Abstract
Background
Administration of small, intermittent doses of glucagon during closed-loop insulin delivery markedly reduces the frequency of hypoglycemia. However, in some cases, hypoglycemia occurs despite administration of glucagon in this setting.
Methods
Fourteen adult subjects with type 1 diabetes participated in 22 closed-loop studies, duration 21.5 ± 2.0 h. The majority of subjects completed two studies, one with insulin + glucagon, given subcutaneously by algorithm during impending hypoglycemia, and one with insulin + placebo. The more accurate of two subcutaneous glucose sensors was used as the controller input. To better understand reasons for success or failure of glucagon to prevent hypoglycemia, each response to a glucagon dose over 0.5 µg/kg was analyzed (n = 19 episodes).
Results
Hypoglycemia occurred in the hour after glucagon delivery in 37% of these episodes. In the failures, estimated insulin on board was significantly higher versus successes (5.8 ± 0.5 versus 2.9 ± 0.5 U, p < .001). Glucose at the time of glucagon delivery was significantly lower in failures versus successes (86 ± 3 versus 95 ± 3 mg/dl, p = .04). Sensor bias (glucose overestimation) was highly correlated with starting glucose (r = 0.65, p = .002). Prior cumulative glucagon dose was not associated with success or failure.
Conclusion
Glucagon may fail to prevent hypoglycemia when insulin on board is high or when glucagon delivery is delayed due to overestimation of glucose by the sensor. Improvements in sensor accuracy and delivery of larger or earlier glucagon doses when insulin on board is high may further reduce the frequency of hypoglycemia.
Keywords: artificial pancreas, glucagon, glucose sensor, hypoglycemia, type 1 diabetes
Introduction
Advances in the field of artificial pancreas systems indicate that home use of these systems is rapidly approaching.1–5 To maximize safety, each component of such a system needs to be scrutinized to predict and overcome potential pitfalls. In general terms, an artificial pancreas is comprised of a glucose sensor that sends data to a computer algorithm that, in turn, determines the amount of insulin to be delivered by an insulin pump. This type of system is more accurately known as closed-loop insulin delivery, given it lacks many of the functions and inputs of a mammalian pancreas.
Closed-loop glycemic control, however, need not be limited to insulin delivery and can include delivery of glucagon, a 29 amino acid hormone produced by the normal endocrine pancreas in response to hypoglycemia. Secretion and action of glucagon is typically abnormal in patients with type 1 diabetes.6,7 This type of dual hormone delivery for closed-loop control was first proposed by Kadish8 in 1963 and has been demonstrated to be effective in adults with type 1 diabetes by our group3 and by El-Khatib and colleagues.4 Glucagon acts to rapidly raise blood glucose in both persons with and without diabetes by catabolizing liver glycogen.9 In general terms, in an insulin and glucagon closed-loop system, insulin is delivered nearly continuously to treat and prevent hyperglycemia, and glucagon is delivered intermittently to treat and prevent hypoglycemia. When used in this manner, glucagon decreased hypoglycemia by two-thirds, from an average of 40 min/day in the hypoglycemic range to 15 min/day.3
Two current limitations in closed-loop control are sensor inaccuracy and the slow onset and offset of insulin. There are multiple potential sources of sensor error, one being sensor drift, which is poorly understood and unpredictable.10,11 If a sensor is overestimating the glucose level, an inappropriate amount of insulin would be called for by the closed-loop system, thus increasing the risk of hypoglycemia. The long duration of action of insulin is also problematic and predisposes patients to hypoglycemia, because even the most short-acting insulin analogs currently available have a duration of action of approximately 8 h when delivered subcutaneously.12 For this reason, the discontinuation of insulin delivery during impending hypoglycemia often cannot prevent hypoglycemia.
Glucagon is a good candidate for preventing insulin- induced hypoglycemia due to its rapid onset of action after subcutaneous injection. In our study of insulin and glucagon closed-loop control, glucagon prevented most, but not all, cases of hypoglycemia. Here we describe the factors that influenced the response to glucagon and how such knowledge can be used to optimize glucagon delivery in the future.
