Abstract
Polo-like kinases (Plks) are a family of serine/threonine protein kinases that are involved in the regulation of the various stages of the cell cycle. Plk2 and Plk3, two members of this family, are known to interact with calcium- and integrin-binding protein 1 (CIB1). Activity of both Plk2 and Plk3 is inhibited by CIB1 in a calcium-dependent manner. However, the physiological consequences of this inhibition are not known. Here, we show that overexpression of CIB1 inhibits T47D cell proliferation. Overexpression of CIB1 or knockdown of Plk3 using shRNA produced a multinucleated phenotype in T47D cells. This phenotype was not cancer cell specific, since it also occurred in normal cells. The cells overexpressing CIB1 appear to undergo proper nuclear division, but are unable to complete the process of cytokinesis, thus forming large multinucleated cells. Both CIB1 overexpression and Plk3 knockdown disrupted microtubule organization and centrosomal segregation, which may have led to incomplete cytokinesis. The observed effect of CIB1 overexpression is not due to the inhibition of Plk2 by CIB1. Plk3 and CIB1 both colocalize at the centrosomes, however, localization of CIB1 is dependent on the expression of Plk3. Furthermore, expression of Plk3 blocks the multinucleated phenotype induced by expression of CIB1 in these cells. These results suggest that CIB1 tightly regulates Plk3 activity during cell division and that either over- or underexpression results in a multinucleated phenotype.
Keywords: T47D, CIB1, Plk3, multinucleation, cytokinesis, microtubules, centrosomes
1. Introduction
The polo-like kinases (Plks), members of a family of serine/threonine kinases, have been shown to play an important role in controlling ordered progression through the cell cycle (Eckerdt et al., 2005). Plks regulate the progression of several mitotic events, such as centrosome maturation, mitotic spindle assembly, regulation of the anaphase-promoting complex, and cytokinesis (Archambault and Glover, 2009). There are four members of Plk’s in the mammalian cells; Plk1, Plk2/Snk, Plk3/Fnk and Plk4/Sak (Clay et al., 1993; Fode et al., 1994; Li et al., 1996; Simmons et al., 1992). Polo boxes are also shown to be involved in protein-protein interactions. They bind to the phosphorylated serine or threonine residues of the binding partners and regulate subcellular localization of Plks (Park et al.).
Deregulation of Plks is associated with defects in microtubule organization, centrosome numbers, and aneuploidy (Simizu and Osada, 2000). Although Plk1 and Plk2 expression is carefully regulated during the various stages of the cell cycle, Plk3 expression is shown to increase during the G1 phase (Zimmerman and Erikson, 2007), but remains mostly unaltered during cell cycle progression (Ouyang et al., 1997). It is thus believed that the regulation of the activity of Plk3 or its subcellular localization may dictate its function during the cell cycle, but little is known about the mechanism by which the activity of Plk3 is regulated.
Using the yeast two-hybrid system, Plk3 was shown to interact with CIB1 (Kauselmann et al., 1999), which was originally identified to interact with the integrin αIIb subunit (Naik et al., 1997). CIB1 mRNA and protein expression is widespread, including notable expression in cancer cell lines (Naik et al.; Shock et al., 1999). We have recently shown that CIB1 inhibits Plk3 activity in a Ca2+-dependent manner (Naik et al.). Here we investigated the physiological relevance of inhibition of Plk3 activity by CIB1. We show that CIB1-overexpression disrupted microtubule integrity and centrosomal segregation, thus impeding cytokinesis and resulting in multinucleated cells. Knockdown of Plk3 expression by RNAi mimicked the cellular phenotype observed upon CIB1-overexpression. Furthermore, overexpression of Plk3 rescued the CIB1-induced multinucleate phenotype. Taken together, our results suggest that CIB1 tightly regulates Plk3 activity during cell division and altered expression of CIB1 results in multinucleate phenotype.
2. Materials and methods
2.1. Cell culture, shRNA design and transfection
T47D, K562, HeLa and DAMI cell lines were obtained from ATCC. These cells were maintained as per the ATCC instructions. HUVECs (Lonza) were maintained as per the supplier instructions. Stable transfection using pcDNA3.1 (Mock) or pcDNA3.1-CIB1 expression vector was performed as described (Naik and Naik, 2003b). Plk3 specific shRNA used in this study has been described previously (Naik et al., 2010). A 23-nucleotide (5’-TTCCTGACGAAGCAGGAGAT-3’) sequence was used to design of shRNA specific for CIB1. The empty vector (pSUPER) or the resultant shRNA constructs were transiently transfected-using Lipofectamine (Invitrogen) following the manufacture’s instructions.
2.2. Cell proliferation assay
Cell proliferation assays were performed as described (Keely et al., 1995). Briefly, CIB1-and mock-transfected cells were seeded (1×104 cells/well) in triplicate onto 24-well plates pre-coated with or without 30 µg/ml of collagen type I (Sigma). At two-day intervals, cells from triplicate wells were counted. In a separate set of experiments the cells were incubated with 3H-thymidine (0.2 µCi; (Amersham) and the extent of 3H-thymidine incorporation was determined using scintillation counter.
2.3. Cell morphology
Mock or CIB1-transfected cells were stained with Diff-Quik® (Dade Behring). K562 and DAMI cells were allowed to attach to fibrinogen pre-coated wells for 4h at 37°C before staining.
2.4. Flowcytometry
Cell suspensions (1×106 cells/ml) were fixed in 70% ice-cold methanol for 1h and further incubated for 1h at RT in propidium iodide solution (0.1% Triton X-100, 50 µg/ml RNase A, and 50 µg/ml propidium iodide). DNA content was analyzed using FACScan Caliber (Becton Dickinson).
2.5. Cell Adhesion
Mock-transfected or CIB1-overexpressing cells were serum-starved overnight and plated in triplicates (50,000 cells/well) on BSA or collagen pre-coated 96 well plates and were allowed to adhere for 1h at 37°C. Non-adherent cells were washed and adherent cells were stained with crystal violet. Dye was extracted and quantified at 560 nm using a plate reader (Dynatech). In a separate set of experiments, the adherent cells were counted from ten different fields of view per well in triplicates.
2.6. Time-lapse microscopy
Mock or CIB1-overexpressing cells were plated on 8-chambered coverglass slide (Nunc) and live cell images were captured using Zeiss Axiovert 200 microscope equipped with controlled environmental chamber at 37°C and 5% CO2.
2.7. Immunofluorescence
Immunofluorescence was performed as described previously (Naik and Naik, 2003a). Anti-CIB1 antibody was produced as described (Naik et al., 1997). FNK, polyclonal antibody was a generous gift from Douglas K. Ferris; NCI, Baltimore, MD. Anti-Plk3 monoclonal (BD Biosciences), β– and γ-tubulin antibodies (Sigma), FITC-phalloidin, SYTO-13, or Topro-3; nuclear stains (Invitrogen). Mouse anti-Plk1 was purchased from ZYMED Laboratories. SNK/Plk2 and FNK were purchased from Santa Cruz Biotechnology. Anti-rabbit α/β tubulin and acetyl-α-tubulin were obtained from Cell Signaling. FITC or TRITC-conjugated secondary antibodies were obtained from Jackson laboratories. The stained cells were visualized using a Zeiss LSM510 laser confocal microscope.
2.8. Centrosome duplication assay
CIB1-or mock-transfected cells were treated with 4 mM hydroxyurea (HU; Sigma) for 40h at 37°C. Cells were fixed with cold methanol and stained as described in immunofluorescence. Each experiment was performed at least three times and 150–300 cells were counted in each case.
2.9. Mitotic shake-off
Cells were synchronized by mitotic shake off as described (Nakajima et al., 2003). Synchronized cells were treated with or without 35 µM ML-7 for 72h; fixed, and stained as described in immunofluorescence.
2.10. Immunoprecipitation
Immunoprecipitations were performed as described previously (Naik and Naik, 2003b). In brief, mock or CIB1-overexpressing cells lysed with ice-cold lysis buffer. Lysates (500 µg/ml) were immunoprecipitated with anti-CIB1 or Plk3 or Plk2 (Santa Cruz) or isotype-specific antibody using protein G-Sepharose beads (Amersham). Immunocomplex were separated by SDS-PAGE and detected by Western blotting (Naik et al., 2008). Densitometric analysis of band intensity was performed using a Bio-Rad (Richmond) Gel Doc 2000 system.
