Abstract
Adult bone marrow (BM) harbors several small populations of cells which may contribute to cardiac and endothelial repair, such as endothelial progenitor cells (EPCs), mesenchymal stromal cells (MSCs) and very small embryonic-like cells (VSELs) expressing several markers of pluripotent stem cells (PSCs), such as Oct-4, Nanog and SSEA-1. Such cells were identified in mice bone marrow, peripheral blood and solid organs as well as in umbilical cord blood (UCB) and peripheral blood (PB) in humans. The adult BM-derived VSELs may undergo differentiation into cells derived for all three germ layers, including cardiomyocytes and vascular endothelial cells. VSELs can be isolated using a multiparameter live cell sorting technique with special gating strategy based on their small size, expression of stem cell markers (Sca-1 in mice, CXCR4 and CD133 in humans) and absence of hematopoietic lineage markers (CD45− Lin−). Experiments in murine models of myocardial infarction (MI) demonstrated population of VSELs expressed also early markers of cardiac and endothelial lineages (GATA-4, Nkx2.5/Csx, VE-cadherin, von Willebrand factor) which migrated to stromal-derived factor-1 (SDF-1) and other chemoattractant gradient and underwent rapid mobilization into peripheral blood in experimental MI mice models. Recently, we demonstrated the mobilization of VSELs expressing PSC, early cardiac and endothelial markers in patients with acute MI. In addition to BM, VSELs were also identified in several murine solid organs including the heart and brain, as well as in umbilical cord blood and peripheral blood in adult humans. We hypothesized that VSELs are quiescent progeny of epiblast-derived PSCs that are deposited during organogenesis in developing organs. In experimental MI intramyocardial injection of VSELs was more efficient than that of HSCs at improving left ventricular ejection fraction and attenuation of myocardial hypertrophy. VSELs can be useful in translational studies of cardiovascular repair.
Keywords: very small embryonic-like cells, bone marrow, cardiomyocyte
Introduction
In the last decade the concept of myocardial regeneration was rapidly translated from basic science and animal experiments to application of bone marrow (BM) – derived stem and progenitor cells in multiple clinical trials including patients with acute myocardial infarction (MI) and ischemic cardiomyopathy (Abdel-Latif et al., 2007). So far, in the majority of clinical trials heterogenous population of non-selected bone marrow-derived mononuclear cells was used and currently there is no proof that any particular type of cells might be superior to others for myocardial repair in patients with MI and ischemic cardiomyopathy (Dimmeler et al., 2008).
Parallel to ongoing clinical trials with BM cells, investigating the optimal cell delivery route, dose and target population of patients, new concepts emerged which aim to enhance the capability of BM cells to induce the functional recovery of the myocardium or identifying new, more potent cell populations including cardiac stem cells (Messina et al., 2004), genetically engineered bone marrow progenitor cells (eg. Guided Bone Marrow-derived Mesenchymal Cardiopoietic Cells) (Sasaki et al., 2006), allogeneic mesenchymal stromal cells (Pittenger & Martin, 2004) and recently, various types of pluripotent stem cells (PSC), including iPS (Martinez-Fernandez et al., 2009) and very small embryonic-like stem cells (VSELs) (Kucia et al., 2006). The progress of regenerative cardiovascular medicine generated the need for safe, ethically acceptable and therapeutically efficient sources of PSCs. VSELs seem to be an optimal population of cells for studies on cardiac repair as they can be efficiently isolated from adult BM, expanded in culture and differentiated. This paper reviews the recent data on VSELs and their potential application in experimental and clinical research.
Structural and molecular characteristics of VSELs
VSELs were initially discovered in adult mice using fluorescence activated cell sorting (FACS)-based live cell sorting technique which allowed the isolation of a rare population of Sca-1+lin−CD45− cells enriched for early embryonic markers (Oct-4, Nanog, SSEA-1, Rex1, Dppa3, Rif-1). Consistently with previous SDF-1 chemotaxis-based studies FACS-isolated cells expressed chemokine receptor CXCR4 (Kucia et al., 2006). The morphology of the cells was investigated using transmission electron microscopy which showed their distinctive morphology and size differentiating VSELs from HSC in particular in terms of size (3–6 µm vs. 6–8 µm for HSC), chromatin structure and nucleus/cytoplasm ratio. Based on their small size, presence of PSC markers, distinct morphology (open-type chromatin, large nucleus, narrow rim of cytoplasm with multiple mitochondria) and ability to differentiate into all three germ layers, including mesoderm-derived cardiomyocytes, these cells were named very small embryonic-like stem cells (Kucia et al., 2006; Kucia et al., 2008; Wojakowski et al., 2007).
Presence of characteristic markers of VSELs was confirmed using several complementary research tools. Real-time RT-PCR (RQ-PCR) showed increased levels of mRNA for PSC markers (Oct-4, Nanog, SSEA-1, Rex-1) and it was confirmed on protein level using immunofluorescent staining and ImageStream system (ISS). Also it was recently demonstrated that in VSELs the promoters of Oct4 and Nanog contain transcriptionally active chromatin excluding the possibility of amplification of pseudogenes (Shin et al., 2009).
Comparison of murine BM-derived VSELs with other populations of cells demonstrated that VSELs are smaller than hematopoietic stem cells (6.50 µm), monocytes and granulocytes and erythrocytes (4.90 µm). However, they are larger than platelets (2.12 µm) (Ratajczak et al., 2009).