Methods
Fourteen adult subjects with type 1 diabetes underwent 22 human closed-loop experiments lasting 21.5 ± 2.0 h as published previously.3 One subject withdrew early from the study because of repeated intravenous catheter failures, for a total of 21 closed-loop experiments in 13 subjects who received automated insulin and glucagon delivery. Hormone delivery rates were determined by the fading memory proportional derivative (FMPD) algorithm.13,14 Each subject wore two glucose sensors, and data from the more accurate sensor after a 2 h assessment period was used as the input into the FMPD algorithm. Arterialized venous blood glucose (VBG) levels were measured every 10 min for assessment of sensor accuracy, detection of hypoglycemia, and data analysis purposes. If neither glucose sensor was reading accurately, defined as deviating by more than 20% (for VBG 75 mg/dl or higher) or 20 mg/dl (<75 mg/dl) from the reference VBG value, then the VBG level was used as the input into the algorithm.
In addition to a basal component, the subcutaneous insulin delivery was dosed according to the difference between the glucose levels and the glucose target (proportional error) and the slope of glucose level (derivative error). The insulin delivery rate was modified by a fading memory (exponential decay) of past proportional and derivative errors, with more recent errors affecting the rate more heavily than remote errors.
Glucagon was delivered subcutaneously via a syringe pump and was given at times of impending hypoglycemia as determined by the FMPD algorithm, but in the opposite direction compared to insulin. Glucagon delivery was increased for negative proportional errors (glucose levels below target) and negative derivative errors (falling glucose levels) and was also modified by the glucose history. Seven subjects received glucagon delivered in a prolonged fashion, termed low-gain glucagon delivery, and six subjects received glucagon delivered in a short-lived but brisk fashion at times of impending hypoglycemia, termed high-gain glucagon delivery. Episodes of high-gain glucagon delivery that exceeded a dose of 0.5 mcg/kg were analyzed. The analysis was confined to glucagon delivery that was triggered by low and/or falling sensed glucose values and was separated from a meal by at least 1 h in either direction, yielding a total of 19 episodes. To be classified as a success (or failure), VBG did not (or did) fall below 70 mg/dl over a 60 min time period beginning at the start of glucagon delivery.
Insulin on board was estimated at the start of glucagon delivery and compared between successes and failures using a model that we derived from data published by Holmes and associates.15 From the time of delivery, the amount of insulin on board, in units, was modeled to rise in a linear fashion for 30 min and maintain constant at this peak level for an additional 60 min. After this 90 min period, the amount of insulin considered to be on board from a discrete time point was decreased using the exponential decay function: ae-kt, where a is the amount of insulin infused over a five minute time interval, e is Euler’s number (2.717), k is the decay constant (0.012 min-1), and t is the elapsed time in minutes since the end of the 60 min plateau. After 9 h, insulin was considered to be completely cleared. For comparison, serum free insulin levels were measured by ELISA (Mercodia) in seven subjects just prior to each episode of glucagon delivery, as well as 5, 20, and 40 min after glucagon delivery.
Data are expressed as mean ± standard error of the mean. Sensor accuracy was calculated by comparing sensor glucose to reference blood glucose values.16 If there was no VBG value available at the start of glucagon delivery, then the values done just prior to starting the glucagon and just after the initiation of glucagon were averaged. In general, these values were from blood drawn 5 min prior to and 5 min after the start of glucagon delivery, respectively. The same was true for calculating sensor bias at the start of glucagon delivery. Comparisons were made using unpaired t tests. Calculations were performed using Excel 2007 version 12 (Microsoft Corporation, Redmond, WA).