2.11. Statistical analysis
Data analysis was performed using Standard statistical tests (mean value, SEM, and paired Student’s t test). Results from at least three separate experiments were expressed as mean ± SEM. P≤ 0.05 were regarded as statistically significant.
3. Results
3.1. CIB1 inhibits cell proliferation
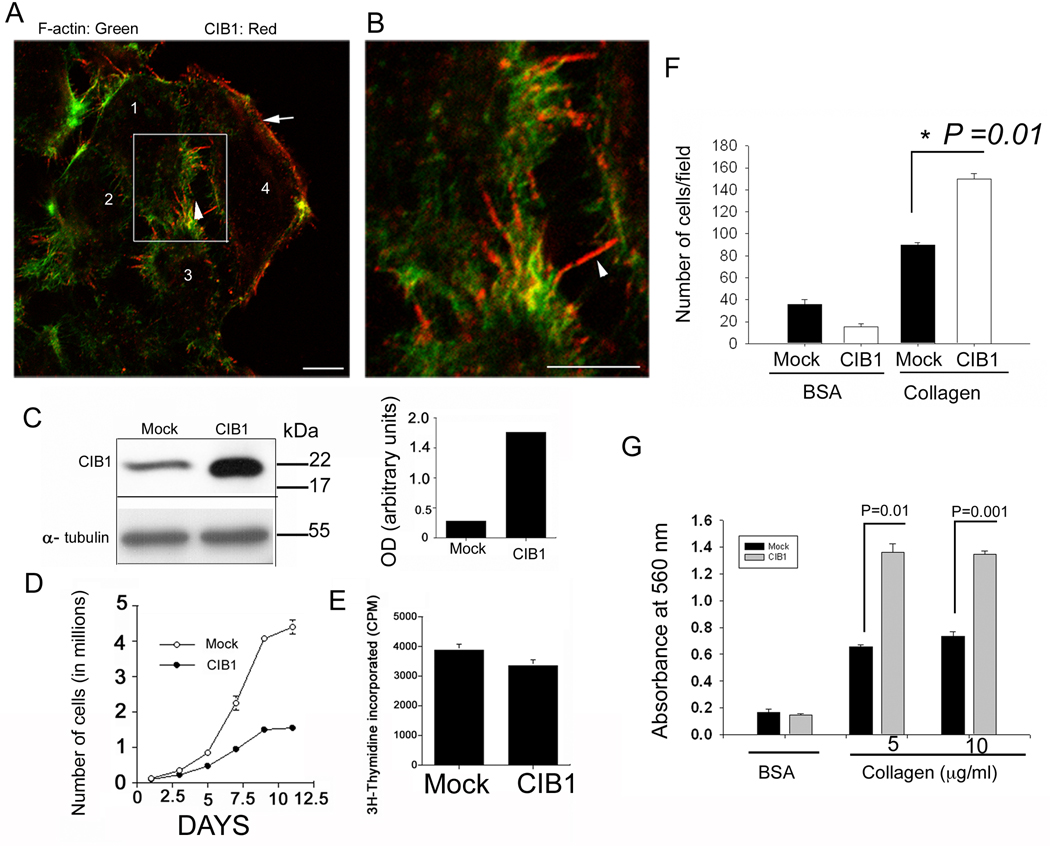
We have recently shown that CIB1, a myristoylated Ca2+-binding protein, is upregulated during breast cancer progression (Naik et al.). When its cellular distribution was analyzed, CIB1 was found to concentrate at the tip of the filopodia and membrane periphery of T47D, a ductal breast carcinoma cell line (Fig. 1A &B), a pattern consistent with previous findings in platelets (Naik and Naik, 2003b). Western blot analysis showed that a detectable amount of CIB1 expression is endogenous to this cell line (Fig. 1C), as had been reported previously (Shock et al., 1999). During microscopic examination, we noticed that many CIB1-overexpressing cells were larger and more spread than mock-transfected cells. A cell proliferation assay performed on collagen-coated dishes showed about a 75% reduction in the rate of proliferation of CIB1-overexpressing cells (Fig. 1D). A trypan blue dye exclusion viability test (Pusztai et al., 1993) indicated that very few dead cells were present in either CIB1-overexpressing or mock cell populations, ruling out the possibility of apoptosis being responsible for the declined rate of proliferation upon CIB1 overexpression. Surprisingly, we found that the rate of DNA synthesis in CIB1-overexpressing cells as determined by 3H-thymidine incorporation is similar to that of mock-transfected cells, (Fig. 1E) ruling out the possibility of a delayed S-phase. We also found that CIB1-overexpressing cells show significantly more adhesion to collagen than the mock cells as determined by both cell number and staining (Fig. 1F&G) excluding anoikis as a possible cause.
Fig. 1.
Overexpression of CIB1 inhibits cell proliferation (A) Immunofluorescence staining of T47D cells with anti-CIB1. Numbers indicate different cells in the view. Arrow indicate CIB1 staining at the membrane ruffle and arrow head indicate filopodia (B) High magnification of boxed area in A. Bar; 10 µm (C) Western blot analysis of cell lysates from mock- and CIB1-overexpressing T47D cells using anti-CIB1 antibody (top panel). Anti-α-tubulin blot (bottom panel) indicate equal loading, densitometric analysis of C (right panel). (D) Cell proliferation of mock-and CIB1-overexpressing T47D cells as determined by cell counting. (E) 3H-thymidine incorporation in mock- and CIB1-overexpressing T47D cells from 6-days samples performed in triplicate. (F&G) Cell adhesion of mock-and CIB1-overexpressing T47D cells on collagen as substratum assayed by counting the number of adherent cells (F) or amount of crystal violet retained by adherent cells measured at absorbance of 560 nm (G).
3.2. CIB1-induces multinucleation in both normal and cancer cells
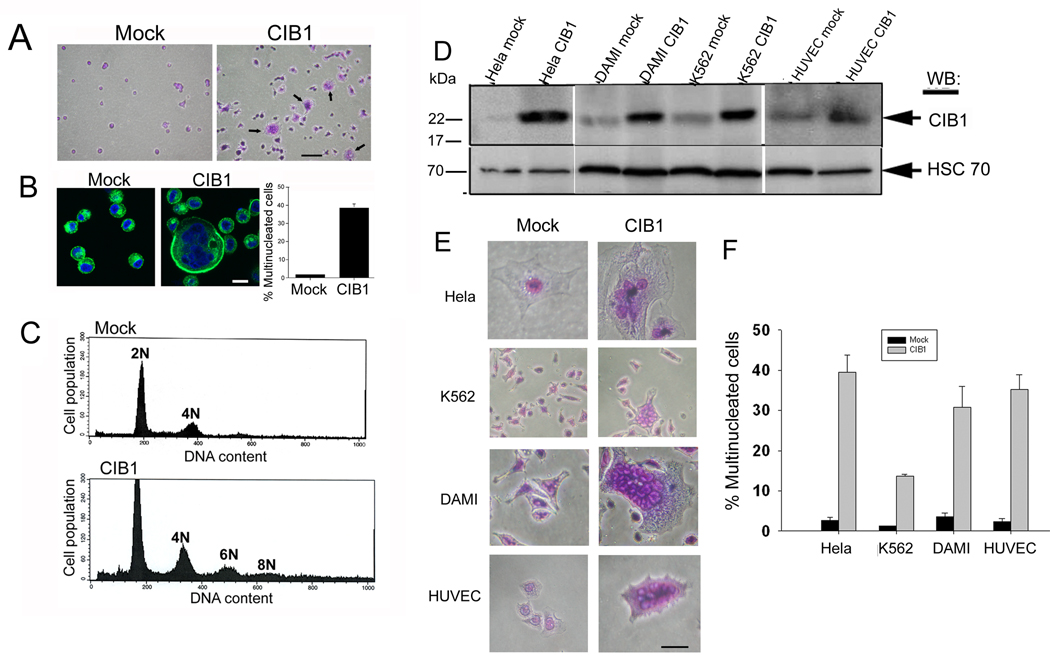
When cells adhered to collagen were stained and observed microscopically, we found that while mock cells exhibited a single nucleus (Fig. 2A), a number of CIB1-overexpressing cells appeared to contain multiple nuclei and were more spread (Fig. 2A). Each nucleus was of similar size and distinct from the others, but they appeared to be bunched together in a circular fashion (Fig. 2B). Conversely, only a few mock cells were double nucleated and none contained multiple nuclei (Fig. 2B). Quantitation of the extent of multinucleation revealed that about 40% of CIB1-overexpressing cells contained two or more nuclei, as compared to only 5% of mock cells (Fig. 2B). Interestingly, we found that several of the CIB1 overexpressing cells had three nuclei. Propidium iodide labeling followed by FACS analysis further confirmed that CIB1-overexpressing cells exhibit multinucleation, whereas the mock cells showed a normal DNA content (Fig. 2C). The presence of a 6N peak in the FACS analysis could be due to the presence of cells with three nuclei, suggesting the possibility of asymmetric nuclear division. To address cell-type specificity of this phenomenon, we overexpressed CIB1 in a variety of human cell lines that do express CIB1 endogenously (Fig. 2D). A multinucleated phenotype was observed in all established cell lines as well as HUVECs, the primary endothelial cells (Fig. 2E). Co-transfection of the green lantern plasmid was used to confirm that the multinucleated cells were the ones that were overexpressing CIB1 (data not shown). The quantitation of this data indicated that nearly 40% of the CIB1-overexpressing cells were multinucleated (Fig. 2F). The lower percentage (18%) obtained in K562 cells is probably due to the lower transfection efficiency obtained in these types of cells that grow in suspension. Thus, these results suggest that CIB1 is capable of inducing multinucleation in both cancerous as well as normal cells independent of cell type.