In contrast to HSC, VSELs are primarily non-hematopoietic cells and their represent respective immunophenotype – absence of pan-hematopoietic marker (CD45−) and hematopoietic lineage markers (Lin−). Also freshly isolated and expanded VSELs do not from hematopoietic colonies when standard co-culture protocol using C2C12 myoblasts are used (Zuba-Surma & Ratajczak, 2010).
There are some differences of the VSEL phenotype between mice and humans (Table). Murine VSELs express Sca-1 antigen, whereas in human cord blood and peripheral blood of adults the population of VSELs consists of lin− CD45− positive for CXCR4, CD133 and CD34 antigens as confirmed on the mRNA level by RQ-PCR and protein level by IF and ISS. Expression of PSC markers Oct-4 and SSEA-4 was confirmed in population of small cells (~6–8 µm), therefore human VSELs are larger than murine. Presence of Oct-4 was confirmed in approximately 50–60% of VSELs isolated from human peripheral blood (Zuba-Surma & Ratajczak, 2010).
Table.
Major differences between isolation of murine bone marrow-derived and human umbilical cord blood-derived cells.
| Murine BM-VSELs | Human VSELs | |
|---|---|---|
| SURFACE MARKERS | Sca-1+, CXCR4+, CD133+, CD34+, SSEA-1+, AP+, c-Met+, LIF-R+, CD45−, Lin−, HLA-DR−, MHC I −, CD90−, CD29−, CD105− | CXCR4+, CD133+, CD34+, SSEA-4+, AP+, c-Met+, LIF-R+, CD45−, Lin−, HLA-DR−, MHC I −, CD90−, CD29−, CD105− |
| SIZE | 4–5 µm | 6–8 µm |
| FREQUENCY | 0.03% of nucleated cells | 0.01% of nucleated cells |
| TISSUES | Bone marrow, peripheral blond, brain, muscle, thymus, heart, liver, kidney, testes, spleen, lung, retina, pancreas, fetal liver | Bone marrow, umbilical cord blood, peripheral blood |
| ANTIBODIES USED FOR FACS-BASED ISOLATION | anti mouse-Ly-6/E (Sca-1), anti mouse-CD45, anti mouse-lineage: CD45R/B220, Gr-1, TCRαβ, TCRγδ, CD11b, Ter119 | anti-human-CD133, anti-human-CD45, anti-human-lineage: CD2,CD3, CD14, CD66b, CD24, CD56, CD16, CD19, CD235a |
Strategy of isolation of VSELs
Figure 1 illustrates the protocol for FACS-based live cell sorting from BM and PB samples. Initially BM is flushed from the femurs and nucleated cells are isolated by lysis of erythrocytes instead of using the Ficoll-based protocol as the latter might have led to the losing of very small cells. This is particularly important because VSELs represent very small population of cells, approximately 0.030 ± 0.008% of total BM cells in mice. Cell lysis is used also for isolation of VSELS from PB and CB (Zuba-Surma et al., 2008).
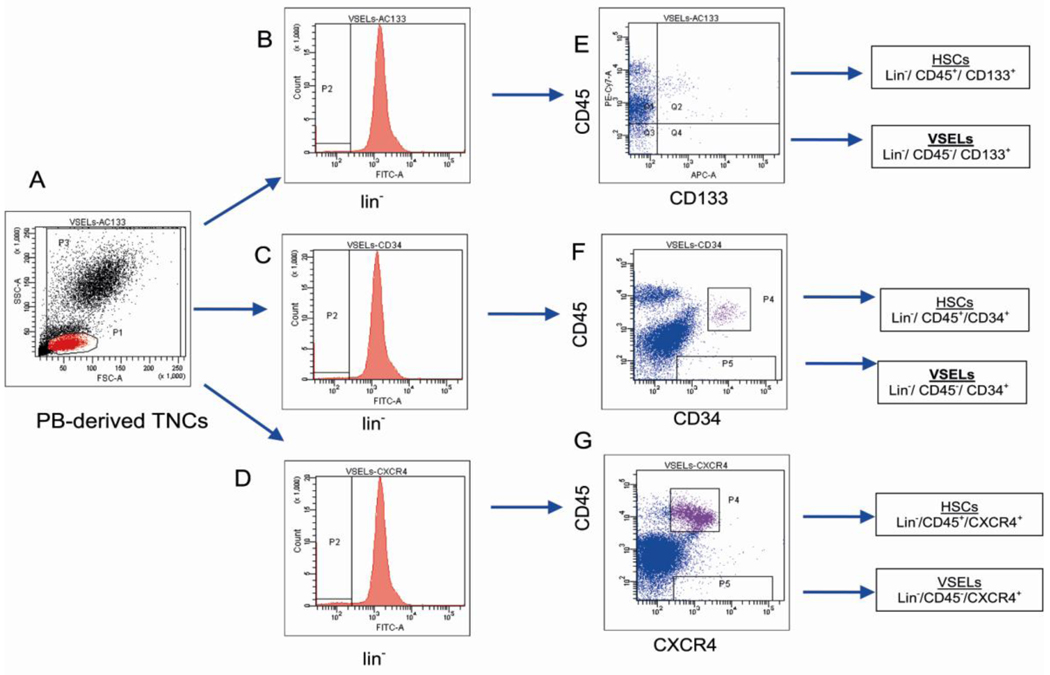
Fig. 1. Gating strategy for isolating VSELs by fluorescence- activated cell sorting (FACS).
