Abstract
The subunit composition of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors (AMPARs) is an important determinant of AMPAR biophysical properties and trafficking. To date, AMPAR subunit composition has been quantitatively evaluated only for the hippocampus, where different experimental approaches have yielded different results. Here, we used quantitative co-immunoprecipitation to characterize GluA1-3 associations in the adult rat nucleus accumbens, dorsal striatum, prefrontal cortex, and hippocampus, and blue native electrophoresis (BNE) to study GluA1-3 assembly state. In all brain regions, co-immunoprecipitation experiments showed that ~90% of GluA1 was associated with GluA2 or GluA3 (most was GluA1A2). All regions contained a small number of GluA1A3 receptors. Homomeric GluA1 receptors may also exist. More than half of the GluA2 (53%-65% depending on the region) was not associated with GluA1. However, this represents an over-estimate of the percent of GluA2 present in GluA2A3 receptors, based on BNE results demonstrating that the majority of GluA2 exists as dimers, rather than functional tetrameric receptors. Relatively more GluA1 was present in tetramers. Together with other findings, our results suggest a dominant role for GluA1A2 receptors in all brain regions examined. They also help explain why different results for hippocampal AMPAR subunit composition were obtained using co-immunoprecipitation, which assesses the total cellular pool of AMPARs including partially assembled AMPARs in intracellular compartments, and electrophysiological approaches, which can selectively assess tetrameric (functional) AMPARs on the cell surface.
Keywords: AMPA receptor assembly, AMPA receptor subunit composition, dorsal striatum, nucleus accumbens, hippocampus, prefrontal cortex
1. Introduction
α-Amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptors (AMPARs) mediate the majority of fast excitatory transmission in the brain. AMPARs are tetramers (dimers of dimers), composed of various combinations of the subunits GluA1-4 (Cull-Candy et al., 2006; Greger and Esteban, 2007). GluA1-4 subunits were formerly termed GluR1-4 or GluRA-D (see Collingridge et al., 2009). The hippocampus (HPC) is the only brain area in which AMPAR subunit composition has been quantitatively evaluated. Wenthold et al. (1996) used co-immunoprecipitation (co-IP) while Lu et al. (2009) used a single-cell genetic approach combined with electrophysiology. These studies agree that GluA1A2 receptors constitute a major AMPAR population but differ with respect to the abundance of GluA2A3 receptors (see Discussion).
Subunit composition plays a critical role in determining biophysical properties of the AMPAR (Dingledine et al., 1999; Palmer et al., 2005). Most strikingly, the presence of the GluA2 subunit controls Ca2+ permeability of the AMPAR channel (see Discussion). Other results suggest that subunit composition plays an important role in determining AMPAR trafficking “rules”. According to the leading theory, synaptic insertion of GluA1A2 receptors occurs in an activity-dependent manner, whereas GluA2A3 receptors constitutively cycle in and out of synapses (Malinow, 2003). This may reflect differences in regulatory phosphorylation events and protein-protein interactions between subunits with a long C terminus, such as GluA1, and those with shorter C termini (GluA2 and GluA3) (Song and Huganir, 2002; Derkach et al., 2007). AMPAR gating and trafficking are also regulated by AMPAR auxiliary subunits. The best characterized are transmembrane AMPA receptor regulatory proteins (TARPs; Kato et al., 2010) although others have been discovered recently (Schwenk et al., 2009; von Engelhardt et al., 2010).
Here, we used a previously established quantitative co-IP technique (Wenthold et al., 1996; Sans et al., 2003) to determine AMPAR subunit composition in the nucleus accumbens (NAc), the dorsal striatum (DS), and the prefrontal cortex (PFC). These brain areas were chosen because of their role in drug addiction, which involves plasticity of AMPAR transmission. Drug-induced AMPAR plasticity has been studied extensively in the NAc (Kalivas, 2009; Wolf and Ferrario, 2010; Bowers et al., 2010) but also occurs in other regions, including the PFC (e.g., Van den Oever et al., 2008; Ghasemzadeh et al., 2009b) and the DS (e.g., Kim et al., 2009; Ferrario et al., 2010). We also analyzed the HPC. This was done primarily in order to compare our findings to previous results (Wenthold et al., 1996; Lu et al., 2009), but it is notable that drugs of abuse also produce AMPAR plasticity in the HPC (e.g., Song et al., 2007; Billa et al., 2010). To aid in the interpretation of our co-IP results, we used blue native electrophoresis (BNE), which is performed under non-denaturing conditions and thus preserves protein complexes (Schägger et al., 1994; Wittig and Schägger, 2009), to determine the state of assembly for GluA1, GluA2, and GluA3.
2. Results
2.1 Assessment of AMPAR species in membrane preparations
The co-IP approach to determining AMPAR subunit composition is based on the assumption that AMPAR subunit antibodies pull down functional AMPARs, which are tetramers (Mano and Teichberg 1998; Rosenmund et al. 1998; Greger and Esteban 2007), because the presence of partially assembled subunits would complicate the results. Wenthold et al. (1996) used 3H-AMPA binding to address this issue and validate their co-IP results, on the assumption that only functional AMPARs bind glutamate agonists. However, it is possible that monomers or dimers also bind glutamate agonists. We therefore took a different approach to addressing this issue. We analyzed our membrane preparations using BNE, which maintains protein associations during electrophoresis and can therefore determine the state of oligomerization of protein complexes (Schägger et al. 1994; Wittig and Schägger 2009). Our goal was to determine if GluA1-3 are detected in complexes corresponding to the predicted size of tetramers, dimers or monomers. For BNE studies aimed at quantifying AMPAR species in the NAc, results were based on analysis of 3 different membrane preparations, while BNE results for the HPC, DS, and PFC were based on the analysis of 2 runs of a single membrane preparation (each preparation was comprised of tissue pooled from 3-12 rats, depending on the brain region).