Fig. 2.
Ectopic expression of CIB1 induces multinucleation. (A) Light microscopic images of mock- and CIB1-overexpressing cells. Arrows indicate multinucleated cells. Bars: 20 µm. (B) Immunofluorescence images of cells from A; stained for F-actin (green) and DNA (blue). Quantitation of the extent of multinucleation observed. Bar 5 µm. (C) FACS analysis of DNA content of mock or CIB1-overexpressing T47D cells. (D) Ectopic expression of CIB1 in various cell lines as indicated, lysates were Western blotted with anti-CIB1 (upper blots) and reprobed with anti-HSC-70 (lower blots). (E) Light microscopic images of mock- or CIB1-overexpressing cells. Bar: 10 µm. (F) Quantitation of percent of multinucleated cells from E. All data and images shown are representative of at least three separate experiments.
3.3. CIB1 regulates cytokinesis
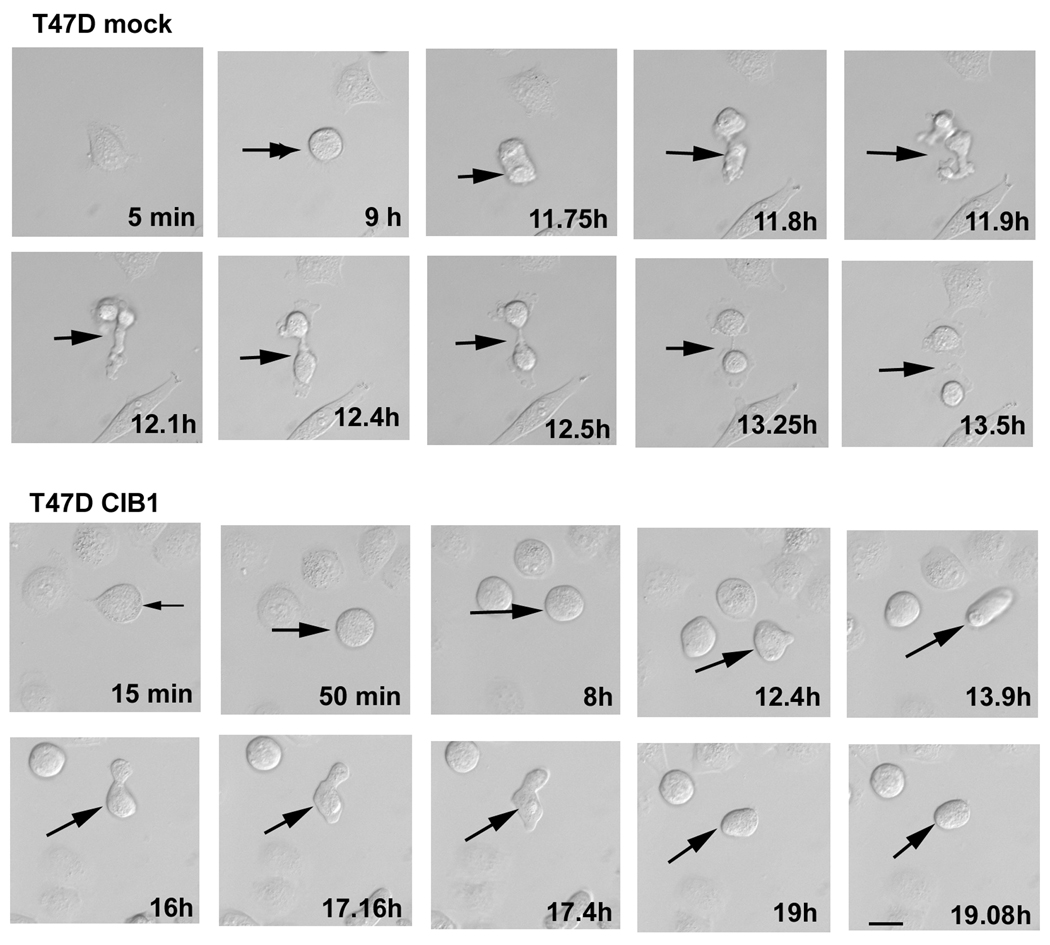
When cells were observed live for a period of 20h using time-lapse microscopy, we found that mock cells showed normal cleavage furrow formation with a distinct cytoplasmic bridge that eventually severed to release daughter cells (Fig. 3; T47D Mock). Interestingly, in CIB1-overexpressing cells, the cleavage furrow formed and the daughter cells tried to separate, but could not and eventually aborted the process, fusing into a single round cell (Fig. 3; T47D CIB1). Further analysis of 50–60 dividing cells from three separate experiments revealed that mock-transfected cells took an approximately 1.5h for completion of cytokinesis. In contrast, this time was prolonged substantially in CIB1-overexpressing cells, where it took 4h before the cytokinesis was aborted. These results suggest that the CIB1-induced multinucleate phenotype is probably due in part to aberrant cytokinesis.
Fig. 3.
Overexpression of CIB1 causes incomplete cell division. (A) Live time-lapse microsopic images of mock- or CIB1- overexpressing T47D cells taken after every 5min for a period of 24h. Arrows indicate cells undergoing late stages of cell division. Shown are representative images of 50–60 dividing cells from three separate experiments. Bar: 10 µm.