Human VSELs were isolated from umbilical cord blood (CB)-derived total nucleated cells (TNCs) stained for: i) CD45 panleukocytic antigen, ii) hematopoietic lineages markers (Lineage/ Lin) and iii) CD133 stem cell antigen. Based on the size predefined beads (standard diameters of 1, 2, 4, 6, 10, and 15 µm) the gate R1 was set up to include all objects larger than 2µm (Panel A). TNCs were further visualized on the dot plot including region R1 and showing forward scatter (FSC) vs. side scatter (SSC) signals of cells that are related to the size and granularity/complexity of the cell, respectively (Panel B). Cells from region R1 were analyzed for hematopoietic lineages markers expression and only Lin− events were included into region R2. Population from region R2 was subsequently visualized based on CD45 and CD133 antigens (Panel D). Lin−/CD45+/CD133+ cells (VSELs) were sorted as events enclosed in logical gate including regions R1, R2 and R4, while Lin−/CD45−/CD133+ hematopoietic stem/progenitor cells (HSPCs) from gate including regions R1, R2 and R3. Percentages show the average content of each cellular subpopulation (± SEM) in total nucleated cells.
For sorting, cells are stained with Sca-1 (in mice) or CD133 (in humans), anti-CD45, anti-hematopoietic lineages markers (Lin) and CXCR4. Cells are sorted using a multiparameter, live sterile cell sorting systems (MoFlo, Beckman Coulter or FACSAria, Beckton Dickinson) (Kucia et al., 2006). To validate the inclusion of small events (2–10 µm) in the extended lymphate synthetic beads of defined size (1 –15 µm) are used (Zuba-Surma et al., 2008). Strategy of gating includes the extended lymphocyte gate which contains lymphocytes based on their size and granularity but on the left side extends to include events sized > 2 µm. Such gate contains approximately 95% of VSELs (Zuba-Surma et al., 2008; Zuba-Surma & Ratajczak, 2010). ISS is particularly useful tool to confirm the presence of viable cells in sorted material, because this area of the cytogram contains a substantial number of cellular debris. Therefore decoding of the particular events on cytogram using ISS allows to confirm their typical morphology, presence of nucleous and colocalization of VSEL markers (Zuba-Surma et al., 2008; Zuba-Surma & Ratajczak, 2010).
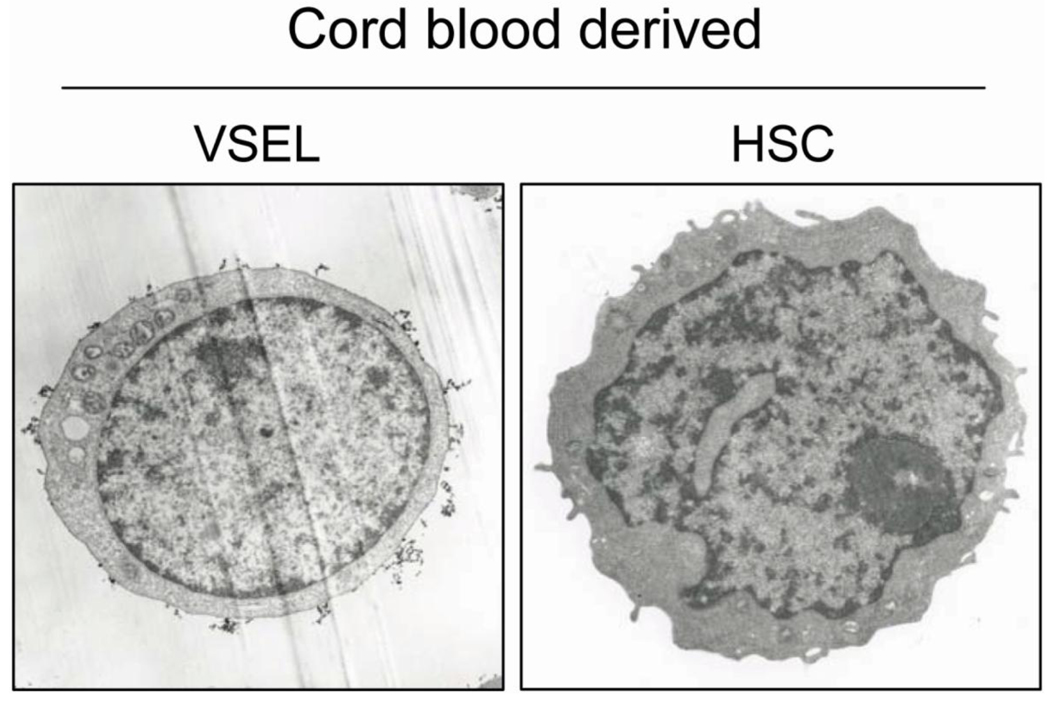
Comparison of VSELs and HSC using the ISS is shown in figure 2.
Fig. 2. Representative images of human peripheral blood- derived VSEL and HSPC by ImageStreamX system.
Human blood cells were stained for markers distinguishing VSELs such as: i) CD45 panleukocytic antigen (APC-Cy7, cyan), ii) hematopoietic lineages markers (FITC, green) and iii) stem cell antigens CD133 (PE, yellow) and CD34 (APC, violet). Nuclei were stained with Hoechst 33342 dye. Images were collected by imaging flow cytometer – ImageStreamX system. VSELs and HSPCs were distinguished based on CD45 antigen expression.
