Abstract
The present study was conducted to examine cytokine profiles in the masseter muscle before and after complete Freund’s adjuvant (CFA)-induced inflammation and possible sex differences in the cytokine levels. Age matched male and female Sprague Dawley rats were injected with CFA in the mid-region of the masseter muscle. Muscle tissue surrounding the injection site was extracted 6 hrs, 1, 3 and 7 days after the injection to measure TNF-α, IL-1β, IL-6 and IL-4 levels with Luminex multi-analyte profiling (xMAP) technology. The cytokine levels were compared to those obtained from naïve rats. CFA injection into the masseter muscle led to a significant time effect in the level of TNF-α compared to that of naive rats. The pattern of changes in TNF-α level after CFA injection was significantly different between the male and female rats owing to the differences in basal levels. CFA injection induced significant time-dependent increases in the levels of IL-1β and IL-6 in the masseter muscle in both male and female rats. The level of IL-4 was slightly, but significantly, reduced in both sexes at 6 hrs and 3 days after CFA-induced inflammation. No significant sex differences were observed in the levels of IL-1β, IL-6 or IL-4. The results provided novel information about distinct cytokine profiles during CFA-induced muscle inflammation, and the basis for further pursuing contributions of each cytokine in pain processing and analgesic responses in both sexes.
Keywords: complete Freund’s adjuvant, TNF-α, IL-1β, IL-6, IL-4, sex, muscle
Musculoskeletal pain conditions including neck pain, back pain, orofacial muscle pain and other chronic widespread pain conditions such as fibromyalgia are prevalent and severe pain conditions. While most of our knowledge about chronic types of pain is based on cutaneous pain models, muscle pain conditions, including exercise-induced muscle pain and orofacial muscle pain related to temporomandibular disorders, appear to utilize different mechanisms in the development and maintenance compared to cutaneous pain [21,34].
Many chronic musculoskeletal pain conditions have been shown to originate from muscle inflammation [1,15,20,22]. Cytokines released locally during inflammation modulate nociceptive processing via multiple mechanisms. Cytokines can directly sensitize nociceptors and increase neuronal sensitivity to heat, mechanical and chemical stimuli [17,18,24,26,35]. Inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α, contribute to pain and hyperalgesia indirectly by inducing the production of inflammatory agents that sensitize nociceptors [2,28]. In addition, inflammatory cytokines are powerful modulators of the expression of many receptors involved in pain and analgesia. For example, TNF-α significantly up-regulates TRPV1 in trigeminal sensory neurons [19]. In the opioid system, IL-1β, IL-6, TNF-α and IL-4 induce mu-opioid receptor expression in neuronal and non-neuronal cells [3,4, 30]. Similarly, in the cannabinoid system, cytokine-stimulated whole blood elevates CB1 and CB2 mRNA and protein levels when compared to non-stimulated blood [16]. Thus, changes in cytokine profiles in the local tissue during injury or inflammation can provide potent modulation of nociceptive signaling processes.
Studies based on cutaneous tissue have demonstrated distinct changes in cytokine profiles after inflammation and tissue injury: e.g., tissue injury induces bradykinin release leading to the release of TNF-α, which results in the release of IL-6 and IL-1β [28]. There are, however, distinct differences in carrageenan-induced changes in cytokine profiles when cutaneous hindpaw tissue is compared to gastrocnemius muscle in female Sprague Dawley rats [20]. TNF-α is elevated significantly in the hindpaw but not in the muscle. Both IL-1β and IL-6 are elevated at an earlier time point in the cutaneous hindpaw tissue compared to the muscle tissue. These data confirm that there is a specific cytokine profile associated with muscle inflammation, at least in the female rats.
However, since pain responses from males and females vary a great deal in many chronic musculoskeletal pain conditions, and the two sexes also respond differently to inflammation [11], it is important to examine whether there are sex differences in the cytokine profiles in muscle under inflammatory conditions. At present, there is no study that has directly assessed potential sex differences in the local cytokine levels under inflammatory conditions. Thus, the objective of this project was to establish the cytokine profiles in the masseter muscle before and after complete Freund’s adjuvant (CFA)-induced inflammation in both male and female rats. We chose to analyze the levels of three pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and one anti-inflammatory cytokine IL-4 because of their known roles in inflammation and as modulators of receptor expression and nociceptive signaling at the primary afferent level.