Results
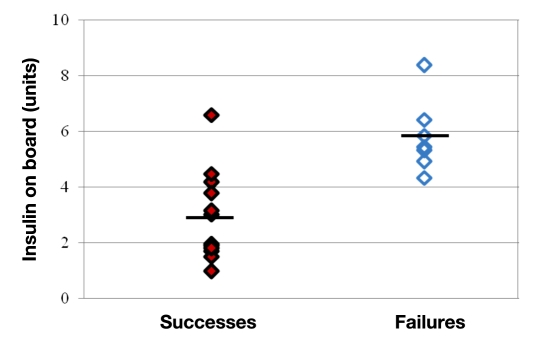
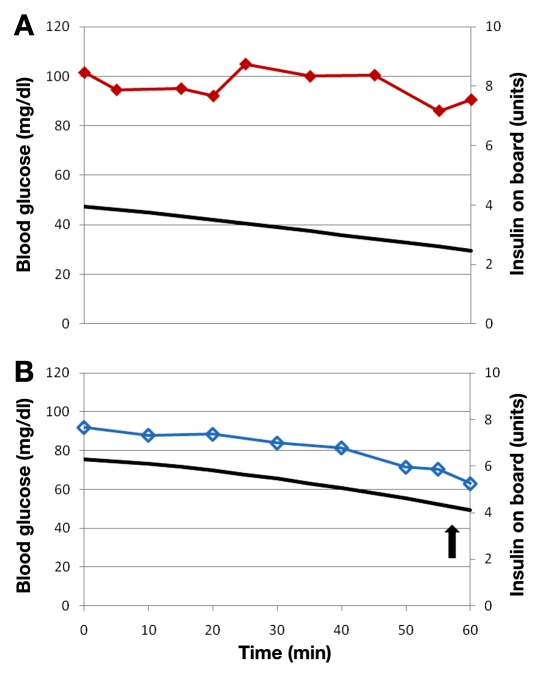
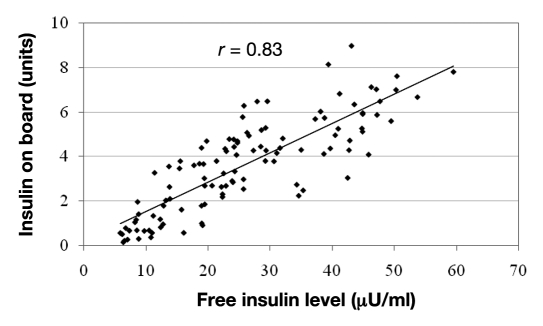
Hypoglycemia occurred in the hour after glucagon delivery in 7 of these 19 episodes. Estimated insulin on board at the start of glucagon delivery was significantly higher in the failures compared to successes (5.8 ± 0.5 versus 2.9 ± 0.5 U, p < .001) as shown in Figure 1. A representative example from a closed-loop study is shown in Figure 2, where hypoglycemia was avoided when insulin on board was low but did occur when insulin on board was high. The estimation of insulin on board appears to be accurate, as it correlated strongly with free insulin levels (r = 0.83, p < .001) as demonstrated in Figure 3. Because insulin sensitivity varies from individual to individual, the estimated insulin on board was also calculated as a percentage of the individual’s total daily requirement of insulin. This percentage was also significantly higher in the failures (10.9 ± 1.0 versus 7.2 ± 1.0%, p = .03).
Figure 1.
Episodes where hypoglycemia was successfully avoided (successes) are noted by solid diamonds, and those where hypoglycemia occurred in the hour after glucagon delivery (failures) are noted by empty diamonds. Mean values are indicated by black lines. Note that the estimated insulin on board is significantly (p < .001) higher in failures compared to successes.
Figure 2.
Panels A and B are VBG tracings taken from a single subject during the same closed-loop study. Time zero is when glucagon was delivered. Insulin on board is noted by a solid curve. Blood glucose is noted by solid diamonds (panel A) or empty diamonds (panel B). In panel A, insulin on board was low (less than 4 U), blood glucose remained stable, and hypoglycemia did not occur. In panel B, insulin on board was higher (above 6 U) and hypoglycemia did occur (as indicated by an arrow).
Figure 3.
Free insulin levels compared to estimated insulin on board in seven subjects. Note the close correlation between the two, demonstrating the accuracy of this estimation method.
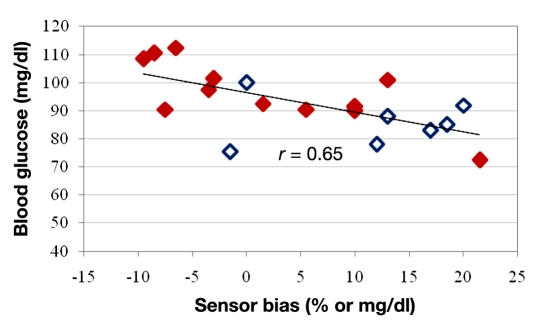
Late initiation of glucagon delivery only when blood glucose is nearly in the hypoglycemic range would be expected to result in overt hypoglycemia. Indeed, VBG at the start of glucagon delivery was significantly lower in failures relative to successes (86 ± 3 versus 95 ± 3 mg/dl, p = .04). When the VBG was less than 90 mg/dl at the start of glucagon delivery, hypoglycemia occurred in 87.5% of cases (7 of 8). Glucagon was delivered based on the glucose sensor reading, so sensor bias, in particular, overestimation of the VBG, might be expected to lead to a delay in the start of glucagon delivery. Indeed, sensor bias was highly correlated with starting glucose (r = 0.65, p = .002) as shown in Figure 4.