Functional features of VSELs
Freshly sorted VSELs can be expanded in coculture with C2C12 murine myoblast feeder layer. After 7 days of co-culture, approximately 5–10% of all VSELs form sphere-like clusters consisting of a few hundred cells resembling embryoid bodies (VSEL-derived spheres, VSEL-DSs). VSEL-DSs express placenta-like alkaline phosphatase (PLAP). Expanded population of VSELs isolated from VSEL-DS retain the pluripotent capacity and in cultures with differentiating media give rise to cellular lines from all three germ layers, such as mesodermal cardiomyocytes, ectodermal neural cells and endodermal pancreatic cells (Kucia et al., 2006).
Although freshly isolated VSELs are primarily non-hematopoietic cells without the radioprotection capability in lethally irradiated mice, when cultured in hematopoiesis permissive environment (OP-9 feeder layer) eventually differentiate in to HSC. Moreover VSEL-derived HSCs give rise to hematopoietic colonies in vitro and are able to reconstitute hematopoiesis in lethally irradiated mice (Ratajczak, 2008). The differentiation potency of was documented also in circulating murine VSELs after injection of G-CSF. Consistently with BM cells, rapidly mobilized VSELs showed up-regulation of PSC markers. Additionally levels of PSC markers in circulating VSELs were comparable to ES-D3 murine embryonic cell line and the cells were capable of differentiation into cells derived from all three germ layers. These findings support not only the pluripotency of VSELs, but also the hypothesis that mobilized cells might contribute to tissue repair (Kucia et al., 2008).
Pluripotency of VSELs
VSELs neither complemnent blastocyst development nor form teratomas after injection into experimental animals and we identified a molecular mechanism responsible for this phenomenon – that is modification of imprinted genes. Accordingly, we noticed that murine Oct-4+ VSELs do not proliferate in vitro if cultured alone and that the quiescence of these cells is epigenetically regulated by DNA methylation of genomic imprinting, which is an epigenetic program that ensures the parent-of-specific monoallelic transcription of imprinted genes. The imprinted genes play a crucial role in embryogenesis, fetal growth, totipotential status of the zygote, and pluripotency of developmentally early stem cells. The expression of imprinted genes is regulated by DNA methylation on differential methylated regions (DMRs), which are CpG-rich cis-elements in their loci.
We noticed that VSELs freshly isolated from murine BM erase the paternally methylated imprints (e.g., Igf2-H19, Rasgrf1 loci), however they hypermethylate the maternally methylated ones (e.g., Igf2 receptor (Igf2R), Kcnq1-p57KIP2, Peg1 loci). According to the parental conflict theory, paternally expressed imprinted-genes (Igf2, Rasgrf1) enhance the embryo growth and maternally expressed genes (H19, p57KIP2, Igf2R) inhibit cell proliferation, the unique genomic imprinting pattern observed on VSELs demonstrates growth-repressive imprints in these cells. Based on our data, VSELs highly express growth-repressive genes (H19, p57KIP2, Igf2R) and downregulate growth-promoting ones (Igf2, Rasgrf1), which explains the quiescent status of VSELs.
It is physiological mechanism (similar one operates at level of primordial germ cells) that prevent these cells from tumor formation (Shin et al., 2009).
Sources of VSELs
So far the presence of VSELs was confirmed in mice and humans. After initial findings in murine BM, this population of cells was identified also in murine peripheral blood, fetal liver and in other adult tissues including solid organs: brain, retina, kidneys, pancreas, skeletal muscles spleen, and thymus. Among the solid organs probably the richest source of VSELs is brain. Although the pluripotent features of VSELs, including formation of VSEL-DS was confirmed for cells derived from fetal liver these findings need to be confirmed for adult organs (Zuba-Surma et al., 2009). In humans VSELs were found in umbilical cord blood and peripheral blood. Currently we investigate the presence of these cells in human bone marrow and cardiac tissue (Ratajczak et al., 2009; Ratajczak et al., 2008)
Hypothetical role of VSELs in adult organisms
We hypothesized that VSELs are derived from epiblast-stem cells and form a pool of quiescent PSC deposited in the BM, heart and other tissues during early organogenesis (Ratajczak et al., 2007). VSELs display some features of primordial germ cells such as expression of CXCR4, PLAP, Stella, Fragilis, Nobox and Hdac6.
We hypothesize that VSELs are progeny of epiblast cells which migrated to developing tissues and organs. Therefore and that they may serve as a reserve pool of pluripotent cells capable of tissue repair (Ratajczak et al., 2007). VSELs may therefore participate in the turnover of resident tissue-specific progenitor cells located throughout the organs and capable of proliferation and differentiation upon activation by tissue damage, ie. stroke, myocardial infarction (Ratajczak et al., 2007). Of course pluripotent cells have potential of inducing carcinogenesis and this particular issue needs to be investigated. So far it seems that VSELs isolated from adult BM are in quiescent state which may be a physiological mechanism of prevention of teratoma formation (Ratajczak et al., 2007).
Circulation of VSELs
Acute myocardial infarction (MI) and ischemic stroke induce generalized inflammatory response reflected by increased local and blood levels of chemoattractants (phospholipids, kinins, chemokines, cytokines, growth factors, components of the complement cascade) leading to subsequent mobilization of stem and progenitor cells from BM. These circulating cells represent predominantly committed lineages (monocytes, granulocytes, lymphocytes) however significantly less numerous subpopulations of tissue-committed progenitors as well as multi- and pluripotent cells which may play a role in cardiovascular repair. Numerous studies confirmed that MI induces rapid mobilization of hematopoietic stem cells (HSC), endothelial progenitor cells (EPC), mesenchymal stromal cells (MSC) and pluripotent VSELs (Krankel et al., 2008; Kucia et al., 2004; Massa et al., 2005; Wojakowski et al., 2004).