Age matched adult male and female Sprague-Dawley rats (8 weeks old; 250–300g for males and 225–260g for females) were used in this experiment. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The levels of cytokines in the masseter muscle were analyzed in naïve rats, and rats treated with CFA at 6 hrs, 1, 3 and 7 days following the CFA treatment. All groups, except for the 6 hr time point, consisted of 6 rats. The 6 hr group consisted of 4 rats. CFA (Sigma; 1:1 in isotonic saline; 50μl) was injected directly in the mid-region of masseter muscle to induce local inflammation. In additional groups of naïve and CFA-inflamed rats (1 day post CFA) plasma samples were collected by cardiac puncture to assess whether the changes cytokine levels in the muscle result from local inflammatory response or systemic reaction. Estrus cycle in female rats was not determined in this study.
All animals were euthanized by decapitation under pentobarbital anesthesia (100mg/kg). Masseter muscle surrounding the injection site (approximately 100mg) was extracted and homogenized in RIPA buffer (Cell Signaling Technology, Inc., Danvers, MA) containing complete protease inhibitor cocktail tablets (Roche Diagnostics, Berkeley, CA). The amount of RIPA buffer added was nine times the weight of the tissue sample. The total protein concentration in lysate was determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA) and the amount of protein was normalized to that of the control sample. Total protein of 2mg (250μl, 8μg/μl) was used in the cytokine assay for each sample.
The Luminex multi-analyte profiling (xMAP) technology (Luminex corp.) was used to measure cytokine levels. Bead sets were coated with capture antibody for various cytokines (TNF-α, IL-1β, IL-4 and IL-6). Fluorescence detection antibodies were then applied to bind the cytokine-capture antibody complex on the bead set. Multiple cytokines in the samples were then recognized by the differences in bead sets with fluoregenic emission detection using flow cytometric analysis [29]. Each sample was measured in triplicate. Two-Way ANOVA was used to compare the cytokine levels between naïve and CFA groups and between male and female rats. All comparisons between multiple groups were followed by a post hoc test (Bonferroni). Data are presented as mean ± SEM and differences were considered significant at p<0.05.
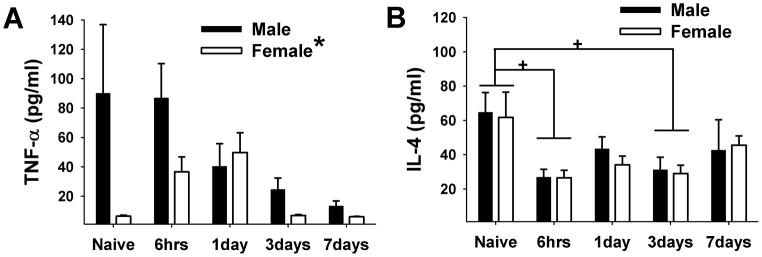
In male rats, the mean level of TNF-α in the masseter muscle was at its highest (90.1±46.7 pg/ml) under the naïve condition (Fig 1A). The injection of CFA into the masseter muscle induced a gradual decrease in the TNF-α level. The mean TNF-α level was reduced to 86.9±23.3, 40.4±15.4, 24.7±7.6, and 13.3±3.4 pg/ml after 6 hrs, 1, 3 and 7 days, respectively. In female rats, the mean level of TNF-α in the masseter muscle was 6.4±0.65 pg/ml under the naïve condition, about 14 fold less than the level in naïve male rats. However, after CFA injection, the level of TNF-α in female rats increased to 36.6±10.2 pg/ml after six hours and 49.7±13.5 pg/ml after one day before returning to the level under the naïve condition after three days. Significant sex differences between male and female rats were observed due to the low basal level in female rats (F=6.417, p=0.015). There was also a significant time effect on inflammation-induced TNF-α levels (F=2.767, p=0.038), but post hoc analysis did not detect significant group differences. A significant group difference was found only between naïve and CFA 7day male rats. No other individual group comparisons were significant.