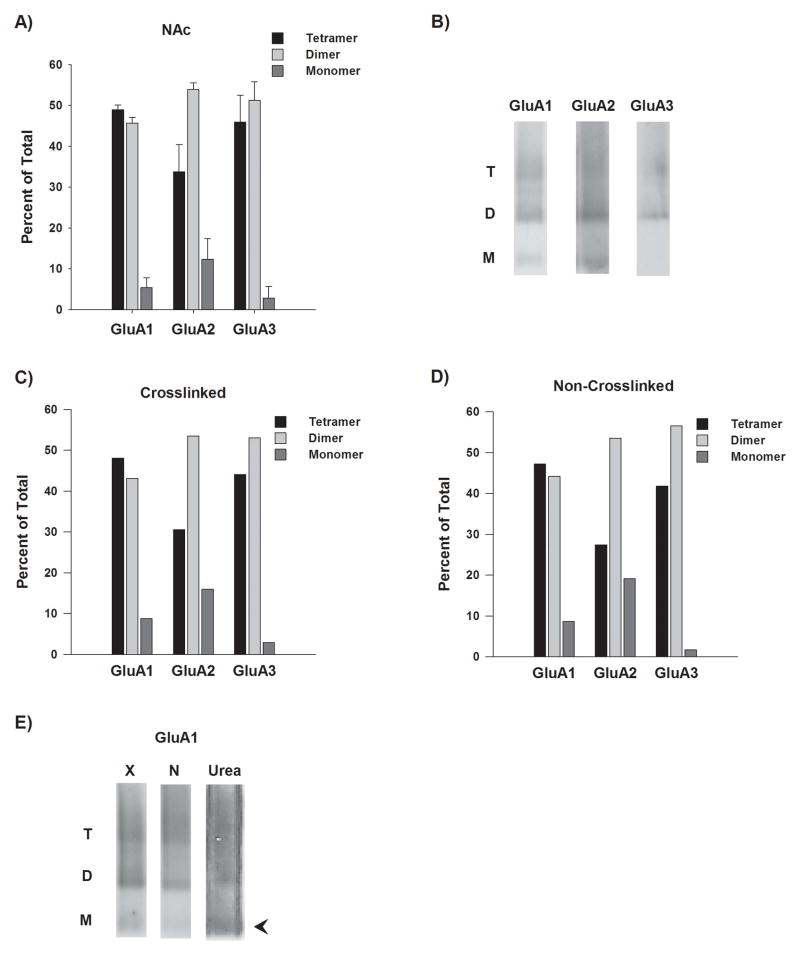
NAc membrane preparations were analyzed with BNE followed by immunoblotting with antibodies to GluA1, GluA2 and GluA3. Each antibody detected three bands which we identified as monomer, dimer and tetramer species (Fig. 1A, B). Based on the ~100 kDa molecular weight of each AMPAR subunit, the predicted molecular weights of these species are ~100, ~200 and ~400 kDa, respectively. Unfortunately, protein complexes do not migrate at their predicted molecular weight during BNE due to effects of tertiary/quaternary structure (see legend to Fig. 1 for more information). However, if the molecular weight of the putative monomer band in Fig. 1 is assigned a value of 1, then the putative dimer is ~2.3 and the putative tetramer is ~4, supporting our identification of these bands. Should higher molecular weights have been predicted based on associations between AMPARs and TARP auxiliary subunits? TARPs may associate only with tetrameric AMPARs, not monomers or dimers (Vandenberghe et al., 2005). In cerebellar neurons, only a single TARP molecule appears to be associated with each tetrameric AMPAR (Kim et al., 2010; but see Shi et al., 2009 for results indicating variable stoichiometry in hippocampal neurons). A single TARP (~36kDa) would not be expected to produce a change in the migration of the ~400 kDa AMPAR tetramer that would be detectable with BNE.
Figure 1.
BNE reveals the state of assembly of GluA1, GluA2, and GluA3 in solubilized membrane preparations from the adult rat NAc. Panel A: For each subunit, the percent of total subunit immunoreactivity represented by tetramer, dimer and monomer species was determined from BNE followed by immunoblotting. Results are based on analysis of 3 different NAc membrane preparations and expressed as mean ± S.E.M. Panel B: Representative BNE immunoblots showing the migration patterns for GluA1-3 species (T, tetramer; D, dimer; M, monomer). Molecular weights were estimated by comparison to NativeMark protein standards (Invitrogen). For each subunit, the molecular weight for each assembly state was similar: ~700kD for tetramers, ~400kD for dimers, and ~175kD for monomers. Differences from predicted molecular weights (i.e., ~100kD for monomers) are attributed to the fact that proteins are not denatured in BNE and their tertiary/quaternary structure may cause a slower migration. However, if the monomer size is set at 1, then the dimer is ~2.3 and the tetramer is ~4. Panels C and D: Crosslinking surface AMPARs before preparing NAc membranes does not alter the assembly state revealed by BNE. A membrane impermeant crosslinking agent (BS3) was used in order to selectively crosslink surface expressed tetrameric receptors in NAc tissue (see Sections 2.1 and 4.4). Non-crosslinked samples were processed in parallel but in the absence of BS3. Membranes were prepared and analyzed by BNE and immunoblotting to determine the percent of total subunit immunoreactivity represented by GluA1-3 tetramer, dimer, and monomer species in crosslinked (panel C) and non-crosslinked (panel D) preparations. These percentages (derived from results from one membrane preparation) were similar to those in Panel A, suggesting that dimer and monomer species do not originate from tetramers that dissociate during BNE. Panel E: Immunoblots showing the migration pattern for GluA1 in crosslinked (X) and non-crosslinked (N) NAc tissue (left panels) as well as a NAc membrane preparation denatured with 7M Urea (right panel) prior to BNE. Note the more abundant monomer band in the denatured sample (arrowhead). Dissociation is incomplete because the sample separates from urea as it runs on the gel.
In NAc membranes, GluA1 and GluA3 immunoreactivity were approximately equally distributed between tetramer and dimer bands, with only a small amount present as monomers (Fig. 1A). However, relatively more GluA2 was present as dimers. This is consistent with prior studies of adult rat brain (Greger et al., 2003) and has important implications for interpretation of co-IP results, since it implies that a significant portion of the GluA2 pulled down or left behind in an IP experiment may consist of intracellular dimers rather than functional tetramers (see Section 2.2 and Discussion).
A control experiment was conducted to demonstrate that the presence of monomers and dimers in our NAc membrane preparation was not due to the dissociation of tetramers during electrophoresis. In this experiment, NAc slices were incubated with vehicle or with the bi-functional protein crosslinking reagent bis(sulfosuccinimidyl)suberate (BS3) prior to membrane solubilization. BS3 does not cross membranes and therefore selectively modifies surface-expressed proteins. Crosslinking was performed in order to keep surface (or functional) AMPARs, which are tetramers, intact during BNE by covalently linking AMPAR subunits within the tetrameric receptor. Based on the length of the spacer arm in the bi-functional BS3 molecule (11 angstroms), it is expected to crosslink within a terameric receptor rather than between tetramers (Safferling et al., 2001). If tetramers dissociate during BNE, crosslinking should prevent this and thus the crosslinked sample should contain relatively more tetramers. Instead, we found that crosslinked and non-crosslinked samples contained similar proportions of tetramers, dimers and monomers (Fig. 1C, D). Note that dimers and monomers are present in the crosslinked sample because it still contains intracellular proteins; although BS3 selectively modifies surface-expressed proteins, these are not separated from other proteins in the sample prior to BNE.