It is hypothesized that stem cells residing in BM and other tissues in order to reduce the cardiac ischemic injury the BM cells must undergo mobilization and subsequently home and engraft into the myocardium (Kucia et al., 2005).During experimental MI in mice there is a rapid mobilization of VSELs from the BM to the peripheral blood. Kucia et al. showed that MI induces release of non-hematopoietic Sca-1+lin−CD45− cells enriched for early cardiac markers (GATA-4 and Nkx2.5/Csx), which migrated along the SDF-1 gradient (Kucia et al., 2004; Wojakowski et al., 2009). ese findings suggested for the first time that circulation of BM-derived VSELs may be an important mechanism of myocardial repair.
Consistently, in humans very small number of VSELs [0.8–1.3 cells/µL] as well as HSC (CD45+) and EPC (CD34+CD133+VEGFR2+) is continuously mobilized from BM into peripheral blood. Circulating VSELs express PSC markers and low levels of cardiac-specific markers (Massa et al., 2005; Wojakowski et al., 2009). This may be an important physiological mechanism which allows to maintain the homeostasis of the pool of tissue-resident stem cell niches (Tang et al., 2010). By contrast in acute MI, the number of VSEL significantly increased early after the onset of symptoms along with increased expression PSC and cardiac markers in isolated VSELs. Populations of CD34+CD133+VEGFR2+ EPC and CD34+CXCR4+ cells increased significantly in acute MI, however the absolute numbers of cells were higher than VSELs (Wojakowski et al., 2009).
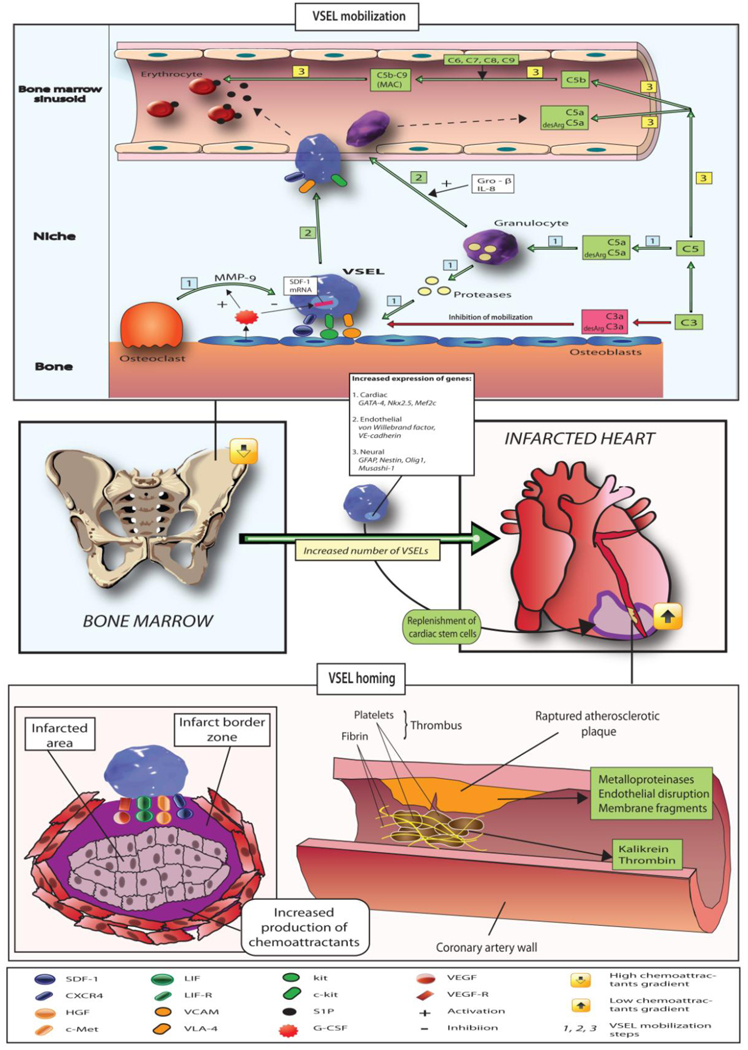
Trafficking of stem cells involves the mobilization into peripheral blood, homing, adhesion and engraftment into the target tissue. This process, as a part of inflammatory response and tissue repair requires signaling mediated primarily by chemokines, activation of the complement system as well as some phospholipids (sphingosine-1-phosphate) which are the most important regulators of cell trafficking, survival and function. Numerous hematopoietic and inflammatory cytokines known to increase progenitor cell mobilization are markedly up-regulated in acute coronary syndromes, and also increased to lesser degree in CAD. The processes are regulated by α-chemokine stromal derived factor-1 (SDF-1), and its receptor CXCR4 and other important cytokine–receptor systems regulating the stem cell mobilization and homing are leukemia inhibitory factor (LIF) – LIF receptor, hepatocyte growth factor (HGF) – c-met axis, stem cell factor-CD117 axes. All of them are up-regulated and activated in acute myocardial necrosis (Kucia et al., 2004; Kucia et al., 2005; Kucia et al., 2006). Figure 3 summarizes the mobilization and homing of VSELs.
Fig. 3. The mechanism of mobilization and homing of VSELs in acute myocardial infarction and ischemic stroke.