Figure 1.
Mean levels of TNF-α and IL-4 in masseter muscle in naïve and rats treated with CFA. (A) Significant sex differences were observed between male and female rats. There was also a significant time effect, but the post hoc test did not reveal any significant group differences. A significant reduction of TNF-α level 7 days following CFA treatment was noted only in male rats. (B) The levels of IL-4 decreased slightly but significantly at the 6 hr and 3 day time points in both sexes. No sex difference was observed. *, + denote significant group and time effects, respectively with p<0.05, n=4–6/group.
The mean level of IL-4 decreased slightly but significantly after the CFA injection (F=4.065, p=0.007) (Fig 1B). Under the naïve condition, the mean levels of IL-4 were 64.7±11.5 and 61.8±14.7 pg/ml for male and female, respectively. Six hours after the CFA injection, the mean levels of IL-4 decreased significantly to 27.0±4.5 and 26.5±4.4 pg/ml in male and female respectively. A second phase of slight decrease in the IL-4 level was observed three days after CFA injection. No significant sex difference was detected (F=0.138, p=0.712).
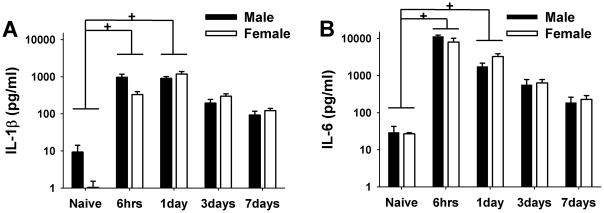
The mean levels of IL-1β in the masseter muscle were low in both male (9.3±4.9 pg/ml) and female rats (1.0±0.5 pg/ml) under the naïve condition (Fig 2A). Injection of CFA into the masseter muscle induced a substantial increase in the IL-1β levels in both sexes (~1000 pg/ml) as early 6hrs and remained significantly increased for one day (F=43.258, p=<0.001). Seven days after CFA injection, the levels of IL-1β in both male and female rats decreased to approximately 100 pg/ml. No significant difference in the IL-1β level was detected between naïve and CFA 3 or 7 day groups. There appeared to be a slight delay in the increase and decrease in the level of IL-1β in females compared to males; however no significant sex difference was observed (F=0.548, p=0.463).
Figure 2.
Mean levels of IL-1β and IL-6 in masseter muscle in naïve and rats treated with CFA. (A) The levels of IL-1β increased significantly after 6 hrs and 1 day after CFA injection in both sexes. No significant sex difference was observed. (B) The levels of IL- 6 increased significantly after 6 hrs and 1 day following CFA injection in both male and female rats. No significant sex difference was observed. + denotes significant time effect with p<0.05, n=4–6/group. Y axis is in a log scale.
The pattern of inflammation-induced changes in the level of IL-6 was similar to that of IL-1β. The mean level of IL-6 was 29.6±13.3 pg/ml in both male and female rats under the naïve condition (Fig 2B). Injection of CFA into the masseter muscle induced a significant and substantial increase in IL-6 levels in both sexes (F=79.190, p<0.001). Six hours after the CFA injection, the level of IL-6 rose to approximately 10,000 pg/ml, more than a 300 fold increase from the naïve condition. The mean level of IL-6 remained significantly increased after one day, which then gradually decreased to approximately 200 pg/ml after 7 days. There were no significant differences in the IL-6 level between naïve and CFA 3 or CFA 7 day groups. No significant sex difference was detected (F=0.940, p=0.337).
In order to rule out the possibility that the CFA-induced changes in cytokine levels in local tissue did not result from systemic reactions the plasma levels of all 4 cytokines collected 1 day after CFA inflammation were compared to those of naïve rats. The plasma levels of TNF-α, IL-1β, and IL-4 were below the detection level in both naïve and CFA treated rats. The plasma level of IL-6 was detectable but there was no significant difference between naïve and CFA treated rats (t=1.127, p>0.05).