A second control experiment was designed to confirm that the tetramers and dimers detected in NAc membranes with BNE can be chemically dissociated into monomers. BNE is performed in the absence of detergents in order to maintain native conformations. Therefore, in order to denature our samples, we treated them with 7M urea before electrophoresis. Whereas the monomer band was barely detected in “native” samples, it became prominent in the urea-treated sample (Fig. 1E, arrowhead). These results further confirm that the high molecular weight band is a tetramer of AMPAR subunits, because its dissociation with urea resulted in an increase in abundance of monomers (Fig. 1E). Note that the denatured sample is “streaky” due to the fact that the urea comes out of solution during BNE. Separation of urea from the sample probably accounts for incomplete dissociation of tetramers and dimers.
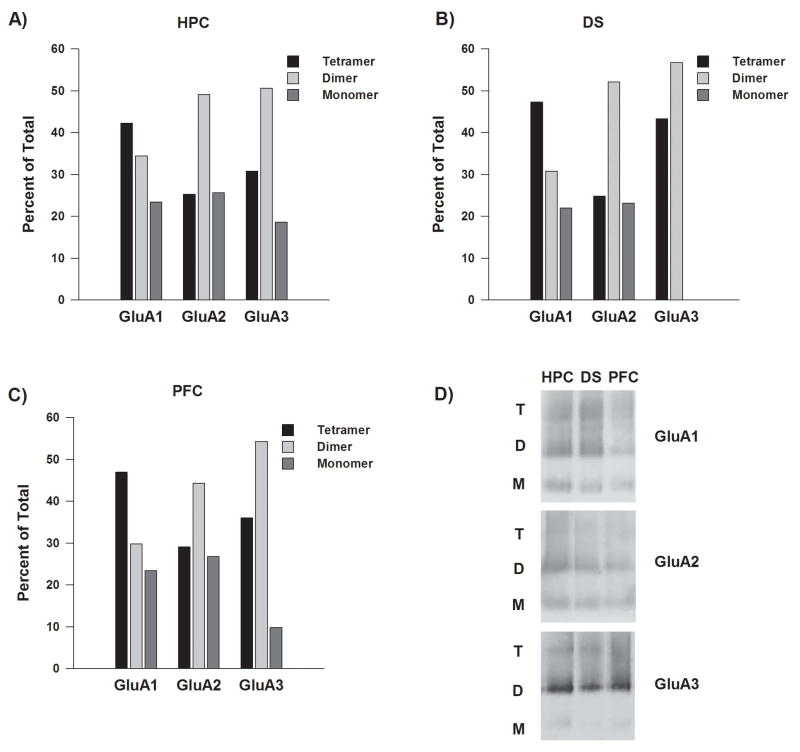
Membrane preparations from HPC, DS and PFC were also analyzed with BNE (Fig. 2). For GluA1, tetramers were more prevalent than dimers or monomers. For GluA2 and GluA3, dimers were most prevalent. Apparent regional variability could reflect the fact that only one membrane preparation (pooled from multiple rats) was analyzed with BNE for each of these brain regions. We did not analyze additional membrane preparations because the present results are sufficient to show that dimers and monomers are contributing to our co-IP results (Sections 2.2-2.5). Even if the exact proportions of each species were determined, this information could not be used to “correct” co-IP results because it cannot be assumed that all species contribute equally in each co-IP experiment.
Figure 2.
BNE reveals the state of assembly of GluA1, GluA2, and GluA3 in solubilized membrane preparations from the adult rat hippocampus (HPC), dorsal striatum (DS) and prefrontal cortex (PFC). Panels A-C: For each subunit in each brain region, the percent of total subunit immunoreactivity represented by tetramer, dimer and monomer species was determined from BNE followed by immunoblotting. Results are expressed as the average of two runs (both from the same membrane preparation) that yielded similar results. Panel D: Representative BNE immunoblots showing the migration patterns for GluA1-3 species (T, tetramer; D, dimer; M, monomer). See legend to Figure 1, Panel B for more information on this analysis.
2.2 Co-IP results in NAc
For NAc and other regions (below), membrane preparations were solubilized with Triton X-100 and then subjected to IP using antibodies to GluA1, GluA2/3, GluA4 and the combinations of GluA1+GluA2/3 or GluA2/3+GluA4 antibodies, as described previously (Wenthold et al., 1996; Sans et al., 2003). Unbound fractions were run on gels and immunoblotting was used to determine the amount of subunit remaining after IP. We analyzed the unbound fraction to avoid signal interference with IgG heavy chains, the issue of antigen release from agarose beads, and the potential for “false positives” due to nonspecific aggregation in the bound fraction (see Wenthold et al., 1996). For each blot, a standard curve was generated by running 5%, 25%, 50%, 75%, and 100% of the supernatant after the control IgG IP. The percent of total AMPAR subunit remaining in each experimental sample was determined by interpolation from this standard curve Quantification of AMPAR species in NAc and other brain regions was performed in the same membrane preparations used for BNE experiments. For each brain region, three separate membrane preparations (each generated by pooling tissue from 3-12 rats) were analyzed (Fig. 3).
Figure 3.
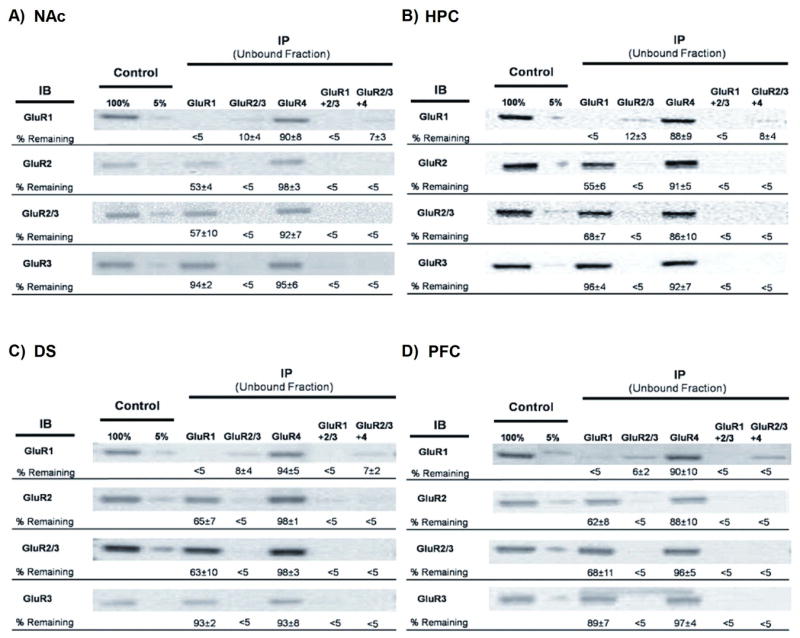
The percent of AMPAR subunits remaining in the unbound fraction after immunoprecipitation (IP) of solubilized membrane preparations. Panels A-D show results from the nucleus accumbens (NAc), hippocampus (HPC), dorsal striatum (DS) and prefrontal cortex (PFC). The AMPAR subunit antibodies used to IP are indicated at the top, while the antibodies used for immunoblotting (IB) are indicated on the left. The left two lanes show control tissue representing 5% and 100% of the supernatant after the control IgG IP. To generate a full standard curve, five control samples were run on every blot (5%, 25%, 50%, 75% and 100%) but only the 5% and 100% samples are shown because they indicate the range of immunoreactivity detected. The percent of immunoreactivity remaining after IP with AMPAR subunit antibodies is calculated from the standard curve and indicated under each lane (mean ± SEM from 3 separate experiments with different membrane preparations). Levels of immunoreactivity lower than the 5% control sample were considered not detectable.