3.4. Downregulation of Plk3 expression results into multinucleated phenotype
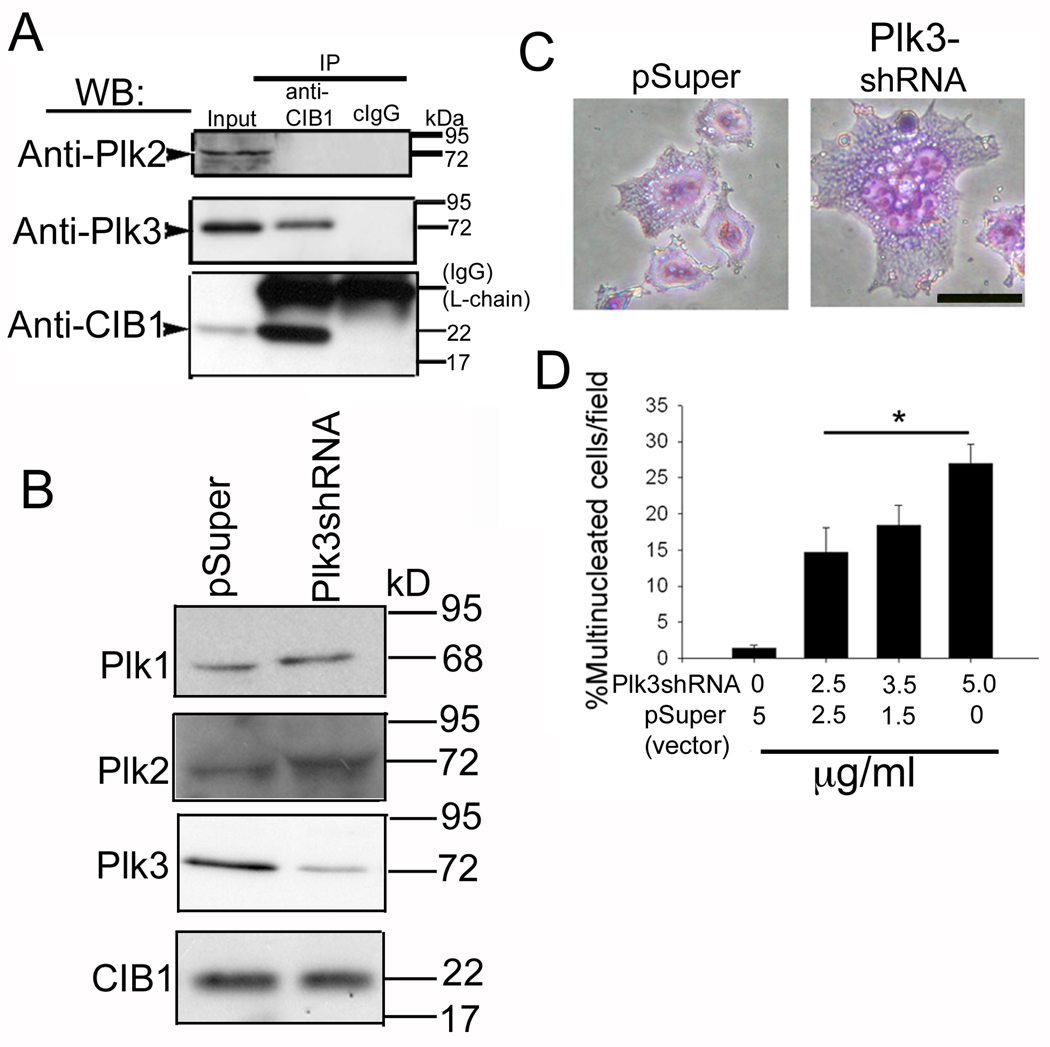
Although it was originally identified as an integrin binding protein, CIB1 has been shown to bind a variety of proteins with diverse cellular functions. CIB1 has been shown to inhibit the activity of Plk2 and Plk3, which are know to regulate cell cycle (Ma et al., 2003; Naik et al.). Coimmunoprecipitation experiments showed that, although both Plk2 and Plk3 were expressed in T47D cells, only Plk3 specifically associated with CIB1 (Fig. 4A). The fact that CIB1 associates and inhibits Plk3 activity and overexpression of CIB1 induces multinucleation suggested that inhibition of Plk3 by CIB1 is the cause for the observed multinucleation. This notion was further supported by the down-regulation of Plk3 expression, which resulted in similar multinucleated phenotype. Transfection of Plk3-shRNA resulted in an almost 60–70% reduction in the expression of Plk3 protein (Fig. 4B). This knockdown had no effect on endogenous expression CIB1 (Fig. 4B). Similarly, the levels of Plk1 and Plk2 were unaffected, suggesting the knockdown to be specific to Plk3 (Fig. 4B). As expected, control (pSuper-transfected) cells had a single nucleus, whereas populations of Plk3-shRNA-transfected cells were large and had multiple nuclei (Fig. 4C). Quantitation of multinucleated cells showed that over 25% of the cells per field had two or more nuclei as compared to less than 2% of the cells transfected with the control vector (Fig. 4D). The extent of multinucleation was significantly (P<0.01) and dose-dependently increased when increasing amounts of the Plk3-shRNA construct were transfected, suggesting a relationship between Plk3 knockdown and multinucleation (Fig. 4D).
Fig. 4.
Knockdown of Plk3 expression results in multinucleated cells. (A) Immunoprecipitate of T47D cell lysates using anti-CIB1 or cIgG1 and immunoblotted for Plk2, Plk3, and CIB1 as indicated. (B) Immunoblots of cells transfected with pSuper or Plk3-shRNA construct probed with anti-Plk1 or anti-Plk2 or anti-Plk3 or anti-CIB1 as indicated. (C) Light microscopic images of cells from B. Bar: 10 µm. (D) Quantitation of the number of multinucleated cells as a function of Plk3 shRNA concentration (*P<0.01).
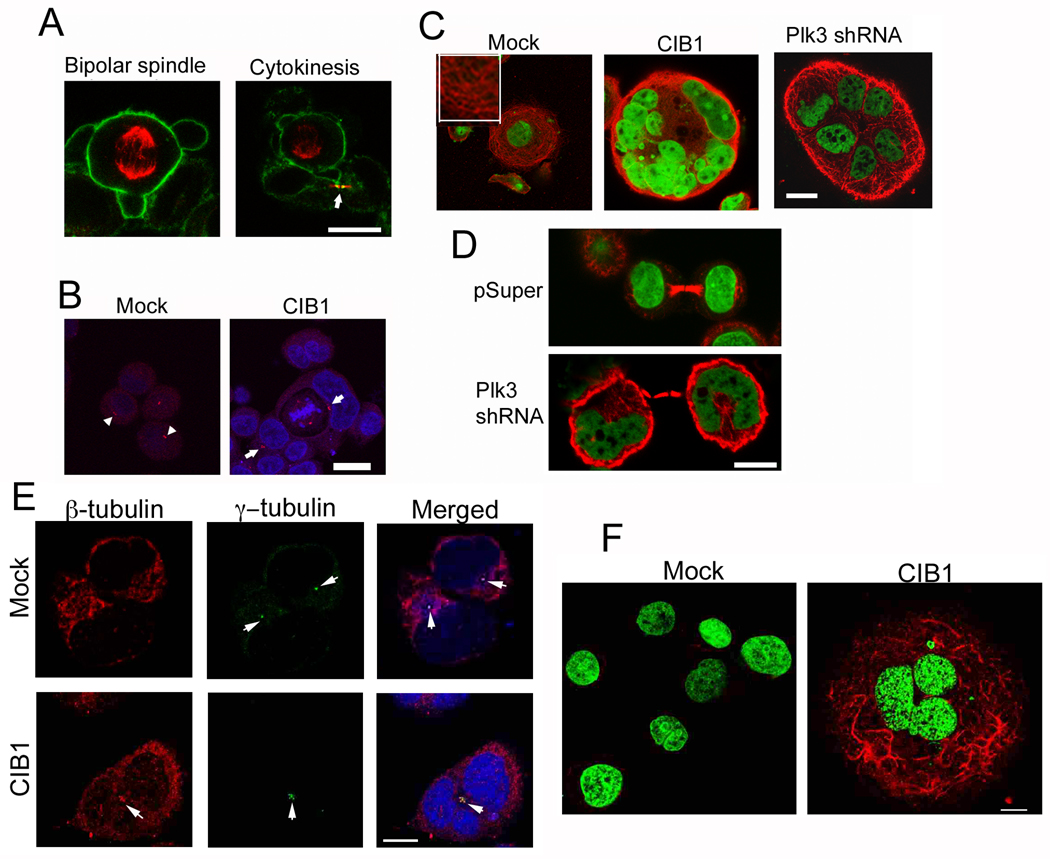
3.5. Microtubule integrity is regulated by both CIB1 and Plk3
In observing mitotic cells, mock-transfected cells underwent typical centrosomal separation, bipolar spindle formation, chromosomal segregation, and cytokinesis (Fig. 5A). As depicted in Figure 5B, we noticed several instances of CIB1-overexpressing cells with two nuclei; however only one nucleus was actively dividing and multiple centrosomes were visible, indicative of asynchronous nuclear division. This is consistent with FACS analysis where a 6N peak was distinctly visible in CIB1-overexpressing cells (Fig. 2C). When cells were stained for microtubules, mock cells exhibited long, highly organized microtubule filaments, whereas microtubules in CIB1-overexpressing cells appeared disorganized, forming a nest-like structure surrounding the nuclei (Fig. 5C). Similar microtubule disorganization was evident in multinucleated cells resulting from Plk3 downregulation (Fig. 5C; right panel). The nest-like appearance of microtubule could result from displacement of the microtubules by the multiple nuclei found in these cells. Normal cytokinetic bridges were visible in multinucleated cells that arise due to downregulation of Plk3 (Fig. 5D) as well as overexpression of CIB1, (data not shown) suggesting that the process is similar in both situations. To test the possibility that the microtubule network is uncoupled from the centrosomes, the microtubule organizing center (MTOC) in CIB1 overexpressing cells, we found that in mock cells the β-tubulin network appear to originate from the MTOC as expected. In contrast, in CIB1 overexpressing cells, the MTOC were bunched together and the microtubule network appeared to be distinct from the MTOC (Fig 5E). To determine if CIB1 stabilizes the microtubule network, we analyzed the presence of acetylated α-tubulin as a marker for microtubule stability. We found that mock cells had no detectable acetylated tubulin. In contrast, multinucleated CIB1 overexpressing cells showed high levels of acetylated tubulin strands nesting the nuclei (Fig 5F). Taken together, these observations are consistent with the idea that Plk3 is involved in microtubule organization and dynamics during the cell cycle, and that CIB1 may act to regulate Plk3 activity during this process.