Figure 4.
Comparison of sensor bias to VBG at the start of glucagon delivery. Episodes in which hypoglycemia was successfully avoided (successes) are noted by solid diamonds and those where hypoglycemia occurred in the hour after glucagon delivery (failures) are noted by empty diamonds. Note that overestimation of blood glucose (positive sensor bias) correlates with a lower starting VBG, which is associated with glucagon failure.
Repeated doses of glucagon could become increasingly less effective if multiple doses resulted in depletion of liver glycogen. For this reason, prior cumulative glucagon dose was examined for each episode of glucagon delivery but was not associated with failure versus success (411 ± 89 versus 448 ± 68 mcg).
Discussion
Automated glucagon delivery markedly decreases the frequency of hypoglycemia during closed-loop glycemic control. Glucagon acts to raise blood glucose by converting liver glycogen into circulating glucose. It is effective in people both with and without diabetes; however, the effect has been shown to be delayed and less pronounced in those with type 1 diabetes.7 Patients with poor glycemic control may have an impaired ability to store glycogen in the liver, although this impairment can be partially reversed by improved control of blood glucose.17 Because closed-loop glycemic treatment offers an opportunity to improve glycemic control, it may result in improved responsiveness to glucagon.
Our results suggest that high insulin on board at the time of glucagon delivery will reduce the likelihood that glucagon delivery will successfully prevent hypoglycemia. Insulin on board provides an estimate of serum insulin levels but not of insulin effect. Insulin effect refers to the lowering of blood glucose via the movement of glucose into cells and the inhibition of glucose release from the liver. There is a delay between the appearance of insulin in blood and its effect, and the magnitude of this effect is determined by tissue sensitivity to insulin.18 We acknowledge that an estimation of insulin effect, rather than insulin levels, would likely serve as a better estimate of the risk for glucagon action failure. Based on these results, a higher amount of glucagon should be called for by the algorithm at times of high insulin on board in order to avoid overt hypoglycemia. In a reciprocal fashion, a lower amount of glucagon could be called for at times of low insulin on board in order to minimize the overall amount of glucagon delivered, thus reducing the risk of adverse effects such as nausea, rebound hyperglycemia, and liver glycogen depletion.
Sensor overestimation of glucose clearly led to a delay in glucagon delivery. Not surprisingly, delivery of glucagon at a lower starting glucose value resulted in more cases of hypoglycemia. None of the currently approved continuous glucose sensors are as accurate as modern capillary blood glucose (CBG) monitors, and for this reason, none is approved to replace CBG monitoring. Closed-loop glycemic control systems require the input of CBG or arterialized VBG values for glucose sensor calibration. For current versions of these systems, calibration will need to be relatively frequent to maintain sensor accuracy and to detect periods when the degree of inaccuracy might compromise closed-loop safety.
The reasons for sensor error are complicated. Calibration error and sensor drift are among the more common causes of sensor error.19,20 To the degree that the problem of sensor error cannot be solved, there are several algorithm choices that can help to minimize the frequency of hypoglycemia resulting from sensor over-estimation of glucose. These choices include selecting a higher glucose target, reducing insulin delivery gain factors, and increasing gain factors for glucagon delivery.
Conclusions
Hypoglycemia is a barrier to optimal glycemic control in patients on standard insulin therapy20 and is also a major concern in closed-loop glycemic control.21 Although most chronic diabetes-related complications are caused by hyperglycemia, people with diabetes are often more fearful of hypoglycemia, which can cause acute complications such as seizure, loss of consciousness, and even death. Glucagon is widely used for the treatment of severe hypoglycemia and, when given in low intermittent doses, has now been shown to be effective in preventing hypoglycemia in closed-loop systems. Glucagon, however, may fail to prevent hypoglycemia in cases when insulin on board is high or when glucagon delivery is delayed due to sensor overestimation of glucose. Improvements in sensor accuracy and delivery of larger or earlier glucagon doses when insulin on board is high may further reduce the frequency of hypoglycemia.
Acknowledgments
We thank the staff and research subjects who carried out these studies at the Oregon Clinical and Translational Research Institute, which is supported by Grant UL1 RR024140 from the National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research.