Analysis of the unbound fraction of IP’ed NAc tissue shows that we were successful in capturing 100% of the target subunits (Fig. 3A). For example, when the unbound fraction from the GluA1 IP was immunoblotted with GluA1 antibody, no immunoreactivity was detected. The same is true for antibodies to GluA2/3 (Fig. 3A) and GluA4 (data not shown). The same results were obtained in the other regions analyzed (see Sections 2.3-2.5). The ability to IP 100% of the target subunit enables this method to be used for quantitative studies. Anti-GluA2 and GluA3 antibodies did not reliably provide complete pull-down of their target subunits and thus were used only for immunoblotting.
In the remainder of the Results section, we will describe co-IP results and use them to infer the extent to which particular GluA subunits are associated in each region. For clarity, we will not attempt to factor the unassembled/partially assembled pools of GluA subunits into each estimate of GluA subunit associations, although it should be noted that all of our calculations therefore overestimate associations in tetrameric receptors due to the existence of the other species. However, in the case of GluA2, which is found mainly in dimers, we will draw upon the BNE data for the purpose of reconciling IP results (present study; Wenthold et al., 1996) with those obtained using a single-cell genetic approach combined with electrophysiology (Lu et al., 2009).
In assessing NAc results (Fig. 3A), we began by estimating the percent of GluA1 in NAc membrane preparations that is associated with GluA2 or GluA3. IP with GluA2/3 antibody left 10% of GluA1 in the unbound fraction, indicating that ~90% of GluA1 is complexed with GluA2 or GluA3. What portion of GluA1 is with GluA2 versus GluA3? We found that IP with GluA1 left ~94% of the GluA3 in the unbound fraction. This indicates the existence of a small pool of GluA1A3 receptors. Thus, we estimate that upwards of 80% of GluA1 is complexed with GluA2 in NAc tissue. IP with GluA1 antibody left behind ~53% of the GluA2 and ~57% of the GluA2/3 immunoreactivity, indicating that about half of the GluA2 is not associated with GluA1. These results could be interpreted to suggest that the 53% of GluA2 not associated with GluA1 is present in GluA2A3 receptors. However, BNE analysis shown in Fig. 1A indicates that most GluA2 in NAc membranes is not assembled into tetrameric receptors. Together with results from IP with GluA2/3 antibody, these results indicate that the major functional AMPAR population in the NAc is GluA1A2, although GluA2A3 receptors are also present.
A final issue is the abundance of homomeric GluA1 receptors. IP with GluA2/3+GluA4 antibodies removed 93% of the GluA1 immunoreactivity. The remaining 7% of the GluA1 represents homomeric GluA1 receptors (tetramers) and/or GluA1 dimers or monomers. The contribution of this 7% to the total AMPAR pool depends on what fraction of this total pool contains GluA1, but our results set an upper limit. These results, along with evidence for GluA1A3 receptors (see previous paragraph), suggest the existence of a small number of GluA2-lacking AMPARs. This is consistent with results of electrophysiological studies in the adult rat NAc (see Discussion).
The GluA4 antibody pulled down very small amounts of GluA1, GluA2, GluA2/3 and GluA3 immunoreactivity in NAc (Fig. 3A) and other regions (Fig. 3B-D), consistent with prior hippocampal results (Wenthold et al., 1996). The significance is unclear given low GluA4 expression in these regions (Petralia and Wenthold, 1992). Furthermore, GluA4 does not contribute to AMPAR currents in the HPC (Lu et al., 2009) or to AMPARs in striatal medium spiny neurons (Bernard et al., 1997; Stefani et al., 1998). GluA4 is expressed predominantly during early postnatal development (e.g., Zhu et al., 2000).
2.3 Co-IP results in hippocampus
IP with GluA2/3 antibody left ~12% of GluA1 in the unbound fraction, indicating that 88% of GluA1 is complexed with GluA2 or GluA3 (Fig. 3B). IP with GluA1 left ~96% of the GluA3 in the unbound fraction. This indicates the existence of a small pool of GluA1A3 receptors. Thus, we estimate that upwards of 80% of GluA1 is complexed with GluA2 in hippocampal tissue. IP with GluA1 antibody left ~55% of GluA2 and ~68% of GluA2/3 immunoreactivity. Based on BNE results, a major portion of this “left-over” GluA2 (and GluA3) is likely to represent partially assembled receptors (Fig. 2A). IP with GluA2/3+GluA4 antibodies left ~8% of GluA1 in the unbound fraction, indicative of a small population of homomeric GluA1 receptors and/or GluA1 dimers or monomers.
In a prior study of HPC using the same methods, IP with GluA2/3 antibody left ~19% of GluA1 immunoreactivity in the unbound fraction, IP with GluA1 antibody left ~31% of the GluA2, ~53% of the GluA2/3 and ~90% of the GluA3, and IP with GluA2/3+GluA4 antibodies left ~19% of the GluA1 (Wenthold et al., 1996). Differences from our results may reflect differences in rat age or strain, dissections, or antibody lots.
2.4 Co-IP results in dorsal striatum
Analysis of DS is shown in Fig. 3C. Results obtained after IP with GluA2/3 antibody indicate that, of the total GluA1 pool, ~92% is associated with GluA2 or GluA3. A small population of GluA1A3 is indicated because GluA1 IP removed ~7% of the GluA3. After IP with GluA1, ~65% of GluA2 and ~63% of GluA2/3 immunoreactivity remained, representing a combination of GluA2A3 receptors and unassembled/partially assembled subunits. Based on GluA1 remaining after IP with GluA2/3+GluA4 antibodies, ~7% of all GluA1 is in homomeric receptors and/or GluA1 dimers or monomers.