Fig. 5.
Microtubule integrity is regulated by both CIB1 and Plk3. (A) Immunofluorescence images of mock cells undergoing normal cell division stained for β-tubulin (red) and F-actin (green). Arrow indicate cytokinetic bridge. (B) Mock or CIB1-overexpressing cells stained for DNA (blue) and γ-tubulin (red). Arrows indicate centrosomes. (C) Cells transfected with pSuper, CIB1 construct, and Plk3-shRNA. Cells were stained for β-tubulin (red) and DNA (green). (Inset) High magnification of anti-β-tubulin staining in the mock cells. Images shown are representative of about 20% of the total cell population. (D) Cells from C were immunostained for β-tubulin (red) and DNA (green). (E) Cells from B were stained for β-tubulin (red), γ-tubulin (green) and DNA (blue). Arrowheads indicate centrosomes. (F) Mock or CIB1-overexpressing cells stained for DNA (green) and acetyl-α-tubulin (red). Bars: A–F, 1µm.
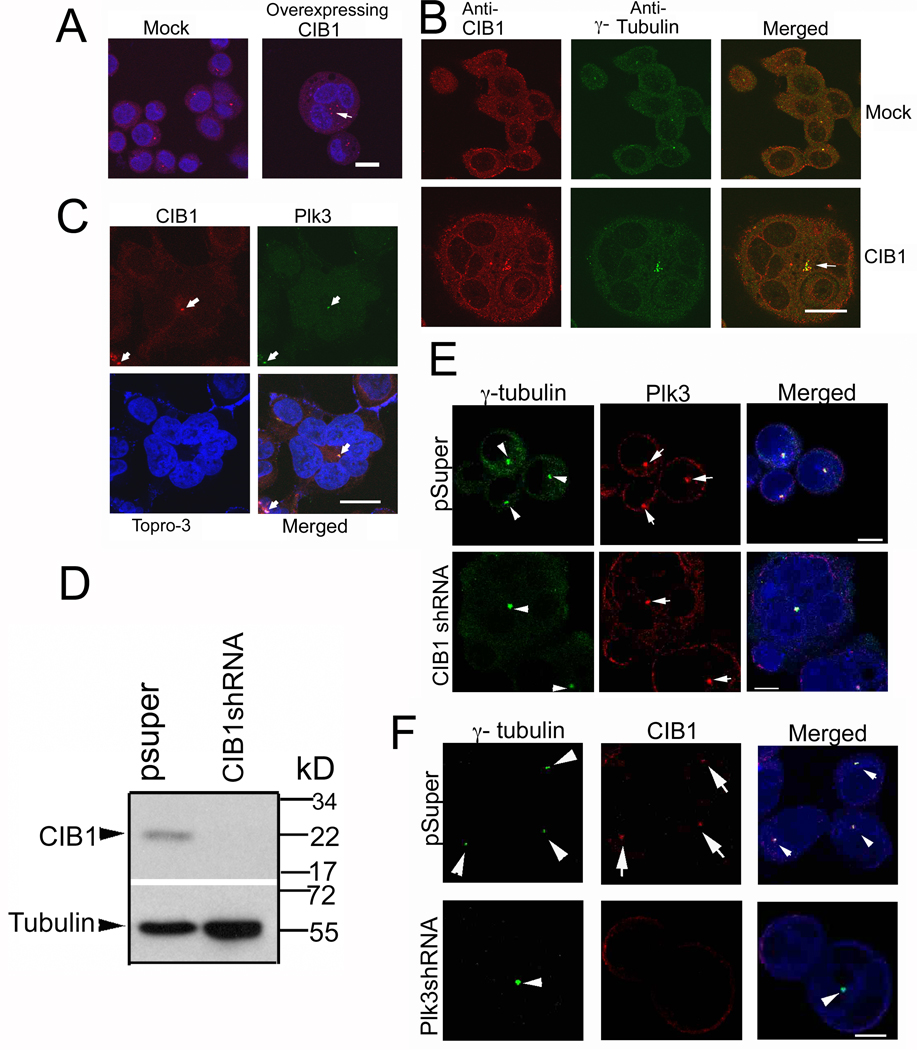
3.6. CIB1 and Plk3 colocalizes at the centrosomes
When immunostained for γ-tubulin, a centrosomal marker, we observed that CIB1-overexpressing multinucleated T47D cells possessed multiple centrosome pairs (Fig. 6A, right panel) as compared to mock cells, which only showed one per cell (Fig. 6A, left panel). Similar multiple centrosomes were evident in multinucleated cells generated by knockdown of Plk3 using shRNA (data not shown). Interestingly, all the centrosomes were grouped together in the center with nuclei surrounding in a pericentric fashion, possibly due to defective centrosomal segregation (Fig. 6A). From this observation, it seems unlikely that the observed multinucleate phenotype is due to the fusion of multiple cells, since one would not expect all centrosomes to be congregated together after such an event. Immunofluorescence analysis indicated that although CIB1 colocalized with the centrosomes in both mock and CIB1-overexpressing cells, its localization was more intense and prominent in the CIB1-overexpressing cells (Fig. 6B). Furthermore, we found that Plk3 and CIB1 colocalized at areas representative of centrosomes (Fig. 6C). We next determined the effect of downregulation of endogenous CIB1 on cell morphology. We were able to downregulate more than 90% of CIB1 using shRNA specific to CIB1 (Fig. 6D). We observed multinucleated phenotype similar to CIB1 overexpression, suggesting that optimum level of CIB1 is necessary for proper progression of cell cycle. However, this downregulation of CIB1 did not affect localization of Plk3 to the centrosomes (Fig. 6E). Interestingly, the localization of CIB1 to the centrosomes was entirely dependent on Plk3, since Plk3 knockdown resulted in the loss of CIB1 at the centrosomes (Fig. 6F). This suggests that CIB1 may play a role in the physiological regulation of centrosomal activity, perhaps through its inhibitory effect on Plk3.
Fig. 6.
CIB1 and Plk3 colocalize at MTOC. (A) Confocal images of Mock- and CIB1-overexpressing cells immunostained for DNA (blue) and γ-tubulin (red). Arrow indicates multiple centrosomes. (B) Cells from A were immunostained for CIB1 (red) and γ-tubulin (green), arrow indicates centrosomes. (C) Cells from A were immunostained for CIB1 (red), Plk3 (green), and DNA (blue). Arrows indicate putative position of centrosomes. Images shown are representative of about 20% of the total cell population. (D) Immunoblots of T47D cells stably transfected with pSuper or CIB1 shRNA, blotted using anti-CIB1 and reprobed using anti-tubulin as a loading control. (E) Confocal images of cells from D immunostained for DNA (blue), Plk3 (red) and γ-tubulin (green). Arrowheads indicate centrosomes and arrows indicate Plk3. (F) Confocal images of T47D cells transfected with pSuper or Plk3 shRNA immunostained for DNA (blue), γ-tubulin (green) and CIB1 (red). Arrowheads indicate centrosomes and arrows indicate CIB1. Bars: A–C, 20µm; E–F, 10µm.
