygen species (ROS). HIF-1 target genes related to cancer are categorized into four groups, with representative genes in each group listed. (B) The role of hypoxia responsive genes in different steps of metastasis. 1) The primary response to hypoxia during primary tumor growth is the induction of angiogenesis through VEGFA. HIFs also enhance cancer stem cell self-renewal ability partly through DLK1. Hypoxia activates the expression of SNAIL, TWIST1, TCF3, ZEB1 and ZEB2 to promote epithelial-mesenchymal transition of tumor cells. 2) Tumor cells acquire additional motility under hypoxia with increased expression of LOX, MET and AMF. Furthermore, hypoxia promotes invasion through basement membrane (BM) and extracellular matrix (ECM) by upregulating proteases such as CTSD, uPAR and MMPs. Increased production of fibronectin by hypoxia facilitates the establishment of a tumor-specific ECM. 3) Hypoxia facilitates intravasation by increasing the expression of VEGFA, miR-372/373 and MMPs. 4) Circulating tumor cells may acquire the resistance to anoikis by activation of TrkB and repression of integrin α5 (ITGA5), both of which are hypoxia targets. 5) After reaching the secondary vasculature, tumor cells extravasate using hypoxia-induced molecules such as ANGPTL4, MMPs and VEGFA. Hypoxia-dependent induction of CXCR4 and LOX mediates tumor cell homing to secondary organs and formation of pre-metastatic niche, respectively. 6) In the parenchyma of the distant organ, hypoxia response factors such as ANGPTL4, CXCR4, DUSP1, CTGF, OPN, IL6, IL8 and MCP1 facilitate tumor-stromal interactions to foster the formation of overt metastasis in distinct organs.
HIF-1α can also be regulated through oxygen-independent mechanisms in a cell type specific manner (1, 2). It can be activated by mutations of PTEN, VHL, succinate dehydrogenase (SDH) or fumarate hydratase (FH). In addition, HIF-1α can be activated by hyperactivity of ERBB2, SRC, endothelin-1 (ET-1), the RAS/MARK pathway, and the PI3K-Akt-mTOR pathway. Moreover, HIF-1α can be stabilized by reactive oxygen species (ROS) through blocking the PHD activities.
HIF-1α is overexpressed in many cancer types and is associated with poor prognosis in cancers of breast, brain, oropharynx, cervix, ovary and uterus (2). Interestingly, HIF-1α is expressed at a higher fraction (69%) in metastases compared with primary tumors (29%) for breast cancer (6). HIF-2α overexpression is associated with poor patient outcome in renal cell carcinoma, non-small-cell lung cancer (NSCLC) and neuroblastoma (7).
Hypoxia Regulation of Metastasis
Metastasis consists of a series of rate-limiting steps (Figure 1B) (8). The initiation of metastasis involves the acquisition of a motile phenotype by tumor cells in a process called epithelial-mesenchymal transition (EMT). Tumor cells then disrupt the integrity of the basement membrane (BM) and interact directly with the underlying extracellular matrix (ECM) to eventually penetrate the blood vessel walls (intravasation) and become circulating tumor cells (CTCs). Successful dissemination depends on the survival of CTCs in blood circulation and subsequent extravasation at the secondary organs. Most disseminated tumor cells (DTCs) remain solitary or as dormant micrometastases until they adapt to the microenvironment of the secondary organs. Tumor initiating capabilities (or cancer stem cell properties) and productive tumor-stromal interactions underlying metastasis organotropism are believed to have fundamental importance in the successful outgrowth of clinically significant metastasis (8). Recent studies implicate regulatory functions of hypoxia in each of these steps (Figure 1B).
Epithelial-mesenchymal transition (EMT)
E-cadherin is a major component of the adherens junctions that maintain epithelial integrity and polarity. Loss of E-cadherin is a hallmark and functional requirement for EMT. Hypoxia induces EMT by HIF-dependent upregulation of transcription repressors of E-cadherin, such as SNAIL (9), TWIST1 (10), TCF3, ZEB1 and ZEB2 (11).