2.5 Co-IP results in prefrontal cortex
Similar to other regions, results in PFC (Fig. 3D) indicated a large GluA1A2 population and a small GluA1A3 population (IP with GluA1 removed ~11% of the GluA3). After IP with GluA1, ~62% of GluA2 and ~68% of GluA2/3 immunoreactivity remained, representing GluA2A3 receptors and unassembled subunits. Unlike other regions, the amount of GluA1 remaining after IP with GluA2/3+GluA4 antibodies was below the defined limit of detection (<5% remaining), although a small amount of immunoreactivity was nevertheless observable in the immunoblots. Thus it is unclear whether the PFC differs significantly from other regions in abundance of homomeric GluA1 receptors or partially assembled GluA1.
3. Discussion
The purpose of this study was to determine the subunit composition of AMPAR populations in the NAc, DS, and PFC, and to compare these regions to the HPC. We used co-IP to determine GluA1-3 subunit associations and BNE to determine the state of assembly of GluA1-3 containing complexes. Together, these approaches indicate a predominant role for GluA1A2 receptors and a smaller role for GluA2A3 receptors. By using BNE results to help interpret co-IP results, our findings offer a way to resolve differences in estimates of hippocampal AMPAR subunit composition obtained by co-IP (Wenthold et al., 1996) versus a single-cell genetic/electrophysiological approach (Lu et al. 2009). In addition, they demonstrate that Western analysis of AMPAR subunit levels in brain homogenates is an inaccurate way to assess the abundance of tetrameric (functional) receptors, because this measure, particularly for GluA2, is contaminated by a very substantial fraction of GluA2 that resides in the ER in a state of partial assembly. Importantly, we confirmed that the presence of GluA monomers and dimers in our membrane preparation did not reflect dissociation of tetramers during electrophoresis, because the relative abundance of these species was not significantly altered when a protein crosslinking reagent was used to covalently link AMPAR subunits within cell surface tetrameric AMPARs prior to preparation of membranes.
3.1 Similar AMPAR populations are detected in all brain regions
A diagram depicting AMPAR subunit associations found in the NAc is provided in Fig. 4. Our results in other brain regions were similar. Furthermore, our hippocampal co-IP results were similar to those of Wenthold et al. (1996). In all regions, co-IP results indicated that most GluA1 was associated with GluA2, indicating a major role for GluA1A2 receptors, whereas a significant amount of GluA2 (~53-65%) was not complexed with GluA1 (see Section 3.2). We also found evidence of a small population of GluA1A3 receptors in all brain regions. Homomeric GluA1 receptors may also be present, based on detection of a small amount of GluA1 that was not IP’ed by GluA2/3+GluA4 antibodies (7%, 7%, 8% and <5% of total GluA1 in NAc, DS, HPC and PFC, respectively). GluA1 monomers or dimers could also explain these latter results.
Figure 4.
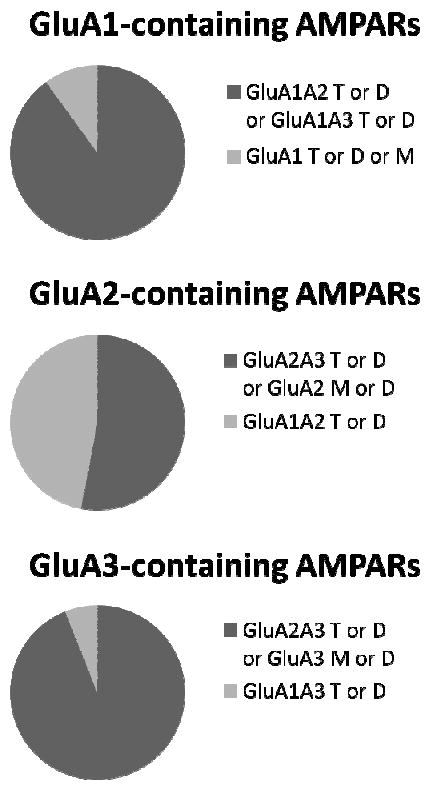
Schematic showing types of AMPAR species within the population of GluA1-containing AMPARs (top), GluA2-containing AMPARs (middle) and GluA3-containing AMPARs (bottom). Because GluA1, GluA2- and GluA3-containing AMPAR populations are only partially overlapping, our results cannot determine the percent of all AMPARs that contain a particular subunit or subunit combination. Abbreviations: T, tetramer; D, dimer; M, monomer. Top: About 90% of GluA1 is complexed with GluA2 (GluA1A2 T or D) or GluA3 (GluA1A3 T or D) (dark portion). GluA1A2 species are much more prevalent, but GluA1A3 species exist (see legend to bottom panel). About 10% of GluA1 was not pulled down with GluA2/3 antibody, indicating a population composed of GluA1 T, D or M (light portion). Middle: After IP with GluA1 antibody, ~53% of the GluA2 remained (dark portion), representing GluA2A3 T or D or GluA2 M or D. The remainder is GluA1A2 T or D (47%; light portion). Bottom: IP with GluA1 antibody removed ~6% of the GluA3, indicating a small portion of GluA1A3 T or D (light portion). The remainder is GluA2A3 T or D or GluA3 M or D.
An important point is that the GluA1- and GluA2-containing populations are only partially overlapping, so our results cannot determine the percent of all AMPARs that contain GluA1 or the percent that contain GluA2. In other words, we can estimate the abundance of a subset of receptors within the population of GluA1-containing AMPARs (e.g., GluA1A2 receptors represent over 80% of all GluA1-containing AMPARs in the NAc), but we cannot determine what fraction of the total AMPAR population is accounted for by that subset. This accounts for the format used to summarize our results in Fig. 4.