Ischemia induces increased expression of chemoattractants [phospholipids (such as sphingosine-1-phosphate), chemokines (SDF-1), growth factors (VEGF, HGF), cytokines (LIF), and activation of complement cascade]. In healthy subjects very low number of circulating VSELs can be detected in peripheral blood and expression of tissue specific markers is low. After mobilization in the setting of acute ischemia VSELs are mobilized and the mRNA levels for pluripotent markers, early cardiac and neural markers is significantly increased. Tissue ischemia leading to cleavage of SDF-1/CXCR4 in bone marrow and increased expression of chemoattractants in the ischemic organ creates the reversal of chemoattractant gradient leading to the release of VSELs from the bone marrow niches and their homing to injured organ.
Similarly, ischemic stroke triggers the mobilization of VSEL SCs expressing neural markers (GFAP, nestin, beta-III-tubulin, Olig1, Olig2, Sox2, Musashi) which suggest that the same mechanism involving VSELs might mediate the tissue repair in different types of ischemic injury (Paczkowska et al., 2009).
So far we do not have proof sufficient data to prove that mobilization of VSELs into the peripheral blood coexists with their increased number in the target tissue.
Clinical and demographic correlates of VSELs mobilization
Murine experiments showed that the number of circulating VSELs is reduced in older mice, corresponding to lower expression of PSC markers in blood and BM-derived VSELs (Kucia et al., 2008). In our studies in patients with acute MI the mobilization of VSELs was significantly higher in patients younger than 50 years than in older. Also in diabetic patients the number of circulating VSELs was reduced in comparison to non-diabetics. This is an important issue because diabetes is known to be a state associated with increased senescence and deteriorated function of stem and progenitor cells (Orlandi et al., 2010). We found also that the number of circulating VSELs is lower in MI patients with more severely impaired cardiac function. Number of cells was inversely correlated with left ventricular ejection fraction, levels of troponin I and activity of CK-MB corresponding to the extent of the myocardial necrosis (Wojakowski et al., 2009; Wojakowski et al., 2006).
Potential role in cardiovascular repair
So far most data about VSELs in cardiovascular disease addresses the potential role of circulating cells as markers of ischemic injury in humans, whereas animal data suggest possibility of their application in prevention of left ventricular dysfunction after MI.
Our group developed an ex vivo expansion and differentiation protocol which allows differentiation of BM-derived VSELs into cardiomyocytes. In the first step, VSELs are co-cultured with myoblast line (C2C12) where the cells expand and form VSEL-DS.Subsequently, VSELs isolated from VSEL-DS by FACS sorting (EGFP+ VSELs and EGFP− myoblasts) are plated on cardiac differentiation media to expand them into maturing cardiomyocytes. The time-course of changes in expression of early cardiac markers and cardiac structural proteins over 21 days resembles the maturation of cardimyocytes from embryonic stem cells (Kucia et al., 2006; Wojakowski et al., 2010). The possibility of cardiomyocyte differentiation of BM-derived VSELs was a rationale for proof-of-concept experiments. In the murine model of reperfused MI, direct intramyocardial injection of freshly isolated VSELs improved global and regional left ventricular contractility, and reduced myocardial hypertrophy. Dawn et al. showed that direct intramyocardial injection of VSELs 48 hours after coronary artery occlusion had promising effects. Administration of VSELs lead to improved contractility and reduced remodeling as well as reduced myocyte hypertrophy after 35 days of follow-up. In mice that received HSCs no such effects occurred. Interestingly, some, though rare, VSEL-derived GFP+ cardiac myocytes were found to be present in the recipients’ myocardial. Beneficial effects were observed despite use of only small number (10,000 cells) of VSELs. At the same time a much higher number of hematopoietic cells (100,000 cells) was not effective (Dawn et al., 2008). Importantly, when the VSELs expanded over C2C12 cells underwent subsequent cardiopoiesis-guided pre-differentiation for 5 days prior to the intramyocardial injection they were markedly more effective (100,000 cells per mice) than expanded and non-pre-differentiated cells. Cardiopoiesis-guided pretreatment resulted in improved left ventricular ejection fraction, myocardial systolic thickening and attenuated remodeling after 35 days. As shown in previous studies the effect of VSELs injections were probably mediated by paracrine effects because very few cells expressing cardiac markers and derived from the transplanted VSEL were present in the recipients myocardium (Zuba-Surma et al., 2010). The increased efficiency of pre-differentiated VSELs in terms of improved contractility and reduced remodeling were sustained over the 6 months follow-up. Additionally, limited number of VSELs-derived cardiomyocytes was documented (Zuba-Surma EK, AHA 2008, Circulation 2008:116:II204). The mechanisms of the beneficial effects remain elusive. In our murine in vivo studies we employed two different VSELs-based treatment strategies - either freshly isolated VSELs or cardiopoiesis-guided, predifferentiated expanded VSELs. We observed two parallel effects of freshly isolated VSELs on regeneration of myocardium after injection into heart. First one that was related to their differentiation into new myocardiocytes and another based on improvement of LVEF most likely related to paracrine effects. Secondly, we injected cells that were derived from short ex vivo expansion/differentiation of VSELs into cardiomyocytes. In this experiment we observed mostly paracrine effect and we are currently trying to identify factors secreted from VSELs-derived cardyomyocytes that have a beneficial effect on myocardial regeneration.