3.7. Centrosome duplication is not affected by CIB1
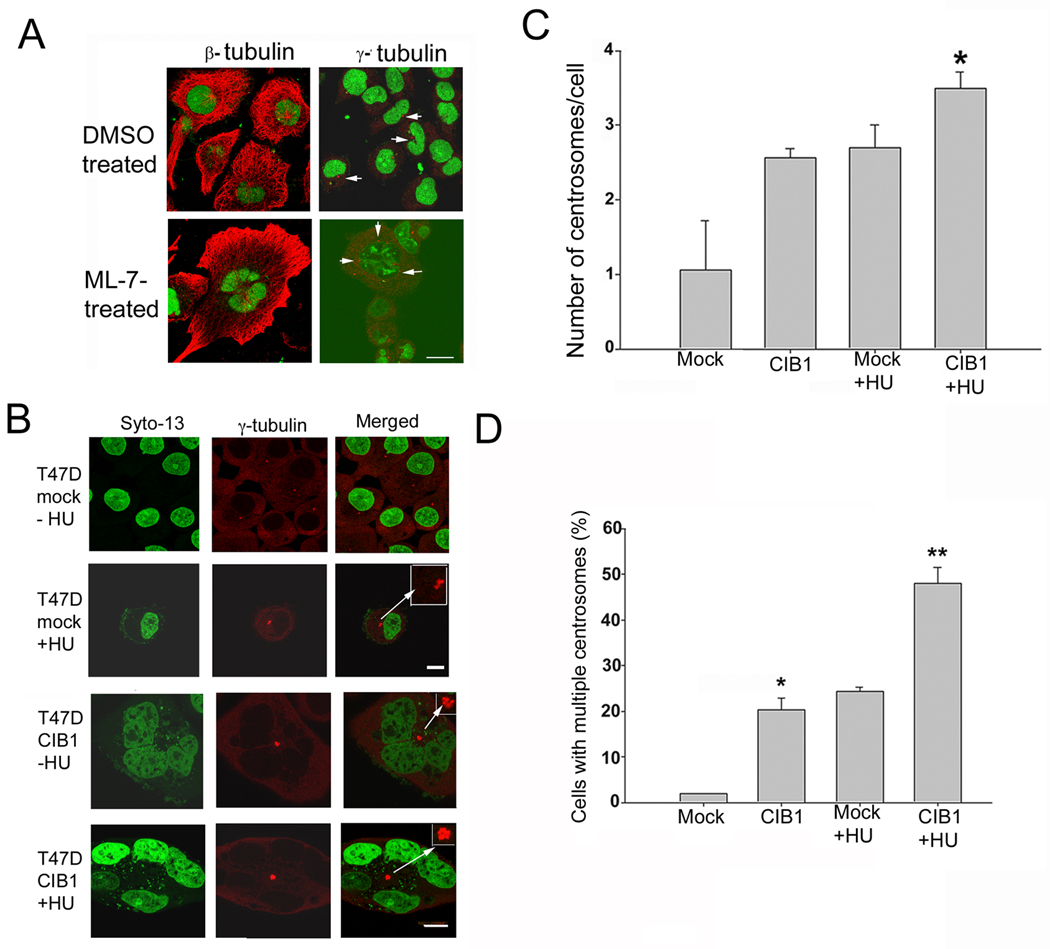
In order to further establish that microtubule disorganization is the cause and not an effect of multinucleation, we treated T47D cells with actomyosin drugs, ML-7 and latrunculin B, which are known to induce multinucleation (Mukhina et al., 2007; Wu et al.). We found that although multinucleation occurred, the microtubule structures were normal upon treatment with ML-7 (Fig. 7A) or latrunculin B (data not shown). Furthermore, centrosomes were well segregated unlike in CIB1 overexpressing cells, suggesting that observed clustering of centrosomes is indeed due to disorganization of microtubule and not a consequence of multinucleation.
Fig. 7.
Overexpression of CIB1 does not affect centromosome duplication. (A) Microtubule (β-tubulin) and centrosome (γ-tubulin) staining of T47D cells synchronized using mitotic shake off and untreated or treated with ML-7 (35 µM for 72h). Arrows indicate centrosomes. (B) Immunofluorescence images of mock- (upper two panels) and CIB1-transfected T47D cells (lower two panels). Cells untreated (first and third panels) and HU-treated (second; fourth panels). Bar, 1µm. Inset show magnified centrosomes. (C) Quantitation of the number of centrosomes per cell in mock- and CIB1-transfected cells, untreated or treated with HU. (D) Quantitation of the number of cells with multiple centrosomes in mock or CIB1-overexpressing cells untreated or treated with HU. Histograms show results from three independent experiments.
It is reasonable to believe that the inhibition of Plk2 may also contribute to the observed effect. If overexpression of CIB1 inhibits Plk2 in these cells, then it should hinder centrosome duplication, because Plk2 has been shown to be required for this process (Warnke et al., 2004). To test this possibility, we treated T47D cells transfected with mock or CIB1 construct with the DNA synthesis inhibitor hydroxyurea (HU), which allows the continuation of multiple rounds of centrosome duplication in the absence of DNA replication for 40h (Meraldi et al., 1999). Immunofluorescence staining with anti-γ-tubulin antibody revealed that in the absence of HU treatment, mock cells contained a single γ-tubulin-positive dot, representing a pair of closely spaced centrosomes; upon HU-treatment, these cells showed more than two centrosomes per cell (Fig. 7B). In contrast, a population of untreated CIB1-overexpressing cells contained more than two centrosomes per cell (as shown above), which further increased upon HU treatment (Fig. 7B). Quantitation of the number of centrosomes per cell indicated that on an average, mock cells showed one centrosome per cell, whereas CIB1-overexpressing cells as well as HU-treated mock cells had 2.7 centrosomes per cell. Interestingly, HU treatment enhanced centrosome numbers from 2.7 to 3.5 centrosomes per cell in CIB1-overexpressing cells (Fig. 7C). When analyzed for the number of cells having multiple centrosomes, less than 1% of mock-transfected cells had multiple centrosomes as compared to >22% of CIB1-overexpressing cells (Fig. 7D). After HU treatment, 25% of mock-transfected and 49% of CIB1-overexpressing cells had multiple centrosomes (Fig. 7D). These results suggest that CIB1 overexpression has no inhibitory effect on centrosome duplication, thus ruling out the possibility that the inhibition of Plk2 by CIB1 may be responsible for the observed multinucleate phenotype.
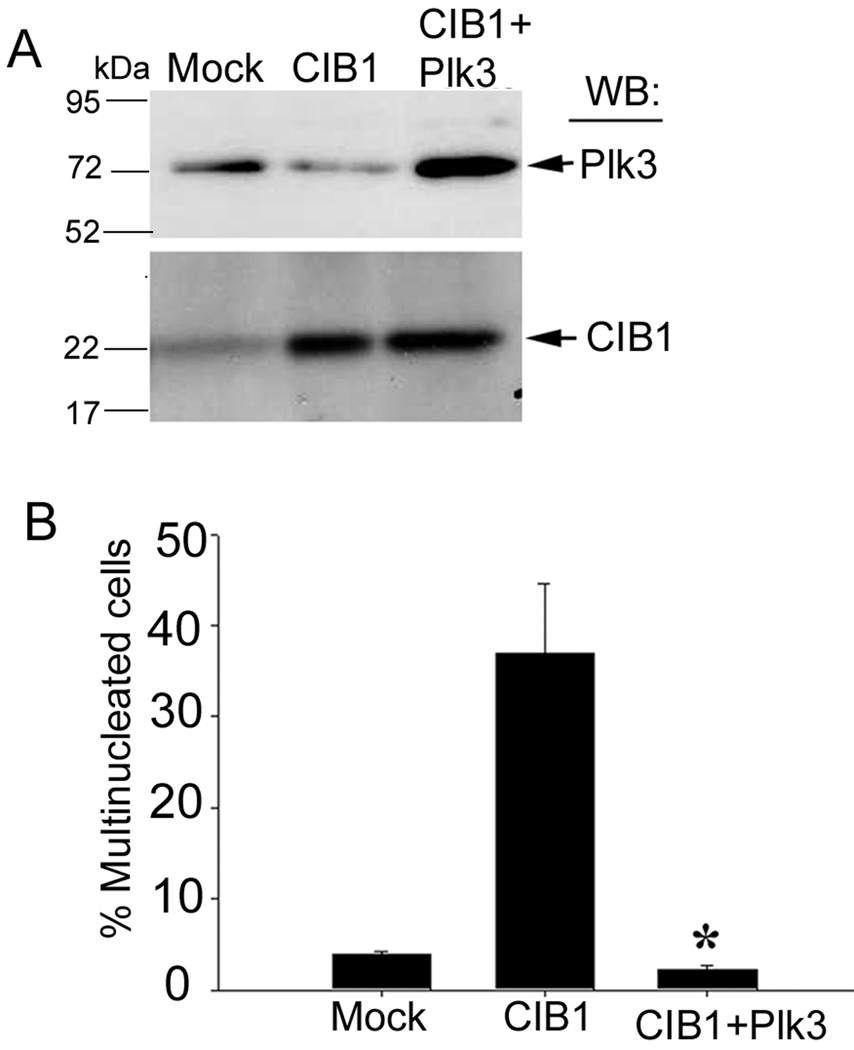
3.8. CIB1-induced multinucleation is rescued by Plk3
To conclusively establish that inhibition of Plk3 activity by CIB1 results in multinucleation in vivo, we performed a coexpression experiment. We reasoned that overexpression of Plk3 should rescue the CIB1-induced multinucleate phenotype. To achieve this, we ectopically overexpressed Plk3 in CIB1-overexpressing cells (Fig. 8A). As expected, CIB1 overexpression produced a multinucleate phenotype (Fig. 8B). Interestingly, coexpression of Plk3 with CIB1 completely blocked the multinucleated phenotype (Fig. 8B). This result suggested that increasing the relative level of CIB1 can cause a multinucleate phenotype, which can be rescued by forced increase of Plk3 levels. Taken together, these results corroborate that inhibition of Plk3 by CIB1 results in a multinucleate phenotype.