Abbreviations
- (CBG)
capillary blood glucose
- (FMPD)
fading memory proportional derivative
- (VBG)
venous blood glucose
References
- 1.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 2.Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AM, Nodale M, De Palma A, Wilinska ME, Acerini CL, Dunger DB. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375(9716):743–751. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
- 3.Castle JR, Engle JM, El Youssef J, Massoud RG, Yuen KC, Kagan R, Ward WK. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33(6):1282–1287. doi: 10.2337/dc09-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2(27):27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klonoff DC, Cobelli C, Kovatchev B, Zisser HC. Progress in development of an artificial pancreas. J Diabetes Sci Technol. 2009;3(5):1002–1204. doi: 10.1177/193229680900300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonson DC, Tamborlane WV, DeFronzo RA, Sherwin RS. Intensive insulin therapy reduces counterregulatory hormone responses to hypoglycemia in patients with type I diabetes. Ann Intern Med. 1985;103(2):184–190. doi: 10.7326/0003-4819-103-2-184. [DOI] [PubMed] [Google Scholar]
- 7.Orskov L, Alberti KG, Mengel A, Møller N, Pedersen O, Rasmussen O, Seefeldt T, Schmitz O. Decreased hepatic glucagon responses in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1991;34(7):521–526. doi: 10.1007/BF00403290. [DOI] [PubMed] [Google Scholar]
- 8.Kadish AH. Automation control of blood sugar a servomechanism for glucose monitoring and control. Trans Am Soc Artif Intern Organs. 1963;9:363–367. [PubMed] [Google Scholar]
- 9.Pontiroli AE, Calderara A, Perfetti MG, Bareggi SR. Pharmaco-kinetics of intranasal, intramuscular and intravenous glucagon in healthy subjects and diabetic patients. Eur J Clin Pharmacol. 1993;45(6):555–558. doi: 10.1007/BF00315314. [DOI] [PubMed] [Google Scholar]
- 10.Dungel P, Long N, Yu B, Moussy Y, Moussy F. Study of the effects of tissue reactions on the function of implanted glucose sensors. J Biomed Mater Res A. 2008;85(3):699–706. doi: 10.1002/jbm.a.31593. [DOI] [PubMed] [Google Scholar]
- 11.Wentholt IM, DeVries JH. An analysis of the SEVEN system: have we reached the summit of needle-type sensor accuracy? J Diabetes Sci Technol. 2009;3(5):1155–1157. doi: 10.1177/193229680900300520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindholm A, Jacobsen LV. Clinical pharmacokinetics and pharmaco-dynamics of insulin aspart. Clin Pharmacokinet. 2001;40(9):641–659. doi: 10.2165/00003088-200140090-00002. [DOI] [PubMed] [Google Scholar]
- 13.Ward WK, Engle J, Duman HM, Bergstrom CP, Kim SF, Federiuk IF. The benefit of subcutaneous glucagon during closed-loop glycemic control in rats with type 1 diabetes. IEEE Sens J. 2008;8(1):89–96. [Google Scholar]
- 14.Gopakumaran B, Duman HM, Overholser DP, Federiuk IF, Quinn MJ, Wood MD, Ward WK. A novel insulin delivery algorithm in rats with type 1 diabetes: the fading memory proportional-derivative method. Artif Organs. 2005;29(8):599–607. doi: 10.1111/j.1525-1594.2005.29096.x. [DOI] [PubMed] [Google Scholar]
- 15.Holmes G, Galitz L, Hu P, Lyness W. Pharmacokinetics of insulin aspart in obesity, renal impairment, or hepatic impairment. Br J Clin Pharmacol. 2005;60(5):469–476. doi: 10.1111/j.1365-2125.2005.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute Performance metrics for continuous interstitial glucose monitoring; approved guideline. CLSI document POCT05-A. 2008;28(33):1–55. [Google Scholar]
- 17.Bischof MG, Krssak M, Krebs M, Bernroider E, Stingl H, Waldhäusl W, Roden M. Effects of short-term improvement of insulin treatment and glycemia on hepatic glycogen metabolism in type 1 diabetes. Diabetes. 2001;50(2):392–398. doi: 10.2337/diabetes.50.2.392. [DOI] [PubMed] [Google Scholar]
- 18.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68(6):1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg SK, Smith J, Beatson C, Lopez-Baca B, Voelmie M, Gottlieb PA. Comparison of accuracy and safety of the SEVEN and the Navigator continuous glucose monitoring systems. Diabetes Technol Ther. 2009;11(2):65–72. doi: 10.1089/dia.2008.0109. [DOI] [PubMed] [Google Scholar]
- 20.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45(7):937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 21.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):3344–3350. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]