Since we used naïve rats rather than vehicle injected rats as the control group we conducted additional experiments to assess whether the vehicle injection significantly alters the local cytokine levels compared to those of naïve rats. The data showed that the vehicle injection did not significantly alter the local levels of TNF–α (t=1.28, p=0.24), IL-6 (t=0.01, p=0.99) and IL-4 (t=2.1, p=0.07). There was a statistically significant difference in the levels of L-1β between naïve and vehicle treated rats (t=3.31, p=0.03). However, the range of IL-1β levels in naïve and vehicle treated groups were comparable (0–30 pg/ml and 17–40 pg/ml, respectively), suggesting that the surge of IL-1β in orders of magnitude following CFA injection is not due to the injection procedure, but to local inflammatory responses.
Few studies have directly compared cytokine levels between males and females. A significantly higher plasma IL-1β level in male patients compared to females was reported following abdominal trauma [8]. A greater concentration of serum IL-6 in men has been implicated in hepatocellular carcinoma, the most common liver cancer in men, while estrogen-mediated inhibition of IL-6 reduces the risk of liver cancer in women [23]. The present study provides the first report regarding sex-based observations on local cytokine profiles following muscle inflammation. Our data showed that CFA-induced inflammation produced similar changes in IL-1β, IL-4 and IL-6 levels between the male and female rats. Inflammation differentially impacted the levels of TNF-α between the sexes, which may result from the difference in the basal levels of TNF-α in the masseter muscle in male and female rats.
In contrast to cutaneous tissue, the level of TNF-α in gastrocnemius muscle does not increase in female rats after carrageenan-induced inflammation [20]. Our data showed that the level of TNF-α in the masseter muscle decreased significantly only at a later stage of CFA-induced inflammation in male rats. However, the level of TNF-α in the masseter muscle in female rats rose slightly at 6 hrs and 1 day, owing to the low basal level. The differences in the basal levels and the pattern of inflammation-induced changes in TNF-α levels in female rats between this study and that of Loram et al [20] could be attributed to the types of muscle (limb vs. craniofacial) or the inflammatory agent (carrageenan vs. CFA). There was large variability in TNF-α level in the masseter under naïve conditions in male rats. We do not know the source of the variability at this point, but it is possible that TNF-α level in local tissue in intact animals may not be stable compared to other cytokines.
The increase in the level of TNF-α during CFA-induced inflammation in female rats was only modest compared to that of IL-1β or IL-6. Also, the level of TNF-α following CFA injection was comparable between male and female rats. These observations suggest that muscle inflammation does not significantly impact the levels of TNF-α. Direct intramuscular injection of a high dose of TNF-α produces mechanical hyperalgesia [32], and sensitizes trigeminal muscle afferents in male rats [12]. The same concentration of exogenous TNF-α could produce different responses in female rats since the basal level is substantially lower in the masseter muscle of female rats. Thus, the contribution of TNF-α at a physiological concentration in muscle pain processing and potential sex specific responses remains to be determined.
IL-1β mediates inflammatory pain and hyperalgesia by inducing a widespread expression of prostanoids both in the periphery and in the CNS [9,31]. In the muscle tissue, IL-1β regulates IL-6 production, which may be involved in muscle injury, degeneration and repair of the muscle [14]. The level of IL-1β increases in human skeletal muscle after eccentric exercise for a prolonged period of time and has also been shown to be a potent hyperalgesic agent [5,9,10]. Similarly, the concentration of IL-1β is elevated in the trapezius muscle of human patients with active myofascial trigger points [34]. In an animal model of muscular hyperalgesia, the surge of IL-1β in the gastrocnemius muscle during carrageenan-induced inflammation occurred at 24 hrs. This followed the initiation of primary muscle hyperalgesia occurring as early as 3 hrs post injection. These observations led the authors to suggest its role in the maintenance of hyperalgesia in muscle tissue [20]. However, carageenan injected in the rat masseter muscle causes an immediate increase (i.e. 1 hr post injection) in the level of IL-1β [27]. In the present study, we also showed a prominent increase in masseteric IL-1β levels following CFA-induced inflammation in both male and female rats. The increase in the levels of IL-1β could be observed from 6 hrs and were maintained until the 3 day time point. Again, the differences could be due to the more potent and prolonged effects of CFA compared to carageenan as well as tissue specific responses.