Invasion, ECM modulation and cell motility
Basement membrane disruption was shown to be promoted by HIF-1α-dependent upregulation of cathepsin D (CTSD), urokinase-type plasminogen-activator receptor (uPAR) and matrix metalloproteinase-2 (MMP2) through a proteolytic cascade (12). Hypoxia promotes the formation of a fibronectin (FN)-rich matrix that can be recognized by integrins expressed in tumor cell surface (12). Hypoxia also upregulates expression of lysyl oxidase (LOX), an extracellular enzyme that covalently modifies collagens to increase focal adhesion kinase activity, cell migration and metastasis (13). Another mechanism of increasing cell motility is through the activation of MET by HIF-1 (14). By overexpressing MET, tumor cells respond to hepatocyte growth factor (HGF), the ligand for MET that is produced at a elevated level by fibroblasts under hypoxia (15), with increased cell migration through tissue parenchyma. Autocrine motility factor (AMF) is another tumor-secreted cytokine that is induced by HIF-1 and VEGF under hypoxia to enhance proliferation, migration and angiogenesis through autocrine or paracrine mechanisms (16).
Intravasation, circulation and extravasation
Besides promoting angiogenesis and lymphangiogenesis, high level of VEGF induced by hypoxia is also associated with increased microvascular permeability and interstitial fluid pressure (IFP), both of which contribute to increased chance of intravasation (17). Hypoxia may also promote intravasation by increasing the production of metalloproteinases. For example, MMP1 and MMP2, synergistic mediators of vascular permeability and intravasation (18), were shown to be HIF-1α targets (19). Moreover, HIF-1 increases expression microRNA miR-372/373, which targets the MMP inhibitory protein RECK to promote intravasation (20).
Since duration from intravasation to extravasation can be as short as several hours, transient hypoxia response in the primary tumor may persist long enough to affect later steps of intravascular movement and extravasation (17). Indeed, transient “priming” of tumor cells with hypoxia in vitro before intravenous inoculation into mice increased colonization (17). Possible mediators of this priming effect include hypoxia responsive factors that regulate resistance to anoikis, such as TrkB (a suppressor of anoikis) (21, 22) and integrin α5 (a sensitizer of anoikis) (23) that plays a role in regulating the survival of CTCs. Extravasation may be promoted by hypoxia response factors VEGF, MMP1 and MMP2 in a similar way to intravasation. ANGPTL4, a key molecule for extravasation in lung, is upregulated by both TGF-β (24) and hypoxia (25), suggesting a possible synergistic priming effect of the two pathways.
Homing and the pre-metastatic niche
Chemokine receptor CXCR4 plays a key role in metastatic homing of tumor cells to organs expressing high level of its ligand SDF1 (26). Hypoxia may increase metastatic homing by inducing CXCR4 expression in renal cell carcinoma (27), ovarian cancer (28), breast cancer (25), and NSCLC (29). Hypoxia in the primary tumor may influence the metastatic seeding at distant organs even before tumor cell dissemination. This is achieved through the regulation of the pre-metastatic niche that was reported to form in distant organs by hematopoietic bone marrow derived cells under the influence of soluble factors released from primary tumors (30). Interestingly, a critical mobilizing factor is LOX, a known hypoxia target, which recruits CD11b+ myeloid cells to form the niche to facilitate the colonization of metastatic tumor cells (31).
Proliferation at the secondary organs
Some organs contain relatively hypoxic areas, including the hematopoietic stem cell niche region in the bone marrow and the pericentral region of the liver. Metastases in these organs may experience hypoxia immediately after extravasation. Otherwise, metastasis colonies that continue to grow will eventually become hypoxic when the growing tumor outstrips its blood supply. Indeed, we recently demonstrated the existence of hypoxia in early bone and lung metastases of breast cancer in a xenograft model using a dual-color bioluminescence imaging system (25). Hypoxia condition was partially relieved when angiogenesis was induced.