So far results of a single clinical trial using population of selected CD34+CXCR4+ cells which is enriched for VSELs are available. The objective of Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) study (NCT00316381) was to assess the efficacy of intracoronary infusion of autologous BM-derived CD34+CXCR4+ progenitor cells in comparison to non-selected BMMNC on LVEF in patients with acute ST-segment elevation MI and reduced below 40% LVEF. We randomly assigned 200 patients to intracoronary infusion of non-selected MNC (n=80) or selected CD34+CXCR4+ (n=80) BM cells or to the control group without BM cell treatment. CD34+CXCR4+ cells were isolated by two step immunomagnetic selection using the magnetic beads attached to monoclonal antibodies against CD34 and CXCR4 antigens (Miltenyi Biotec). Primary end-point: change of LVEF and volumes measured by MRI before and 6 months after the procedure. After 6 months LVEF increased by 3% (p=0.01) in patients treated with MNC, 3% in patients receiving selected cells (p=0.04) and remained unchanged in CTRL group (p=0.73). There were no significant differences in absolute changes of LVEF between the groups. Absolute changes of LVESV and LVEDV were not significantly different in all groups. Significant increase of LVEF was observed only in patients treated with BM cells who had baseline LVEF < median (37%). Baseline LVEF < median and time from the onset of symptoms to primary PCI ≥ median were predictors of LVEF improvement in patients receiving BM cells. There were no differences in major cardiovascular event (death, reinfarction, stroke, TVR) between groups. The conclusion from the trial is that in patients with acute MI and impaired LVEF treatment with BM cells does not lead to a significant improvement of LVEF or volumes. There was however a trend in favor of cell therapy in patients with most severely impaired LVEF and longer delay between the symptoms and revascularization. The study used a population of cells positive for CXCR4 receptor, however these cells were not pure population of VSELs (Tendera et al., 2009).
Clinical studies using autologous VSELs are needed to validate these promising experimental data.
Summary
The progress of regenerative cardiovascular medicine generated the need for safe, ethically acceptable and therapeutically efficient sources of pluripotent stem cells (PSCs). Pluripotent stem cells, including VSELs, seem to be an optimal population of cells for studies on cardiac repair as they can be efficiently isolated from adult BM, expanded in culture and differentiated. Importantly, it seems that the process of their cardiogenic differentiation follows the same pattern as do embryonic stem cells. We postulate that human VSELs are promising cells for utilization in future clinical studies of patients with ischemic cardiomyopathy. However, their cardiogenic potential should be confirmed in humans and technical issues regarding their isolation, expansion and differentiation need to be addressed.
Acknowledgments
Funding: National Institutes of Health [R01 CA106281-01], European Union structural funds - Innovative Economy Operational Programme, grant No. POIG 01.02-00-109/09 "Innovative methods of stem cells applications in medicine" and Polish Ministry of Science and Higher Education grants 0651/P01/2007/32, 2422/P01/2007/32.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None of the authors have any conflict of interests in regard to the content of this manuscript.
References
- Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167(10):989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- Dawn B, Tiwari S, Kucia M, Zuba-Surma EK, Guo Y, SanganalMath SK, et al. Transplantation of Bone Marrow-Derived Very Small Embryonic-Like Stem Cells Attenuates Left Ventricular Dysfunction and Remodeling After Myocardial Infarction. Stem Cells. 2008;26(6):1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28(2):208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- Krankel N, Katare EG, Siragusa M, Barcelos LS, Campagnolo P, Mangialardi G, et al. Role of Kinin B2 Receptor Signaling in the Recruitment of Circulating Progenitor Cells With Neovascularization Potential. Circ Res. 2008;103(11):1335–1343. doi: 10.1161/CIRCRESAHA.108.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, et al. Cells Expressing Early Cardiac Markers Reside in the Bone Marrow and Are Mobilized Into the Peripheral Blood After Myocardial Infarction. Circ Res. 2004;95(12):1191–1199. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, et al. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood - preliminary report. Leukemia. 2006;21(2):297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, et al. A population of very small embryonic-like (VSEL) CXCR4+SSEA-1+Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20(5):857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell FR, Zuba-Surma EK, Majka M, Ratajczak J, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20(5):857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Jala VR, Dawn B, Ratajczak J, Ratajczak MZ. 1Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells1. Leukemia. 2005;19(7):1118–1127. doi: 10.1038/sj.leu.2403796. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, et al. Trafficking of Normal Stem Cells and Metastasis of Cancer Stem Cells Involve Similar Mechanisms: Pivotal Role of the SDF-1-CXCR4 Axis. Stem Cells. 2005;23(7):879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- Kucia M, Wojakowski W, Reca R, Machalinski B, Gozdzik J, Majka M, et al. The migration of bone marrow-derived non-hematopoietic tissue-committed stem cells is regulated in an SDF-1−, HGF−, and LIF-dependent manner. Arch Immunol Ther Exp. 2006;54(2):121–135. doi: 10.1007/s00005-006-0015-1. [DOI] [PubMed] [Google Scholar]