3.2 Relative importance of GluA1A2 versus GluA2A3 receptors
A major difference exists between estimates of AMPAR populations derived from co-IP experiments (Wenthold et al., 1996; present results) versus a single-cell genetic approach combined with electrophysiological recordings (Lu et al., 2009). The co-IP data suggest that ~50% of GluA2 in the HPC (and other regions) is present in GluA2A3 receptors. However, Lu et al. (2009) found that synaptic AMPARs are ~80% GluA1A2 and ~16% GluA2A3, while ~95% of AMPARs located in extrasynaptic regions of the soma are GluA1A2 complexes. Both techniques are associated with experimental error and difficulties associated with interpreting results in non-overlapping populations (see Section 3.1). However, the most important factor accounting for the difference is that co-IP measures the total cellular pool of AMPARs, including partially assembled AMPARs in intracellular compartments. Thus, co-IP cannot address the question of which AMPARs are targeted to the synapse, whereas electrophysiological techniques can selectively assess synaptic AMPARs (Béïque and Huganir, 2009). Using BNE, we showed that the membrane preparations used in our experiments contain GluA1-3 monomers, dimers and tetramers, indicating that partially assembled AMPAR subunits contribute to our co-IP results. In all brain regions studied, relatively more GluA1 was present in tetramers, whereas relatively more GluA2 and GluA3 subunits were found in dimers. Thus, of the 53-65% of GluA2 that was not pulled down by GluA1 antibody, a major portion is likely present in partially assembled AMPARs. Since partially assembled AMPARs are retained in the endoplasmic reticulum (ER; Greger and Esteban, 2007), our results suggest that only a fraction of the “leftover” GluA2 or GluA3 is present in cell surface GluA2A3 receptors. If this is taken into account when interpreting co-IP data from hippocampal membranes (Wenthold et al., 1996; present results), these data fall into line with electrophysiological results indicating a predominant role for GluA1A2 receptors at CA1 synapses (Lu et al., 2009). Furthermore, our results suggest a similar picture in the NAc, DS and PFC.
Our results are consistent with prior work in cultured neurons and rat brain demonstrating that GluA2 is largely retained in the ER in dimer form, whereas GluA1 is mainly present in tetramers in post-ER compartments (Greger et al., 2003). GluA2 in the ER pool is preferentially associated with GluA3, whereas GluA1A2 heteromers are mostly post-ER (Greger et al., 2002). In line with these results, protein crosslinking experiments in cultured neurons found that ~90% of GluA1 was present on the cell surface, whereas only ~40% of GluA2 and ~60% of GluA3 were surface-expressed (Greger et al., 2002; see also Hall and Soderling, 1997). In similar protein crosslinking experiments using NAc tissue, we have also observed relatively larger intracellular pools of GluA2 and GluA3 compared to GluA1 (Boudreau et al., 2007).
Although these results indicate that GluA1A2 receptors mediate most AMPAR transmission, they do not rule out a significant role for GluA2A3 or GluA1A3. For example, we detect substantial GluA3 surface expression in the NAc (Boudreau et al., 2007) and this measure can be altered by cocaine self-administration (Conrad et al., 2008). Indeed, in vitro studies suggest that most GluA3 is surface expressed (Lee et al., 2004). More efficient GluA3 assembly may be indicated by the fact that less GluA3, compared to GluA1 or GluA2, was found in monomer form in our studies. In fact, GluA3 monomer was below the limit of detection in DS membranes.
Heterogeneity of cell types within a brain region complicates interpretation of IP data. However, most neurons in the NAc and DS are GABAergic medium spiny neurons (90-95%; Meredith and Totterdell, 1999; Bolam et al., 2000), while >95% of neurons in the CA1/CA2 region of adult rat HPC are pyramidal neurons (Bernard and Wheal, 1994). The PFC is a more heterogeneous structure. Despite these differences, similar AMPAR subunit composition was found in all regions.
3.3 GluA2-lacking AMPARs
GluA2 contributes critically to the properties of tetrameric AMPARs. This comes about because GluA2 RNA undergoes a high efficiency editing process that replaces an uncharged glutamine at residue 607 (in the pore-lining region) with a charged arginine (Sommer et al., 1991; Jonas and Bernashev, 1995). Thus, AMPARs containing edited GluA2 subunits have low Ca2+ permeability and a linear current-voltage relationship. GluA2 editing also controls ER retention of GluA2 (Greger et al., 2002). In contrast, GluA2-lacking AMPARs are Ca2+-permeable, exhibit higher conductance, and display inward rectification due to voltage-dependent polyamine block. Alterations in the abundance of GluA2-lacking AMPARs may contribute to a number of disease processes (Cull-Candy et al., 2006; Isaac et al., 2007; Liu and Zukin, 2007).
In all regions studied, the pool of GluA2-lacking AMPARs was less than 10% of the total AMPAR population and probably closer to 5%. Consistent with these results, ~5% of the evoked AMPAR-mediated excitatory postsynaptic current (EPSC) in the adult rat NAc is inhibited by a selective antagonist of GluA2-lacking AMPARs (Conrad et al., 2008). Other electrophysiological studies also suggest a very minor contribution of GluA2-lacking AMPARs in the adult rodent NAc under normal conditions (Kourrich et al., 2007, Mameli et al., 2009; but see Campioni et al., 2009).
We have focused here on the NAc based on strong evidence for the functional importance of cocaine-induced alterations in AMPAR expression, cellular distribution and subunit composition in this region (Kalivas, 2009; Wolf and Ferrario, 2010; Bowers et al., 2010). For example, consistent with a predominant role for GluA1A2 receptors in the NAc, this is the population implicated in neuroadaptations resulting from repeated cocaine injections leading to behavioral sensitization (Boudreau and Wolf, 2005; Boudreau et al., 2007; Kourrich et al., 2007; Ghasemzadeh et al., 2009a; Ferrario et al., 2010). In contrast, GluA2-lacking AMPARs are added to NAc synapses after prolonged withdrawal from extended access cocaine self-administration, and mediate the enhanced cue-induced cocaine seeking observed after such regimens (Conrad et al., 2008; Mameli et al., 2009). Using the same co-IP approach employed herein, we found that both homomeric GluA1 and GluA1A3 receptors may contribute to this effect (Conrad et al., 2008; note that the control rats in this previous study self-administered saline and are therefore not equivalent to the naïve rats analyzed in the present study). Drugs of abuse also alter the abundance of GluA2-lacking AMPARs in the ventral tegmental area (e.g., Bellone and Lüscher, 2006; Argilli et al, 2008; Good and Lupica, 2010) and the HPC (e.g., Song et al., 2007; Billa et al., 2010). Interestingly, whereas surface expression of GluA1-containing AMPARs rapidly increases during cocaine-primed reinstatement following limited access cocaine self-administration (Anderson et al., 2008), GluA2-containing AMPARs may be internalized (Famous et al., 2008). In conclusion, results from animal models of addiction highlight the need to understand AMPAR subunit composition in the normal brain, in order to understand subsequent disease-related perturbations.