Hypoxia response molecules that promote survival, invasion and angiogenesis in the primary tumor may function similarly in the secondary site. In addition, hypoxia may upregulate proteins that mediate interactions with unique stromal cells in the secondary organ. Compatibility of tumor cells with the microenvironment, first proposed as the “seed and soil” hypothesis by Paget (32), plays a predominant role in determining the organ distribution patterns of metastasis (33). Metastatic organotropism was investigated for breast cancer in a series of studies by identifying organ-specific metastasis gene signatures (34). It was shown that tumor cells utilize distinct sets of genes when colonizing different organs. However, how hypoxia influences the organ-specific metastasis was unknown. We recently found that hypoxia affects lung- and bone-metastasis gene signatures in different ways: while hypoxia enhances the expression of a large percentage of genes involved in lung metastasis, it activates a more limited number of bone metastasis genes, such as CXCR4 and DUSP1 (25). Several known hypoxia responsive genes, including connective tissue growth factor (CTGF) (35), osteopontin (OPN) (36), interleukin-6 and −8 (IL-6 and −8) (37, 38), may also contribute to the bone metastasis promoting function of hypoxia. CTGF may promote bone metastasis through its function in osteoclastogenesis and bone resorption (39, 40). Osteopontin (OPN) may promote osteolytic metastasis by interacting with αVβ3-expressed osteoclasts (41). IL-6 and IL-8 have pleiotropic functions in promoting metastasis including angiogenesis, migration, tumor self-seeding and osteolysis during bone metastasis (42–44). Interestingly, a 45-gene hypoxia response signature was identified and showed prognostic power for lung metastasis but not for bone metastasis when applied to transcription profiling data of primary breast tumors, suggesting the selection of the lung metastatic phenotype by hypoxia in the primary site. Despite the different mechanisms of action in organ-specific metastasis, blocking HIF-1α significantly inhibited metastases to both bone and lung in animal models, establishing HIF-1α as a promising therapeutic target for treating metastasis at multiple sites (25, 45).
Cancer stem cells (CSCs)
By definition, metastasis should originate from CSCs with tumor initiating capabilities that allow DTCs to reconstitute growing, heterogeneous secondary tumors (46). Therefore, mechanisms enhancing the CSC phenotype should also increase the efficiency of metastasis. In neuroblastoma, hypoxia induces the expression of Delta-like 1 homolog (DLK1), which was shown to be essential for maintaining stemness and tumorigenicity (47). In glioblastoma, while HIF-1α was upregulated in both CSCs and non-CSCs under hypoxia, HIF-2α upregulation was restricted to CSCs (48). Targeting of either HIF-1α or HIF-2α in CSCs suppressed self-renewal and tumorigenicity (48). Despite the emerging links between hypoxia and CSC regulation, direct demonstration of the enhancement of metastasis by hypoxia in a CSC-dependent manner has not been reported. Cancer types with higher incidence of distant metastasis should be used for such studies.
In addition to the impact of hypoxia in different steps of metastasis discussed above, hypoxia may also promote metastasis through other mechanisms. For example, hypoxia response in stromal cells may be an integral part of the metastasis program. For instance, the expression of monocyte chemoattractant protein-1 (MCP-1), a pro-metastatic factor in multiple organs (49), is induced by hypoxia in fibroblasts (50) and astrocytes (51). MCP-1 produced by the first-tier stromal cells such as fibroblasts may help recruit the second-tier stromal cells, such as macrophages or osteoclast precursors, to promote metastasis. A poorly appreciated yet potentially significant cellular mechanism that promotes metastasis is cell fusion between tumor and host cells or between two tumor cells (52, 53). Hypoxia enhances fusion between hematopoietic progenitor cells and cardiomyocytes (54) and may promote metastasis by increasing frequency of cell fusion in cancer.
Clinical-Translational Advances
As summarized above, hypoxia can activate many steps of metastasis. This connection may partially explain the recent provocative observations that antiangiogenic drugs increased invasiveness and metastasis of tumor cells in preclinical models (55, 56), because these drugs induced hypoxia by blocking neovascularization. Therefore, targeting hypoxia through different approaches (Table 1) at appropriate time windows may significantly reduce tumor-intrinsic and treatment-induced metastasis.