Fig. 8.
Co-overexpression of Plk3 rescues the multinucleation phenotype induced by CIB1. (A) Lysates from mock, CIB1, and CIB1 and Plk3 overexpressing cells were Western blotted with anti-Plk3 (upper panel) and anti-CIB1 (lower panel). (B) Quantitation of % multinucleated cells in mock, or CIB1 or CIB1 and Plk3 overexpressing T47D cells. A total of 50 cells per transfection were counted (*P<0.001). Results shown are representative of three independent experiments.
4. Discussion
In the present study, we sought to determine the physiological relevance of the interaction between Plk3 and CIB1. Our results demonstrate that forced overexpression of CIB1 inhibits the rate of cell proliferation and induces the formation of multinucleated cells. This phenotype is rescued by coexpression of Plk3 and mimicked by a specific downregulation of Plk3. We further show that inhibition of Plk3 or downregulation of its expression results in microtubule disorganization, defective centrosome segregation, and failure of cytokinesis. Taken together, these results provide evidence that CIB1 regulates activity and function of Plk3 and is needed for the proper completion of cell division. These findings are highly relevant to the physiological process of megakaryopoiesis. During megakaryopoiesis hematopoietic progenitor cells undergo endomitosis, a process in which normal cell division is perturbed in such a way that nuclear division occurs without cytokinesis resulting in polyploid cells. Thus our finding can be extended to understand the process of endomitosis.
Although CIB1 has been shown to interact with Plk2 and Plk3 (Kauselmann et al., 1999), the effect of CIB1-overexpression that we observed here is specific to Plk3 for the following reasons; 1) CIB1 only associated with Plk3 in these cells. 2) Specific down regulation of Plk3 mimicked the CIB1 overexpressing phenotype. 3) Coexpression of Plk3 blocked multinucleation induced by CIB1. 4) Plk2 functions at the G1 phase of the cell cycle, whereas we find defects in the late stages of cell cycle. 5) CIB1 does not affect centrosome duplication, which requires Plk2 activity (Warnke et al., 2004). On the contrary, we found an increased number of centrosomes in CIB1-overexpressing cells. Failure to inhibit centrosome duplication by CIB1 indicates that Plk2 is not targeted by CIB1 in these cells. 6) Specific knockdown of Plk2 does not generate multinucleation (Warnke et al., 2004), whereas Plk3 does (Fig. 4). Recently, it has been shown that Plk4+/− embryonic fibroblasts show high incidence of multinucleation (Rosario et al.). However, CIB1 has not been shown to interact with Plk4. Furthermore, Plk4 deficiency causes multinucleation via disruption of RhoGTPase function and aberrant actomyosin ring formation during cytokinesis (Rosario et al., 2010).
It is also known that the polo box is important for the subcellular localization of polo-like kinase family members (Elia et al., 2003; Lee et al., 1998), and it is interesting to note that CIB1 has been shown to bind to the polo box of Plk3, possibly in a phosphorylation independent manner (Kauselmann et al., 1999). Our results show that knockdown of CIB1 does not affect Plk3 localization to the centrosome excluding the possibility that CIB1 may regulate subcellular localization of Plk3.
Expression of Plk3 is rapidly induced upon exposure of serum-starved cells to growth factors, implicating Plk3 in the regulation of cell proliferation (Li et al., 1996). Unlike other members of the polo-like kinase family, Plk3 expression levels remain rather constant during the normal cell cycle (Bahassi el et al., 2002), except that it is slightly upregulated during the G1 phase (Zimmerman and Erikson, 2007). However, in head, neck, and lung carcinomas, it has been shown that Plk3 levels are significantly downregulated, contrasting that of another family member, Plk1 (Dai et al., 2002a; Holtrich et al., 2000; Li et al., 1996). It is thus possible that Plk3 activity is required for normal cell division in such precursor cells, and that its downregulation may aid cancer development. Interestingly, we have shown that CIB1 is upregulated during breast cancer progression (Naik et al.). It is therefore possible that upregulation of CIB1 could be the mechanism by which Plk3 activity is turned off in cells where Plk3 is substantially expressed. The fact that we routinely observed normal DNA synthesis (Fig. 1E) and complete nuclear division upon CIB1-overexpression suggests that CIB1 affects the latter stages of the cell cycle. This is consistent with the implicated role of Plk3 in microtubule organization (Wang et al., 2002). However, it is also possible that inhibition of Plk3 by CIB1 causes an early mitotic defect that results into the observed multinucleated phenotype. Further experimentation will be necessary to address this possibility.
It has been indicated that Plk3 plays an important role in the regulation of the centrosomes (MTOCs) (Dai et al., 2002b; Huang et al., 2004). Our results indicate that knockdown of Plk3 expression or inhibition of its activity causes disorganization of microtubules, thus affecting centrosome separation, but not centrosome duplication. This is consistent with the previous report that Plk3 regulates dynamics of microtubule assembly (Wang et al., 2002). The hypothesis that Plk3 regulates microtubule organization by upregulating the minus end activity, thereby aiding in the microtubule dynamics necessary for successful cellular division (Wang et al., 2002) is supported by our observation that Plk3 localizes to the centrosomes (Figure 6) and inhibition of Plk3 results in stability of microtubules. Plk3 has also been shown to localize to the cell midbody region in exit from mitosis and thereby possibly serves to regulate the process of cytokinesis (Conn et al., 2000). During the process of cytokinesis, the final step of cell division, central spindle microtubules influence the equatorial cortex to promote cleavage furrow formation and ingression; thus failure of cytokinesis could be a result of microtubule disorganization (D'Avino et al., 2005).
Taken together, our results suggest that CIB1 is a regulatory subunit of Plk3, and when overexpressed, it dually affects the normal progression of the cell cycle. First, CIB1 inhibits normal centrosome segregation, and second, it blocks cytokinesis, both possibly by disruption of microtubule integrity (Doxsey, 2001). Thus, in normal cell cycles Plk3 activity is regulated by CIB1 level and an unwanted change in their expression may be a key determinant in the transformation from the normal to malignant phenotypes, making Plk3 and CIB1 potential targets for therapeutic intervention.