4. Materials and methods
4.1 Membrane preparation
Brains from adult male Sprague-Dawley rats (~300 g; Harlan Laboratories, Indianapolis, IN, USA) were obtained after decapitation and the NAc, DS, PFC and HPC (CA1-CA2) were immediately dissected and flash frozen in liquid nitrogen. Three independent membrane preparations were generated for each brain region. For each membrane preparation, tissue from 3-12 rats was pooled, weighed and processed as described by Sans et al. (2003). Briefly, tissue (40-60 mg wet weight/mL) was homogenized in 50 mM Tris-HCl (pH 7.4) containing protease inhibitors (Calbiochem, Gibbstown, NJ, USA). Membranes were pelleted by centrifugation at 100,000 × g for 30 min at 4°C and solubilized in 1% Triton X-100, 50 mM Tris-HCl, and 1 mM EDTA (pH 7.4) for 30-45 min at 37°C. Insoluble material was removed by centrifugation at 100,000 × g for 30 min at 4°C. About 75-85% of the amount of each AMPAR subunit in the starting material was recovered in this detergent solubilized fraction, confirming previous results with the same solubilization procedure (Wenthold et al., 1996). Solubilized tissue was stored at -80°C.
4.2 Immunoprecipitation
Antibodies were obtained from Dr. Robert Wenthold or purchased from Millipore (Billerica, MA, USA): GluA1 (AB1504), GluA2/3 (AB1506), GluA4 (AB1508). 3-5 μg of antibody or control IgG was incubated with 10-20 μL of 50% Protein A agarose slurry (Pierce, Rockford, IL, USA) for 4 h at 4°C. The pellet was collected by centrifugation at 1,000 × g for 30 s and washed three times in TBS containing 0.1% Triton X-100. Membrane preparation was added to the agarose-bound antibody and incubated with agitation overnight at 4°C. The agarose-bound antibody was pelleted by centrifugation at 1,000 × g for 30 s, creating two fractions, the bound (pellet) and unbound (supernatant). The unbound fraction was then subjected to another round of immunoprecipitation. The remaining unbound fraction was then mixed 1:1 with sample treatment buffer (Invitrogen, Carlsbad, CA, USA) and stored at -20°C until further use. For each brain region, each IP experiment was repeated in three different membrane preparations.
4.3 Gel Electrophoresis and Western blotting
For analysis of IP experiments, samples (unbound fractions mixed with sample treatment buffer) were heated to 70°C for 10 min and run on 4-12% Bis-Tris gels (Invitrogen). After electrophoretic separation, proteins were transferred to PVDF membrane for immunoblotting. Membranes were then washed in dH2O and blocked with 1% goat serum with 5% Carnation milk in 0.05% Tween-20 in TBS, pH 7.4, for 1 h at room temperature. Membranes were incubated overnight at 4°C with subunit-specific antibodies that were either provided by Dr. Robert Wenthold or purchased from Millipore: GluA1, 1:500 (AB1504); GluA2/3, 1:2000 (AB1506); GluA2, 1:1000 (AB1768); GluA3, 1:500 (MAB5416). Membranes were washed with TBS-Tween solution, incubated for 60 min with HRP-conjugated anti-rabbit IgG or anti-mouse IgG (Chemicon/Upstate, Billerica, MA, USA: 1:10,000), and washed again with TBS-Tween, followed by TBS. Membranes were rinsed with dH2O, immersed in chemiluminescence (ECL) detecting substrate (GE Biosciences, Piscataway, NJ, USA) for 1 min, and visualized with a VersaDoc system (BioRad, Hercules, CA, USA) (between 5 and 60 s, depending on the antibody). The optical densities of bands were determined using the VersaDoc analysis software. The percentage of total AMPAR subunit remaining in the unbound fraction was calculated on the basis of a standard curve generated using control IgG immunoprecipitated tissue. As noted previously, the GluR2/3 antibody also recognizes the GluR4c splice variant (Wenthold et al., 1996). This is unlikely to affect our results due to very low GluR4 expression in our regions of interest in the adult brain (see Section 2.2). A caveat that applies to quantitative analysis of monomer, dimer and tetramer species in our immunoblots (including BNE immunoblots described in Section 4.4) is that more antibody molecules may bind to multimers (e.g., twice as much GluA2 antibody may bind to a GluA2 dimer compared to a GluA2 monomer), altering signal intensity. However, the extent to which this actually occurs for each antibody is unknown and therefore cannot be corrected for.
4.4 Blue native electrophoresis (BNE)
BNE was conducted as described by Greger et al. (2003). Coomassie blue dye provides a negative charge to the protein complexes, enabling electrophoretic separation. After electrophoretic separation, proteins were transferred to PVDF membrane for immunoblotting. Immunoblotting was performed using the SNAP i.d. protein detection system (Millipore). Membranes were blocked in 1% goat serum with 0.5% nonfat milk in 0.05% Tween-20 in TBS, pH 7.4, or in Bløk-CH (Millipore) for 20 s. Membranes were incubated for 15 min with GluA1 (Pierce, PA137776), GluA2 (Millipore, AB1768), or GluA3 (Cell Signaling, Danvers, MA, USA; 3437S) primary antibodies, followed by washing with TBS-T or blocking buffer and incubation for 15 min with HRP-conjugated anti-rabbit IgG (Millipore). Membranes were washed with TBS-T or block followed by TBS. Membranes were then rinsed with dH2O, immersed in chemiluminescence (ECL) detecting substrate (GE Biosciences) for 1 min, and visualized using HyperFilm ECL. Bands of interest were quantified using TotalLab (Nonlinear Dynamics Ltd., Newcastle, UK). NativeMark protein standards (Invitrogen) were used to estimate molecular weights. For the NAc, BNE experiments were repeated in three different membrane preparations. For the other brain regions, analysis was based on the average of two BNE runs from a single membrane preparation. In a control experiment using NAc membranes, we crosslinked surface expressed proteins to evaluate the extent to which dissociation of tetrameric surface-expressed AMPARs during BNE contributed to the observation of monomer and dimer forms. Crosslinking was carried out as described previously using bis(sulfosuccinimidyl)suberate (BS3), a membrane-impermeant protein crosslinking reagent (Boudreau and Wolf, 2005). Briefly, 400 μm-thick slices of freshly dissected NAc tissue were incubated for 30 min at 4°C in artificial cerebrospinal fluid containing 2 mM BS3. The reaction was quenched with 100 mM glycine for 10 min at 4°C. Tissue was pelleted by centrifugation, flash frozen in liquid nitrogen and stored until membrane preparation was performed as described in Section 4.1.
Acknowledgments
This work was supported by USPHS grants DA015835 and DA00453 to MEW. We are very grateful to the late Dr. Robert Wenthold for his help with the development of methods and for generously supplying many of the antibodies used in these experiments. We thank Dr. Amy Boudreau for assistance in the initial stages of the experiments.