Table 1.
Potential therapeutic agents that target hypoxia and HIF-1
| Agent/approach | Action mechanism | Current status |
|---|---|---|
| Agents blocking HIF-1 function | ||
| Chetomin | Disrupting HIF-1α binding to p300 | Preclinical studies |
| Anthracyclines | DNA intercalator; inhibiting HIF-1 binding to DNA | Clinical use |
| Bortezomib | Proteasome inhibitor; inhibiting HIF-1α transactivation domain | Clinical use |
| Agents attenuating HIF-1 expression | ||
| HIF-1α antisense oligonucleotide | Cleavage of HIF-1α RNA | Preclinical studies |
| HIF-1α siRNA | RNA interference against HIF-1α | Preclinical studies |
| Topotecan | Topoisomerase I inhibitor; inhibiting HIF-1α protein translation | Clinical use |
| Digoxin | Inhibiting HIF-1α protein translation | Clinical trials |
| PX-478 | Inhibiting HIF-1α protein translation | Clinical trials |
| Temsirolimus | mTOR inhibitor; inhibiting HIF-1α protein translation | Clinical use |
| 2-methoxyestradiol | Microtubule-depolymerizing agent; inhibiting HIF-1α at the posttranscriptional level | Clinical trials |
| 17-AAG | Hsp90 inhibitor; inducing HIF-1α degradation | Clinical trials |
| Hypoxia-activated bioreductive prodrugs | ||
| Benzotriene di-N-oxides | ||
| -Tirapazamine | Hypoxic cytotoxicity | Clinical trials |
| Nitroaromatics | ||
| -CB 1954 - | Hypoxic cytotoxicity | Clinical trials |
| -SN 23862 | ||
| -PR-104 | ||
| Quinones | ||
| -mitomycin C | Hypoxic cytotoxicity | Clinical trials |
| -RH-1 | ||
| Tertiary amine N-oxides- | ||
| AQ4N | Hypoxic cytotoxicity | Clinical trials |
| Hypoxia-targeted gene therapy | ||
| HRE-driven BAX | Hypoxia-induced apoptosis | Preclinical studies |
| Gene-directed enzyme prodrug therapy | ||
| HRE-driven P450 reductase | Hypoxia-induced sensitivity to alkylating nitroimidazole | Preclinical studies |
| HRE-driven HSV- thymidine kinase | Hypoxia-induced sensitivity to ganciclovir | Preclinical studies |
| HRE-driven cytosine deaminase | Hypoxia-induced sensitivity to 5- fluorocytosine | Preclinical studies |
Agents targeting HIF-1
There are several ways to target the function or the expression of HIF-1. Interaction of the transactivation domain of HIF-1α with CBP/p300 was blocked with either a dominant-negative polypeptide or the small compound chetomin, which were shown to reduce xenograft tumor growth (57). Anthracyclines (doxorubicin and daunorubicin) inhibited HIF-1 binding to DNA and reduced prostate cancer growth in a mouse model (58). HIF-1α transactivation domain was also inhibited by the proteasome inhibitor bortezomib (59), which is used to treat relapsed multiple myeloma and mantle cell lymphoma. It will be important to investigate how much of the efficacy of bortezomib is due to HIF-1 inhibition.
HIF-1 expression has been perturbed in many ways. Anti-sense oligonucleotide (60) or siRNA (61) targeting HIF-1α showed anti-tumor activity when applied to preclinical models. Agents that inhibit HIF-1α protein translation and showed anti-tumor effects include the topoisomerase I inhibitor topotecan, cardiac glycoside digoxin, and PX-478 (62). Disruption of microtubule polymerization by 2-methoxyestradiol reduced HIF-1α protein level and xenograft tumor growth (63). HIF-1α interacts with the chaperone Hsp90 and the Hsp90 inhibitor 17-AAG induces HIF-1α degradation in a VHL-independent manner (64). Because HIF-1α can be induced by hypoxia-independent signaling pathways, therapeutic agents targeting these pathways, such as mTOR, ERBB2 and MEK, also decreased HIF-1α level and this may partially account for their anti-tumor activities (2).