CFA injected in the rat masseter muscle and the hindpaw have been shown to significantly up-regulate mu and kappa opioid receptors, respectively, in sensory neurons [25,29]. Interestingly, a local injection of IL-1β also significantly up-regulates kappa opioid receptors and treatment with an IL-1 receptor antagonist blocks the CFA-induced up-regulation of kappa opioid receptor expression in sensory neurons, and thereby increasing kappa mediated analgesia in the periphery [29]. Taken together, these data suggest that the surge of IL-1β in local tissue following inflammation contributes to pain and hyperalgesia, as well as analgesia. However, a potential role of IL-1β in mediating sex differences in pain and analgesia may not be explained solely by the local level of IL-1β.
While there is no clear consensus on the role of IL-6 in inflammatory muscle pain, it is likely that IL-6 is involved in multiple aspects of skeletal muscle physiology and pathophysiology. IL-6 is markedly increased during eccentric and concentric contraction of the muscle and involved in protein degradation during muscle damage as well as during muscle regeneration [6,36–38]. Acute injections with IL-6 in the gastrocnemius muscle do not excite or sensitize muscle nocieptors in anesthetized rats [14]. However, local IL-6 injections not only produce muscular hyperalgesia that lasts for several hours but also play a key role in the development of chronic latent hyperalgesia [7]. An immediate rise of intramuscular IL-6 mRNA expression following electrically-induced muscle contraction and carrageenan–induced inflammation in the masseter muscle suggests a multifunctional role for IL-6 across different types of muscle tissue [27]. In addition, our data showing that IL-6 in the masseter muscle increased significantly and dramatically (more than 300 fold) in both male and female rats after CFA-induced inflammation further support the contribution of IL-6 in muscle pain and metabolism in both sexes.
The anti-inflammatory and anti-hyperalgesic effects of IL-4 have been demonstrated [13,40]. However, the information on inflammation-induced changes in IL-4 in the muscle tissue and potential sex differences in its level is not available. In this study, we showed that the levels of IL-4 in the masseter muscle were reduced slightly but significantly in both sexes at the 6 hr and 3 day time points, indicating a pro-inflammatory effect at those time points. It is possible that the reduced level of IL-4 contributed, at least partly, to the increase in IL-1β and IL-6 [39]. IL-4 also contributes to anti-hyperalgesic effects by up-regulating peripheral opioid receptors under inflammatory conditions [4]. Because similar changes were observed in the levels of IL-4 in both sexes, IL-4 mediated anti-hyperalgesic effects are not expected to exhibit sex differences. Since we did not assess the estrus stages for the female rats the potential influence of circulating estrogen on local cytokine profiles cannot be determined in this study. Additional studies with female rats in different estrus stages or with ovariectomized rats should provide more precise information about the role of estrogen in local cytokine responses.
In summary, this study established the cytokine profiles in the masseter muscle before and after CFA-induced inflammation. These observations provided the basis for the potential contribution of each cytokine in inflammation-induced pain and analgesic responses in the muscle tissue in both sexes.