Acknowledgement
The authors would like to thank K. Czymmek for his excellent help with confocal microscopy and D. Vuppalanchi for making the Plk3 shRNA construct. This work was supported by the National Institutes of Health Grant HL57630, National Center for Research Resources Grant 1P20RR155801 (to U.P.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–275. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- Bahassi el M, Conn CW, Myer DL, Hennigan RF, McGowan CH, Sanchez Y, et al. Mammalian Polo-like kinase 3 (Plk3) is a multifunctional protein involved in stress response pathways. Oncogene. 2002;21:6633–6640. doi: 10.1038/sj.onc.1205850. [DOI] [PubMed] [Google Scholar]
- Clay FJ, McEwen SJ, Bertoncello I, Wilks AF, Dunn AR. Identification and cloning of a protein kinase-encoding mouse gene, Plk, related to the polo gene of Drosophila. Proc Natl Acad Sci U S A. 1993;90:4882–4886. doi: 10.1073/pnas.90.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn CW, Hennigan RF, Dai W, Sanchez Y, Stambrook PJ. Incomplete cytokinesis and induction of apoptosis by overexpression of the mammalian polo-like kinase, Plk3. Cancer Res. 2000;60:6826–6831. [PubMed] [Google Scholar]
- D'Avino PP, Savoian MS, Glover DM. Cleavage furrow formation and ingression during animal cytokinesis: a microtubule legacy. J Cell Sci. 2005;118:1549–1558. doi: 10.1242/jcs.02335. [DOI] [PubMed] [Google Scholar]
- Dai W, Liu T, Wang Q, Rao CV, Reddy BS. Down-regulation of PLK3 gene expression by types and amount of dietary fat in rat colon tumors. Int J Oncol. 2002a;20:121–126. [PubMed] [Google Scholar]
- Dai W, Wang Q, Traganos F. Polo-like kinases and centrosome regulation. Oncogene. 2002b;21:6195–6200. doi: 10.1038/sj.onc.1205710. [DOI] [PubMed] [Google Scholar]
- Doxsey S. Re-evaluating centrosome function. Nat Rev Mol Cell Biol. 2001;2:688–698. doi: 10.1038/35089575. [DOI] [PubMed] [Google Scholar]
- Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24:267–276. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, et al. The molecular basis for phosphodependent substrate targeting and regulation of plks by the polo-box domain. Cell. 2003;115:83–95. doi: 10.1016/s0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- Fode C, Motro B, Yousefi S, Heffernan M, Dennis JW. Sak, a murine protein-serine/threonine kinase that is related to the Drosophila polo kinase and involved in cell proliferation. Proc Natl Acad Sci U S A. 1994;91:6388–6392. doi: 10.1073/pnas.91.14.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtrich U, Wolf G, Yuan J, Bereiter-Hahn J, Karn T, Weiler M, et al. Adhesion induced expression of the serine/threonine kinase Fnk in human macrophages. Oncogene. 2000;19:4832–4839. doi: 10.1038/sj.onc.1203845. [DOI] [PubMed] [Google Scholar]
- Huang X, Ruan Q, Fang Y, Traganos F, Darzynkiewicz Z, Dai W. Physical and Functional Interactions Between Mitotic Kinases During Polyploidization and Megakaryocytic Differentiation. Cell Cycle. 2004;3:946–951. [PubMed] [Google Scholar]
- Kauselmann G, Weiler M, Wulff P, Jessberger S, Konietzko U, Scafidi J, et al. The polo-like protein kinases Fnk and Snk associate with a Ca (2+)- and integrin-binding protein and are regulated dynamically with synaptic plasticity. Embo J. 1999;18:5528–5539. doi: 10.1093/emboj/18.20.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely PJ, Fong AM, Zutter MM, Santoro SA. Alteration of collagen-dependent adhesion, motility, and morphogenesis by the expression of antisense alpha 2 integrin mRNA in mammary cells. J Cell Sci. 1995;108:595–607. doi: 10.1242/jcs.108.2.595. [DOI] [PubMed] [Google Scholar]
- Lee KS, Grenfell TZ, Yarm FR, Erikson RL. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc Natl Acad Sci U S A. 1998;95:9301–9306. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Ouyang B, Pan H, Reissmann PT, Slamon DJ, Arceci R, et al. Prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be downregulated in lung carcinomas. J Biol Chem. 1996;271:19402–19408. doi: 10.1074/jbc.271.32.19402. [DOI] [PubMed] [Google Scholar]
- Ma S, Liu MA, Yuan YL, Erikson RL. The Serum-Inducible Protein Kinase Snk Is a G(1) Phase Polo-Like Kinase That Is Inhibited by the Calcium- and Integrin-Binding Protein CIB. Mol Cancer Res. 2003;1:376–384. [PubMed] [Google Scholar]
- Meraldi P, Lukas J, Fry AM, Bartek J, Nigg EA. Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat Cell Biol. 1999;1:88–93. doi: 10.1038/10054. [DOI] [PubMed] [Google Scholar]
- Mukhina S, Wang YL, Murata-Hori M. Alpha-actinin is required for tightly regulated remodeling of the actin cortical network during cytokinesis. Dev Cell. 2007;13:554–565. doi: 10.1016/j.devcel.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik MU, Naik TU, Suckow AT, Duncan MK, Naik UP. Attenuation of junctional adhesion molecule-A is a contributing factor for breast cancer cell invasion. Cancer Res. 2008;68:2194–2203. doi: 10.1158/0008-5472.CAN-07-3057. [DOI] [PubMed] [Google Scholar]
- Naik MU, Naik UP. Calcium-and integrin-binding protein regulates focal adhesion kinase activity during platelet spreading on immobilized fibrinogen. Blood. 2003a;102:3629–3636. doi: 10.1182/blood-2003-05-1703. [DOI] [PubMed] [Google Scholar]
- Naik MU, Pham NT, Beebe K, Dai W, Naik UP. Calcium-dependent inhibition of polo-like kinase 3 activity by CIB1 in breast cancer cells. Int J Cancer. 2010 doi: 10.1002/ijc.25388. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik UP, Naik MU. Association of CIB with GPIIb/IIIa during outside-in signaling is required for platelet spreading on fibrinogen. Blood. 2003b;102:1355–1362. doi: 10.1182/blood-2003-02-0591. [DOI] [PubMed] [Google Scholar]
- Naik UP, Patel PM, Parise LV. Identification of a novel calcium-binding protein that interacts with the integrin alphaIIb cytoplasmic domain. J Biol Chem. 1997;272:4651–4654. doi: 10.1074/jbc.272.8.4651. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J Biol Chem. 2003;278:25277–25280. doi: 10.1074/jbc.C300126200. [DOI] [PubMed] [Google Scholar]
- Ouyang B, Pan H, Lu L, Li J, Stambrook P, Li B, et al. Human Prk is a conserved protein serine/threonine kinase involved in regulating M phase functions. J Biol Chem. 1997;272:28646–28651. doi: 10.1074/jbc.272.45.28646. [DOI] [PubMed] [Google Scholar]
- Park JE, Soung NK, Johmura Y, Kang YH, Liao C, Lee KH, et al. Polo-box domain: a versatile mediator of polo-like kinase function. Cell Mol Life Sci. 2010;67:1957–1970. doi: 10.1007/s00018-010-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai L, Lewis CE, McGee JO. Growth arrest of the breast cancer cell line, T47D, by TNF alpha; cell cycle specificity and signal transduction. Br J Cancer. 1993;67:290–296. doi: 10.1038/bjc.1993.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario CO, Ko MA, Haffani YZ, Gladdy RA, Paderova J, Pollett A, et al. Plk4 is required for cytokinesis and maintenance of chromosomal stability. Proc Natl Acad Sci U S A. 2010;107:6888–6893. doi: 10.1073/pnas.0910941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shock DD, Naik UP, Brittain JE, Alahari SK, Sondek J, Parise LV. Calcium-dependent properties of CIB binding to the integrin alphaIIb cytoplasmic domain and translocation to the platelet cytoskeleton. Biochem J. 1999;342(Pt 3):729–735. [PMC free article] [PubMed] [Google Scholar]
- Simizu S, Osada H. Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nat Cell Biol. 2000;2:852–854. doi: 10.1038/35041102. [DOI] [PubMed] [Google Scholar]
- Simmons DL, Neel BG, Stevens R, Evett G, Erikson RL. Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol Cell Biol. 1992;12:4164–4169. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Xie S, Chen J, Fukasawa K, Naik U, Traganos F, et al. Cell cycle arrest and apoptosis induced by human Polo-like kinase 3 is mediated through perturbation of microtubule integrity. Mol Cell Biol. 2002;22:3450–3459. doi: 10.1128/MCB.22.10.3450-3459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnke S, Kemmler S, Hames RS, Tsai HL, Hoffmann-Rohrer U, Fry AM, et al. Polo-like kinase-2 is required for centriole duplication in mammalian cells. Curr Biol. 2004;14:1200–1207. doi: 10.1016/j.cub.2004.06.059. [DOI] [PubMed] [Google Scholar]
- Wu Q, Sahasrabudhe RM, Luo LZ, Lewis DW, Gollin SM, Saunders WS. Deficiency in myosin light-chain phosphorylation causes cytokinesis failure and multipolarity in cancer cells. Oncogene. 2010;29:4183–4193. doi: 10.1038/onc.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman WC, Erikson RL. Polo-like kinase 3 is required for entry into S phase. Proc Natl Acad Sci U S A. 2007;104:1847–1852. doi: 10.1073/pnas.0610856104. [DOI] [PMC free article] [PubMed] [Google Scholar]