Hypoxia-activated bioreductive prodrugs
These compounds display cytotoxicity in the absence of O2 when the intermediate reaction product, instead of being converted back by O2, further reacts to become toxic end product. Tirapazamine is a prototype of benzotriene di-N-oxides and showed remarkable anti-tumor activity in animal models (65). However, results from Phase III clinical trials were mixed (66). Tirapazamine is currently under evaluation in more Phase III trials. Other bioreductive prodrugs under early phase clinical trials include CB 1954, SN 23862, PR-104 (nitroaromatics), mitomycin C, RH-1 (quinones), and AQ4N (tertiary amine N-oxides) (67). Limitations of bioreductive prodrugs include requirement for reductase activity in the hypoxic areas, toxicity and low efficacy due to rapid consumption (67).
Hypoxia-targeted gene therapy
HRE can be used to drive expression of proteins with intrinsic cytotoxicity to kill hypoxic tumor cells, for example, the apoptosis-inducing factor BAX (68). Similarly, HRE can drive expression of a protein that converts a prodrug into a cytotoxin. This approach, called gene-directed enzyme prodrug therapy, has been shown feasible by expressing HRE-driven flavoprotein cytochrome c P450 reductase (69), HSV thymidine kinase (70) and cytosine deaminase (71).
Conclusions
Mounting evidence suggests hypoxia as an important driving force and master regulator for the multi-step process of metastasis. Targeting hypoxia may become an effective approach to preventing or reducing metastasis. In particular, combining antiangiogenic drugs with HIF-α inhibitors may minimize the pro-metastatic effects elicited by antiangiogenesis-induced hypoxia and improve the efficacy of current antiangiogenic therapies. Nevertheless, many of the mechanistic and therapeutic investigation of the pro-metastatic role of hypoxia were based on pre-clinical animal models. Therefore, cautions must be applied when extrapolating conclusions drawn from such studies to clinical settings. As hypoxia is likely to have complex and even opposing roles during different stages of tumor development, further studies should focus on clinically relevant tumor models to determine the most appropriate treatment window for targeting hypoxia. As hypoxia is a notoriously heterogeneous and constantly evolving phenotype of a solid tumor, better molecular, pathological and, in particular, noninvasive imaging markers are also needed to improve patient selection and treatment response monitoring for clinical uses of hypoxia inhibitors.
Acknowledgments
We thank members of our laboratory for the critical reading of this manuscript and apologize to those colleagues whose important work can not be cited directly and discussed here owing to space limitations. Grant supports include Champalimaud Foundation, Brewster Foundation, DOD (BC051647), NIH (5R01CA134519) (to Y.K.) and Harold W. Dodds Fellowship of Princeton University (to X.L.).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Harris AL. Hypoxia [mdash] a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–7. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL. Targeting HIF-1 for cancer therapy. Nature Rev Cancer. 2003;3:721–2. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 3.Chi JT, Wang Z, Nuyten DS, et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS medicine. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winter SC, Buffa FM, Silva P, et al. Relation of a hypoxia metagene derived from head and neck cancer to prognosis of multiple cancers. Cancer Res. 2007;67:3441–9. doi: 10.1158/0008-5472.CAN-06-3322. [DOI] [PubMed] [Google Scholar]
- 5.Buffa FM, Harris AL, West CM, Miller CJ. Large meta-analysis of multiple cancers reveals a common, compact and highly prognostic hypoxia metagene. Br J Cancer. 102:428–35. doi: 10.1038/sj.bjc.6605450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1 alpha in common human cancers and their metastases. Cancer Research. 1999;59:5830–5. [PubMed] [Google Scholar]