Abbreviations used
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionate
- BNE
blue native electrophoresis
- co-IP
co-immunoprecipitation
- DS
dorsal striatum
- ER
endoplasmic reticulum
- HPC
hippocampus
- IP
immunoprecipitation
- NAc
nucleus accumbens
- PFC
prefrontal cortex
- TARP
transmembrane AMPA receptor regulatory protein
Footnotes
Disclosure
JMR, MM and MW designed the experiments. JMR and MM conducted the experiments. JMR, MM and MEW wrote the manuscript. The authors have no conflicts of interest to disclose. All authors have approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béïque J-C, Huganir RL. AMPA receptor subunits get their share of the pie. Neuron. 2007;62:165–168. doi: 10.1016/j.neuron.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Bernard C, Wheal HV. Model of local connectivity patterns in CA3 and CA1 areas of the hippocampus. Hippocampus. 1994;4:497–529. doi: 10.1002/hipo.450040502. [DOI] [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billa SK, Liu J, Bjorklund NL, Sinha N, Fu Y, Shinnick-Gallagher, Morón JA. Increased insertion of glutamate receptor 2-lacking α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors at hippocampal synapses upon repeated morphine administration. Mol Pharmacol. 2010;77:874–883. doi: 10.1124/mol.109.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PAC, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: The past, present, and therapeutic future. Neuron. 2010;67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacol. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology. 2010;35:818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience. 2009a;159:414–426. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C. Locomotor sensitization to cocaine is associated with distinct pattern of glutamate receptor trafficking to the postsynaptic density in the prefrontal cortex: early versus late withdrawal effects. Pharmacol Biochem Behav. 2009b;92:383–392. doi: 10.1016/j.pbb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Good CH, Lupica CR. Afferent-specific AMPA receptor subunit composition and regulation of synaptic plasticity in midbrain dopamine neurons by abused drugs. J Neurosci. 2010;30:7900–7909. doi: 10.1523/JNEUROSCI.1507-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB. RNA editing at Arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- Greger IH, Esteban JA. AMPA receptor biogenesis and trafficking. Curr Opin Neurobiol. 2007;17:289–297. doi: 10.1016/j.conb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Hall RA, Soderling TR. Quantitation of AMPA receptor surface expression in cultured hippocampal neurons. Neurosci. 1997;78:361–371. doi: 10.1016/s0306-4522(96)00525-8. [DOI] [PubMed] [Google Scholar]
- Isaac JTR, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Jonas P, Burnashev N. Molecular mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron. 1995;15:987–990. doi: 10.1016/0896-6273(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kato AS, Gill MB, Yu H, Nisenbaum ES, Bredt DS. TARPs differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci. 2010;33:241–248. doi: 10.1016/j.tins.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Kim KS, Yan D, Tomita S. Assembly and stoichiometry of the AMPA receptor and transmembrane AMPA receptor regulatory protein complex. J Neurosci. 2010;30:1064–1072. doi: 10.1523/JNEUROSCI.3909-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Au E, Neve R, Yoon BJ. AMPA receptor trafficking in the dorsal striatum is critical for behavioral sensitization to cocaine in juvenile mice. Biochem Biophys Res Commun. 2009;379:65–69. doi: 10.1016/j.bbrc.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R. AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Mano I, Teichberg VI. A tetrameric subunit stoichiometry for a glutamate receptor-channel complex. NeuroReport. 1998;9:327–31. doi: 10.1097/00001756-199801260-00027. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S. Microcircuits in nucleus accumbens’ shell and core involved in cognition and reward. Psychobiology. 1999;27:165–186. [Google Scholar]
- Palmer CL, Cotton L, Henley JM. The molecular pharmacology and cell biology of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Pharmacol Rev. 2005;57:253–277. doi: 10.1124/pr.57.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Safferling M, Tichelaar W, Kümmerle G, Jouppila A, Kuusinen A, Keinänen K, Madden DR. First images of a glutamate receptor ion channel: Oligomeric state and molecular dimensions of GluRB homomers. Biochemistry. 2001;40:13948–13953. doi: 10.1021/bi011143g. [DOI] [PubMed] [Google Scholar]
- Sans N, Vissel B, Petralia RS, Wang Y, Chang K, Royle GA, Wang C, O’Gorman S, Heinemann SF, Wenthold RJ. Aberrant formation of glutamate receptor complexes in hippocampal neurons of mice lacking the GluR2 AMPA receptor subunit. J Neurosci. 2003;23:9367–9373. doi: 10.1523/JNEUROSCI.23-28-09367.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H, Cramer WA, von Jagow G. Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem. 1994;217:220–230. doi: 10.1006/abio.1994.1112. [DOI] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, Klöcker N. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lu W, Milstein AD, Nicoll RA. The stoichiometry of AMPA receptors and TARPs varies by neuronal cell type. Neuron. 2009;62:633–640. doi: 10.1016/j.neuron.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Song J, Shen G, Greenfield LJ, Jr, Tietz EI. Benzodiazepine withdrawal-induced glutamatergic plasticity involves up-regulation of GluR1-containing α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in hippocampal CA1 neurons. J Pharmacol Exp Ther. 2007;322:569–581. doi: 10.1124/jpet.107.121798. [DOI] [PubMed] [Google Scholar]
- Stefani A, Chen Q, Flores-Hernandez J, Jiao Y, Reiner A, Surmeier DJ. Physiological and molecular properties of AMPA/Kainate receptors expressed by striatal medium spiny neurons. Dev Neurosci. 1998;20:242–252. doi: 10.1159/000017318. [DOI] [PubMed] [Google Scholar]
- Vandenberghe W, Nicoll RA, Bredt DS. Stargazin is an AMPA receptor auxiliary subunit. Proc Natl Acad Sci USA. 2005;102:485–490. doi: 10.1073/pnas.0408269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Goriounova NA, Li KW, Van der Schors RC, Binnekade R, Schoffelmeer AN, Mansvelder HD, Smit AB, Spijker S, De Vries TJ. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci. 2008;11:1053–1058. doi: 10.1038/nn.2165. [DOI] [PubMed] [Google Scholar]
- Von Engelhardt J, Mack V, Sprengel R, Kavenstock N, Li KW, Stern-Bach Y, Smit AB, Seeburg PH, Monyer H. CKAMP44: a brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science. 2010;327:1518–1522. doi: 10.1126/science.1184178. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig I, Schägger H. Native electrophoretic techniques to identify protein–protein interactions. Proteomics. 2009;9:5214–5223. doi: 10.1002/pmic.200900151. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010 Jan 28; doi: 10.1016/j.neubiorev.2010.01.013. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]