Acknowledgments
We thank Jami L. Saloman for helpful comments during the manuscript preparation. This work was supported by NIH-NIDCR grant DE019448 to JYR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armstrong RB. Initial events in exercise-induced muscular injury. Med Sci Sports Exerc. 1990;22(4):429–435. [PubMed] [Google Scholar]
- 2.Bernheim HA, Gilbert TM, Stitt JT. Prostaglandin E levels in third ventricular cerebrospinal fluid of rabbits during fever and changes in body temperature. J Physiol. 1980;301:69–78. doi: 10.1113/jphysiol.1980.sp013189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borner C, Hollt V, Kraus J. Involvement of activator protein-1 in transcriptional regulation of the human mu-opioid receptor gene. Mol Pharmacol. 2002;61(4):800–805. doi: 10.1124/mol.61.4.800. [DOI] [PubMed] [Google Scholar]
- 4.Borner C, Kraus J, Schroder H, Ammer H, Hollt V. Transcriptional regulation of the human mu-opioid receptor gene by interleukin-6. Mol Pharmacol. 2004;66(6):1719–1726. doi: 10.1124/mol.104.003806. [DOI] [PubMed] [Google Scholar]
- 5.Cannon JG, Fielding RA, Fiatarone MA, Orencole SF, Dinarello CA, Evans WJ. Increased interleukin 1 beta in human skeletal muscle after exercise. Am J Physiol. 1989;257(2 Pt 2):R451–5. doi: 10.1152/ajpregu.1989.257.2.R451. [DOI] [PubMed] [Google Scholar]
- 6.Cantini M, Massimino ML, Rapizzi E, Rossini K, Catani C, Dalla Libera L, Carraro U. Human satellite cell proliferation in vitro is regulated by autocrine secretion of IL-6 stimulated by a soluble factor(s) released by activated monocytes. Biochem Biophys Res Commun. 1995;216(1):49–53. doi: 10.1006/bbrc.1995.2590. [DOI] [PubMed] [Google Scholar]
- 7.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152(2):521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ertan T, Keskek M, Kilic M, Gocmen E, Oguz H, Aksaray S, Koc M. Effects of gender difference in early cytokine levels in trauma patients. Bratisl Lek Listy. 2007;108(3):128–132. [PubMed] [Google Scholar]
- 9.Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334(6184):698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 10.Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am J Physiol. 1993;265(1 Pt 2):R166–72. doi: 10.1152/ajpregu.1993.265.1.R166. [DOI] [PubMed] [Google Scholar]
- 11.Flake NM, Hermanstyne TO, Gold MS. Testosterone and estrogen have opposing actions on inflammation-induced plasma extravasation in the rat temporomandibular joint. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R343–8. doi: 10.1152/ajpregu.00835.2005. [DOI] [PubMed] [Google Scholar]
- 12.Hakim AW, Dong XD, Svensson P, Kumar U, Cairns BE. TNFalpha mechanically sensitizes masseter muscle afferent fibers of male rats. J Neurophysiol. 2009;102(3):1551–1559. doi: 10.1152/jn.00326.2009. [DOI] [PubMed] [Google Scholar]
- 13.Hao S, Mata M, Glorioso JC, Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:6. doi: 10.1186/1744-8069-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoheisel U, Unger T, Mense S. Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain. 2005;114(1–2):168–176. doi: 10.1016/j.pain.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Inanici F, Yunus MB. History of fibromyalgia: past to present. Curr Pain Headache Rep. 2004;8(5):369–378. doi: 10.1007/s11916-996-0010-6. [DOI] [PubMed] [Google Scholar]
- 16.Jean-Gilles L, Constantinescu CS. Cytokine Regulation of Cannabinoid Receptors 1 & 2 in Multiple Sclerosis. Proceedings of the British Pharmacological Society. 2007 [Google Scholar]
- 17.Jin X, Gereau RW., 4th Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26(1):246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000;85(1–2):145–151. doi: 10.1016/s0304-3959(99)00262-6. [DOI] [PubMed] [Google Scholar]