- 7.Qing G, Simon MC. Hypoxia inducible factor-2[alpha]: a critical mediator of aggressive tumor phenotypes. Current Opinion in Genetics & Development. 2009;19:60–6. doi: 10.1016/j.gde.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Imai T, Horiuchi A, Wang C, et al. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol. 2003;163:1437–47. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M-H, Wu M-Z, Chiou S-H, et al. Direct regulation of TWIST by HIF-1[alpha] promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 11.Krishnamachary B, Zagzag D, Nagasawa H, et al. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–31. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamachary B, Berg-Dixon S, Kelly B, et al. Regulation of Colon Carcinoma Cell Invasion by Hypoxia-Inducible Factor 1. Cancer Research. 2003;63:1138–43. [PubMed] [Google Scholar]
- 13.Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 14.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–61. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 15.Ide T, Kitajima Y, Miyoshi A, et al. Tumor-stromal cell interaction under hypoxia increases the invasiveness of pancreatic cancer cells through the hepatocyte growth factor/c-Met pathway. Int J Cancer. 2006;119:2750–9. doi: 10.1002/ijc.22178. [DOI] [PubMed] [Google Scholar]
- 16.Funasaka T, Raz A. The role of autocrine motility factor in tumor and tumor microenvironment. Cancer Metastasis Rev. 2007;26:725–35. doi: 10.1007/s10555-007-9086-7. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan R, Graham C. Hypoxia-driven selection of the metastatic phenotype. Cancer and Metastasis Reviews. 2007;26:319–31. doi: 10.1007/s10555-007-9062-2. [DOI] [PubMed] [Google Scholar]
- 18.Gupta GP, Nguyen DX, Chiang AC, et al. Mediators of vascular remodelling coopted for sequential steps in lung metastasis. Nature. 2007;446:765–70. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 19.Shyu K-G, Hsu F-L, Wang MJ, Wang B-W, Lin S. Hypoxia-inducible factor 1[alpha] regulates lung adenocarcinoma cell invasion. Experimental Cell Research. 2007;313:1181–91. doi: 10.1016/j.yexcr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Loayza-Puch F, Yoshida Y, Matsuzaki T, Takahashi C, Kitayama H, Noda M. Hypoxia and RAS-signaling pathways converge on, and cooperatively downregulate, the RECK tumor-suppressor protein through microRNAs. Oncogene. 2010;29:2638–48. doi: 10.1038/onc.2010.23. [DOI] [PubMed] [Google Scholar]
- 21.Douma S, van Laar T, Zevenhoven J, Meuwissen R, van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–9. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 22.Martens LK, Kirschner KM, Warnecke C, Scholz H. Hypoxia-inducible Factor-1 (HIF-1) Is a Transcriptional Activator of the TrkB Neurotrophin Receptor Gene. J Biol Chem. 2007;282:14379–88. doi: 10.1074/jbc.M609857200. [DOI] [PubMed] [Google Scholar]
- 23.Rohwer N, Welzel M, Daskalow K, et al. Hypoxia-Inducible Factor 1α Mediates Anoikis Resistance via Suppression of α5 Integrin. Cancer Res. 2008;68:10113–20. doi: 10.1158/0008-5472.CAN-08-1839. [DOI] [PubMed] [Google Scholar]
- 24.Padua D, Zhang XHF, Wang Q, et al. TGF[beta] Primes Breast Tumors for Lung Metastasis Seeding through Angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X, Yan CH, Yuan M, Wei Y, Hu G, Kang Y. In vivo Dynamics and Distinct Functions of Hypoxia in Primary Tumor Growth and Organotropic Metastasis of Breast Cancer. Cancer Research. 2010;70:3905–14. doi: 10.1158/0008-5472.CAN-09-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 27.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–11. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 28.Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the Chemokine Receptor CXCR4 by Hypoxia. The Journal of Experimental Medicine. 2003;198:1391–402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YL, Yu JM, Song XR, Wang XW, Xing LG, Gao BB. Regulation of the chemokine receptor CXCR4 and metastasis by hypoxia-inducible factor in non small cell lung cancer cell lines. Cancer Biol Ther. 2006;5:1320–6. doi: 10.4161/cbt.5.10.3162. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre–metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-Induced Lysyl Oxidase Is a Critical Mediator of Bone Marrow Cell Recruitment to Form the Premetastatic Niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paget S. Distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3. [PubMed] [Google Scholar]