- Kucia M, Wysoczynski M, Wu W, Zuba-Surma EK, Ratajczak J, Ratajczak MZ. Evidence That Very Small Embryonic-Like Stem Cells Are Mobilized into Peripheral Blood. Stem Cells. 2008;26(8):2083–2092. doi: 10.1634/stemcells.2007-0922. [DOI] [PubMed] [Google Scholar]
- Martinez-Fernandez A, Nelson TJ, Yamada S, Reyes S, Alekseev AE, Perez-Terzic C, et al. iPS Programmed Without c-MYC Yield Proficient Cardiogenesis for Functional Heart Chimerism. Circ Res. 2009;105(7):648–656. doi: 10.1161/CIRCRESAHA.109.203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105(1):199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95(9):911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher A, Dimmeler S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res Cardiol. 2010 doi: 10.1007/s00395-010-0109-0. [DOI] [PubMed] [Google Scholar]
- Paczkowska E, Kucia M, Koziarska D, Halasa M, Safranow K, Masiuk M, et al. Clinical Evidence That Very Small Embryonic-Like Stem Cells Are Mobilized Into Peripheral Blood in Patients After Stroke. Stroke. 2009;40(4):1237–1244. doi: 10.1161/STROKEAHA.108.535062. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Martin BJ. Mesenchymal Stem Cells and Their Potential as Cardiac Therapeutics. Circ Res. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ. Phenotypic and functional characterization of hematopoietic stem cells. Curr Opin Hematol. 2008;15(4):293–300. doi: 10.1097/MOH.0b013e328302c7ca. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Kucia M, Ratajczak J, Zuba-Surma EK. A multi-instrumental approach to identify and purify very small embryonic like stem cells (VSELs) from adult tissues. Micron. 2009;40(3):386–393. doi: 10.1016/j.micron.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4+ stem cells in adult bone marrow and other tissues. Leukemia. 2007;21(5):860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Zuba-Surma E, Machalinski B, Ratajczak J, Kucia M. Very Small Embryonic-Like (VSEL) Stem Cells: Purification from Adult Organs, Characterization, and Biological Significance. Stem Cell Rev. 2008;4(2):89–99. doi: 10.1007/s12015-008-9018-0. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Heeschen C, Aicher A, Ziebart T, Honold J, Urbich C, et al. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci USA. 2006;103(39):14537–14541. doi: 10.1073/pnas.0604144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Zuba-Surma EK, Wu W, Ratajczak J, Wysoczynski M, Ratajczak MZ, et al. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4+ very small embryonic-like stem cells. Leukemia. 2009;23(11):2042–2051. doi: 10.1038/leu.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendera M, Wojakowski W, Ruzyłło W, Chojnowska L, Kepka C, Tracz W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30(11):1313–1321. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- Tang X, Rokosh DG, Guo Y, Bolli R. Cardiac Progenitor Cells and Bone Marrow-Derived Very Small Embryonic-Like Stem Cells for Cardiac Repair After Myocardial Infarction. Circulation J. 2010;74(3):390–404. doi: 10.1253/circj.cj-09-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojakowski W, Kucia M, Zuba-Surma EK, Ratajczak J, Machalinski B, Tendera M, et al. Cardiogenic differentiation of very small embryonic-like cells isolated from adult bone marrow. J Am Coll Cardiol. 2007;49(9, Supplement 1):A43. [Google Scholar]
- Wojakowski W, Tendera M, Kucia M, Zuba-Surma E, Milewski K, Wallace-Bradley D, et al. Cardiomyocyte differentiation of bone marrow-derived Oct-4+CXCR4+SSEA-1+ very small embryonic-like stem cells. Int J Oncol. 2010;37(2):237–247. doi: 10.3892/ijo_00000671. [DOI] [PubMed] [Google Scholar]
- Wojakowski W, Tendera M, Kucia M, Zuba-Surma EK, Paczkowska E, Ciosek J, et al. Mobilization of Bone Marrow-Derived Oct-4+ SSEA-4+ Very Small Embryonic-Like Stem Cells in Patients With Acute Myocardial Infarction. J Am Coll Cardiol. 2009;53(1):1–9. doi: 10.1016/j.jacc.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, et al. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ Stem Cells, and Mononuclear Cells Expressing Early Cardiac, Muscle, and Endothelial Markers Into Peripheral Blood in Patients With Acute Myocardial Infarction. Circulation. 2004;110(20):3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- Wojakowski W, Tendera M, Zebzda A, Michalwska A, Majka M, Kucia M, et al. Mobilization of CD34+, CD117+, CXCR4+, c-met+ stem cells is correlated with left ventricular ejection fraction and plasma NT-proBNP levels in patients with acute myocardial infarction. Eur Heart J. 2006;27(3):283–289. doi: 10.1093/eurheartj/ehi628. [DOI] [PubMed] [Google Scholar]
- Zuba-Surma EK, Guo Y, Taher H, SanganalMath SK, Hunt G, Vincent RJ, et al. Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodeling after myocardial infarction. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba-Surma EK, Kucia M, Abdel-Latif A, Dawn B, Hall B, Singh R, et al. Morphological characterization of very small embryonic-like stem cells (VSELs) by ImageStream system analysis. J Cell Mol Med. 2008;12(1):292–303. doi: 10.1111/j.1582-4934.2007.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba-Surma EK, Kucia M, Rui L, Shin D, Wojakowski W, Ratajczak J, et al. Fetal Liver Very Small Embryonic/Epiblast Like Stem Cells Follow Developmental Migratory Pathway of Hematopoietic Stem Cells. Ann N Y Acad Sci. 2009;1176:205–218. doi: 10.1111/j.1749-6632.2009.04562.x. (Hematopoietic Stem Cells VII) [DOI] [PubMed] [Google Scholar]
- Zuba-Surma EK, Kucia M, Wu W, Klich I, Lillard JW, Jr, Ratajczak J, et al. Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies. Cytometry A. 2008;73A(12):1116–1127. doi: 10.1002/cyto.a.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba-Surma EK, Ratajczak MZ. Overview of very small embryonic-like stem cells (VSELs) and methodology of their identification and isolation by flow cytometric methods. Curr Protoc Cytom Chapter. 2010;9(Unit9):29. doi: 10.1002/0471142956.cy0929s51. [DOI] [PubMed] [Google Scholar]