- 19.Khan AA, Diogenes A, Jeske NA, Henry MA, Akopian A, Hargreaves KM. Tumor necrosis factor alpha enhances the sensitivity of rat trigeminal neurons to capsaicin. Neuroscience. 2008;155(2):503–509. doi: 10.1016/j.neuroscience.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 20.Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain. 2007;8(2):127–136. doi: 10.1016/j.jpain.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Mense S. Muscle pain: mechanisms and clinical significance. Dtsch Arztebl Int. 2008;105(12):214–219. doi: 10.3238/artzebl.2008.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murata Y, Olmarker K, Takahashi I, Takahashi K, Rydevik B. Effects of selective tumor necrosis factor-alpha inhibition to pain-behavioral changes caused by nucleus pulposus-induced damage to the spinal nerve in rats. Neurosci Lett. 2005;382(1–2):148–152. doi: 10.1016/j.neulet.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 24.Nicol GD, Lopshire JC, Pafford CM. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J Neurosci. 1997;17(3):975–982. doi: 10.1523/JNEUROSCI.17-03-00975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunez S, Lee JS, Zhang Y, Bai G, Ro JY. Role of peripheral mu-opioid receptors in inflammatory orofacial muscle pain. Neuroscience. 2007;146(3):1346–1354. doi: 10.1016/j.neuroscience.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Obreja O, Schmelz M, Poole S, Kress M. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain. 2002;96(1–2):57–62. doi: 10.1016/s0304-3959(01)00420-1. [DOI] [PubMed] [Google Scholar]
- 27.Ono T, Maekawa K, Watanabe S, Oka H, Kuboki T. Muscle contraction accelerates IL-6 mRNA expression in the rat masseter muscle. Arch Oral Biol. 2007;52(5):479–486. doi: 10.1016/j.archoralbio.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 28.Poole S, Cunha FQ, Selkirk S, Lorenzetti BB, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-10. Br J Pharmacol. 1995;115(4):684–688. doi: 10.1111/j.1476-5381.1995.tb14987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puehler W, Rittner HL, Mousa SA, Brack A, Krause H, Stein C, Schafer M. Interleukin-1 beta contributes to the upregulation of kappa opioid receptor mrna in dorsal root ganglia in response to peripheral inflammation. Neuroscience. 2006;141(2):989–998. doi: 10.1016/j.neuroscience.2006.03.078. [DOI] [PubMed] [Google Scholar]
- 30.Ruzicka BB, Thompson RC, Watson SJ, Akil H. Interleukin-1 beta-mediated regulation of mu-opioid receptor mRNA in primary astrocyte-enriched cultures. J Neurochem. 1996;66(1):425–428. doi: 10.1046/j.1471-4159.1996.66010425.x. [DOI] [PubMed] [Google Scholar]
- 31.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410(6827):471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 32.Schafers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. 2003;104(3):579–588. doi: 10.1016/S0304-3959(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 33.Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM, Gerber LH. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Sharma NK, Ryals JM, Liu H, Liu W, Wright DE. Acidic saline-induced primary and secondary mechanical hyperalgesia in mice. J Pain. 2009;10(12):1231–1241. doi: 10.1016/j.jpain.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81(1):255–262. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- 36.Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529(Pt 1):237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomiya A, Aizawa T, Nagatomi R, Sensui H, Kokubun S. Myofibers express IL-6 after eccentric exercise. Am J Sports Med. 2004;32(2):503–508. doi: 10.1177/0095399703258788. [DOI] [PubMed] [Google Scholar]
- 38.Tsujinaka T, Ebisui C, Fujita J, Kishibuchi M, Morimoto T, Ogawa A, Katsume A, Ohsugi Y, Kominami E, Monden M. Muscle undergoes atrophy in association with increase of lysosomal cathepsin activity in interleukin-6 transgenic mouse. Biochem Biophys Res Commun. 1995;207(1):168–174. doi: 10.1006/bbrc.1995.1168. [DOI] [PubMed] [Google Scholar]
- 39.Üçeyler N, Sommer C. Cytokine Profiles in Patients with Chronic Widespread Pain. In: DeLeo JA, Sorkin LS, Watkins LR, editors. Immune and Glial Regulation of Pain. IASP Press; Seattle: 2007. pp. 417–427. [Google Scholar]
- 40.Vale ML, Marques JB, Moreira CA, Rocha FA, Ferreira SH, Poole S, Cunha FQ, Ribeiro RA. Antinociceptive effects of interleukin-4, -10, and -13 on the writhing response in mice and zymosan-induced knee joint incapacitation in rats. J Pharmacol Exp Ther. 2003;304(1):102–108. doi: 10.1124/jpet.102.038703. [DOI] [PubMed] [Google Scholar]