- 33.Lu X, Kang Y. Organotropism of Breast Cancer Metastasis. Journal of Mammary Gland Biology and Neoplasia. 2007;12:153–62. doi: 10.1007/s10911-007-9047-3. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 35.Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of Ctgf is directly mediated by Hif-1. AJP - Renal Physiology. 2004;287:F1223–32. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y, Denhardt DT, Cao H, et al. Hypoxia upregulates osteopontin expression in NIH-3T3 cells via a Ras-activated enhancer. Oncogene. 2005;24:6555–63. doi: 10.1038/sj.onc.1208800. [DOI] [PubMed] [Google Scholar]
- 37.Wan J, Ma J, Mei J, Shan G. The effects of HIF-1alpha on gene expression profiles of NCI-H446 human small cell lung cancer cells. J Exp Clin Cancer Res. 2009;28:150. doi: 10.1186/1756-9966-28-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K. Constitutive and Inducible Interleukin 8 Expression by Hypoxia and Acidosis Renders Human Pancreatic Cancer Cells More Tumorigenic and Metastatic. Clinical Cancer Research. 1999;5:3711–21. [PubMed] [Google Scholar]
- 39.Nozawa K, Fujishiro M, Kawasaki M, et al. Connective tissue growth factor promotes articular damage by increased osteoclastogenesis in patients with rheumatoid arthritis. Arthritis Res Ther. 2009;11:R174. doi: 10.1186/ar2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 41.Engleman VW, Nickols GA, Ross FP, et al. A peptidomimetic antagonist of the alpha(v)beta3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. J Clin Invest. 1997;99:2284–92. doi: 10.1172/JCI119404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–26. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ara T, DeClerck YA. Interleukin-6 in bone metastasis and cancer progression. European Journal of Cancer. 2010;46:1223–31. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
44.Bendre MS, Margulies AG, Walser B, et al. Tumor-Derived Interleukin-8 Stimulates Osteolysis Independent of the Receptor Activator of Nuclear Factor-
 B Ligand Pathway. Cancer Research. 2005;65:11001–9. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]
B Ligand Pathway. Cancer Research. 2005;65:11001–9. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar] - 45.Dunn LK, Mohammad KS, Fournier PGJ, et al. Hypoxia and TGF-β Drive Breast Cancer Bone Metastases through Parallel Signaling Pathways in Tumor Cells and the Bone Microenvironment. PLoS ONE. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li F, Tiede B, Massague J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 47.Kim Y, Lin Q, Zelterman D, Yun Z. Hypoxia-Regulated Delta-like 1 Homologue Enhances Cancer Cell Stemness and Tumorigenicity. Cancer Research. 2009;69:9271–80. doi: 10.1158/0008-5472.CAN-09-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z, Bao S, Wu Q, et al. Hypoxia-Inducible Factors Regulate Tumorigenic Capacity of Glioma Stem Cells. Cancer Cell. 2009;15:501–13. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu X, Kang Y. Chemokine (C-C Motif) Ligand 2 Engages CCR2+ Stromal Cells of Monocytic Origin to Promote Breast Cancer Metastasis to Lung and Bone. Journal of Biological Chemistry. 2009;284:29087–96. doi: 10.1074/jbc.M109.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galindo M, Santiago B, Alcami J, Rivero M, Martin-Serrano J, Pablos JL. Hypoxia induces expression of the chemokines monocyte chemoattractant protein-1 (MCP-1) and IL-8 in human dermal fibroblasts. Clin Exp Immunol. 2001;123:36–41. doi: 10.1046/j.1365-2249.2001.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflammation. 2007;4:12. doi: 10.1186/1742-2094-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu X, Kang Y. Cell Fusion as a Hidden Force in Tumor Progression. Cancer Research. 2009;69:8536–9. doi: 10.1158/0008-5472.CAN-09-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu X, Lu X, Kang Y. Organ-specific enhancement of metastasis by spontaneous ploidy duplication and cell size enlargement. Cell Research. 2010 doi: 10.1038/cr.2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S, Shpall E, Willerson JT, Yeh ETH. Fusion of Human Hematopoietic Progenitor Cells and Murine Cardiomyocytes Is Mediated by {alpha}4{beta}1 Integrin/Vascular Cell Adhesion Molecule-1 Interaction. Circulation Research. 2007;100:693–702. doi: 10.1161/01.RES.0000260803.98329.1c. [DOI] [PubMed] [Google Scholar]
- 55.Ebos JML, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated Metastasis after Short-Term Treatment with a Potent Inhibitor of Tumor Angiogenesis. Cancer Cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kung AL, Zabludoff SD, France DS, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 2004;6:33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Lee K, Qian DZ, Rey S, Wei H, Liu JO, Semenza GL. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci U S A. 2009;106:2353–8. doi: 10.1073/pnas.0812801106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Kaluz S, Kaluzova M, Stanbridge EJ. Proteasomal Inhibition Attenuates Transcriptional Activity of Hypoxia-Inducible Factor 1 (HIF-1) via Specific Effect on the HIF-1{alpha} C-Terminal Activation Domain. Molecular and Cellular Biology. 2006;26:5895–907. doi: 10.1128/MCB.00552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dikmen ZG, Gellert GC, Dogan P, et al. In vivo and in vitro effects of a HIF-1alpha inhibitor, RX-0047. J Cell Biochem. 2008;104:985–94. doi: 10.1002/jcb.21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gillespie DL, Whang K, Ragel BT, Flynn JR, Kelly DA, Jensen RL. Silencing of Hypoxia Inducible Factor-1α by RNA Interference Attenuates Human Glioma Cell Growth In vivo. Clinical Cancer Research. 2007;13:2441–8. doi: 10.1158/1078-0432.CCR-06-2692. [DOI] [PubMed] [Google Scholar]
- 62.Onnis B, Rapisarda A, Melillo G. Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med. 2009;13:2780–6. doi: 10.1111/j.1582-4934.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mabjeesh NJ, Escuin D, LaVallee TM, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–75. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 64.Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 2002;277:29936–44. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 65.Brown JM. SR 4233 (tirapazamine): a new anticancer drug exploiting hypoxia in solid tumours. Br J Cancer. 1993;67:1163–70. doi: 10.1038/bjc.1993.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reddy SB, Williamson SK. Tirapazamine: a novel agent targeting hypoxic tumor cells. Expert Opin Investig Drugs. 2009;18:77–87. doi: 10.1517/13543780802567250. [DOI] [PubMed] [Google Scholar]
- 67.Ahn GO, Brown M. Targeting tumors with hypoxia-activated cytotoxins. Front Biosci. 2007;12:3483–501. doi: 10.2741/2329. [DOI] [PubMed] [Google Scholar]
- 68.Ozawa T, Hu JL, Hu LJ, et al. Functionality of hypoxia-induced BAX expression in a human glioblastoma xenograft model. Cancer Gene Therapy. 2005;12:449–55. doi: 10.1038/sj.cgt.7700814. [DOI] [PubMed] [Google Scholar]
- 69.Patterson AV, Williams KJ, Cowen RL, et al. Oxygen-sensitive enzyme-prodrug gene therapy for the eradication of radiation-resistant solid tumours. Gene Ther. 2002;9:946–54. doi: 10.1038/sj.gt.3301702. [DOI] [PubMed] [Google Scholar]
- 70.Ingram N, Porter CD. Transcriptional targeting of acute hypoxia in the tumour stroma is a novel and viable strategy for cancer gene therapy. Gene Ther. 2005;12:1058–69. doi: 10.1038/sj.gt.3302504. [DOI] [PubMed] [Google Scholar]
- 71.Wang D, Ruan H, Hu L, et al. Development of a hypoxia-inducible cytosine deaminase expression vector for gene-directed prodrug cancer therapy. Cancer Gene Ther. 2005;12:276–83. doi: 10.1038/sj.cgt.7700748. [DOI] [PubMed] [Google Scholar]