Abstract
Background
Glucagon is a life-saving medication used in the treatment of hypoglycemia. It possesses poor solubility in aqueous buffers at or near physiological pH values. At low and high pH, at which the peptide can be formulated to concentrations of a milligram or more per milliliter, the chemical integrity of the hormone is limited, as evidenced by the formation of multiple degradation-related peptides. Consequently, the commercial preparation is provided as a lyophilized solid with an acidic diluent and directions for rendering it soluble at the time of use. Any unused material is recommended for disposal immediately after initial use.
Methods
A set of glucagon analogs was prepared by solid-phase peptide synthesis to explore the identification of a glucagon analog with enhanced solubility and chemical stability at physiological pH. The physical properties of the peptide analogs were studied by solubility determination, high-performance chromatography, and mass spectral analysis. The biochemical properties were determined in engineered human embryonic kidney cell line 293 (HEK293) cells that overexpressed either the human glucagon or glucagon-like peptide-1 (GLP-1) receptors linked to a luciferase reporter gene.
Results
We observed the previously characterized formation of glucagon degradation products upon incubation of the peptide in dilute acid for extended periods or elevated temperature. Lowering the isoelectric point of the hormone through the substitution of asparagine-28 with aspartic acid significantly increased the solubility at physiological pH. Similarly, the C-terminal extension (Cex) of the hormone with an exendin-based, 10-residue, C-terminal sequence yielded a peptide of dramatically enhanced solubility. These two glucagon analogs, D28 and Cex, maintained high potency and selectivity for the glucagon receptor relative to GLP-1 receptor.
Conclusions
Glucagon presents unique structural challenges to the identification of an analog of high biological activity and selectivity that also possesses sufficient aqueous solubility and stability such that it might be developed as a ready-to-use medicine. The glucagon analogs D28 and Cex demonstrated all of the chemical, physical, and biochemical properties supportive of further study as potential clinical candidates for treatment of hypoglycemia.
Keywords: glucagon, glucagonCex, glucagonD28, isoelectric point, solubility, stability
Introduction
In 1922, Dr. Charles Kimball and Dr. John Murlin set out to isolate insulin from aqueous pancreas extracts and, in the process, stumbled upon a substance that they reported as possessing “a distinctly hyperglycemic effect.”1 The substance was given the name glucagon. In the years following its discovery, attempts to isolate and purify glucagon proved to be difficult. More than 30 years passed before a pure preparation of glucagon was reported by Behrens and his associates.2 The interplay between insulin and glucagon has become one of the most studied relationships in biochemistry and endocrinology, with insulin acting to lower blood glucose and glucagon functioning in the opposite direction to raise it. Low concentrations of plasma glucose trigger glucagon secretion, a physiological response vital to restoring glucose to a level consistent with sustained health and cognitive function. Glucagon is a hormone secreted by pancreatic α cells,3 and the nature of communication with the other cells of the islet remains a topic of appreciable importance. The glucagon receptor is primarily located in the liver and belongs to the class B, seven-transmembrane, G-protein-coupled receptor family.4 Upon receptor binding, there is an immediate increase in intracellular second messengers, most notably cyclic adenosine monophosphate (cAMP), followed by enhanced glycogenolysis and gluconeogenesis leading to elevation of blood glucose.5
Type 1 diabetes mellitus (T1DM) results from insufficient insulin production, and such individuals are insulin dependent. In the diabetic state, the physiological elements that regulate energy balance are altered. There is an enhanced risk of insulin-induced hypoglycemia, as endogenous sensitivity to low glucose levels is often diminished. This, coupled with the fact that insulin has a low therapeutic index and its excessive action can lead to severe hypoglycemia, represents a fatal flaw in the use of insulin. Moreover, individuals with T1DM often demonstrate little to no glucagon response upon excessive insulin infusion. This has been characterized as a pancreatic α-cell defect that results in insensitivity to insulin-induced hypoglycemia.6 The risk of severe hypoglycemia is the most notable limitation in the use of insulin therapy to normalize glucose in T1DM.7 Outside a hospital setting, exogenous glucagon is the treatment for severe hypoglycemia. However, emergency use of glucagon is complicated by the requirement for reconstitution of the hormone from a solid form immediately prior to parenteral injection.
Glucagon is a peptide hormone consisting of 29 amino acids.8 The amino acid sequence of glucagon presented in single-letter format is shown below. The N-terminal histidine is a free amine that possesses a pKa close to neutrality and the C-terminus is a carboxylic acid that is negatively charged at physiological pH but protonated in the dilute hydrochloric acid commonly used for solubilization.
HSQGT5FTSDY10SKYLD15SRRAQ20DFVQW25LMNT
Peptides are inherently unstable molecules, and glucagon is no exception. The low oral bioavailability of peptide- based medicines necessitates parenteral administration of aqueous solutions. Chemical and physical degradation of glucagon is accelerated in the aqueous environment, and glucagon is only sparingly soluble near physiological pH and requires modestly acidic or basic conditions for solubility at concentrations appropriate for medicinal use. The pharmaceutical glucagon kit employs dilute acid for reconstitution of the peptide. Kirsch studied the degradation process of pharmaceutically relevant acidic solutions of glucagon and reported the major pathways of destruction to be aspartic acid bond cleavage and glutamine deamidation.9 In a related study, Kirsch10 demonstrated that the rates of destruction are reduced as the pH of the glucagon solution is adjusted toward the neutral range.
The physical conformation of glucagon appears to be dependent on the conditions of study. In dilute aqueous solutions, there does not appear to be a single conformation known to be the physiologically relevant form of the hormone.11 Historical evidence suggests three major conformational models for glucagon.12 Blundell and coworkers13 reported a mainly helical structure for glucagon based on X-ray crystal structure analysis. In an aqueous environment, glucagon displays a dynamic range of conformations that is dependent on many factors. Glucagon undergoes a concentration- dependent self-association to trimers, resulting in increased α-helical content.14 This association is of significant relevance to the formulation and resultant stability of the highly concentrated glucagon solution commonly used for pharmaceutical purposes. However, it is well established that concentrated solutions of glucagon can gel and form fibrils composed of aggregated antiparallel β-structures of glucagon.15,16 The formation of amyloidogenic fibrils is a challenge for pharmaceutical use. The translation of the reported in vitro measures of cytotoxicity to questions of clinical efficacy and safety remain points of uncertainty and debate.17
Historically, glucagon proved to be a challenging peptide for chemical synthesis and the application of solid-phase peptide synthesis provided a simplified method.18 This synthetic approach renewed interest in synthetic glucagon and related peptides. Interestingly, the focus of the research was almost exclusively on the development of glucagon antagonists for treatment of hyperglycemia.19 Our work has focused on design of glucagon agonists with enhanced pharmaceutical properties for clinical application. In this study, we present glucagon analogs that were designed to achieve greater solubility at physio-logical pH. It was envisioned that the chemical stability of these glucagon analogs would be much enhanced, and that the pharmacology might benefit from the absence of any physical phase-transition when used in vivo. It is our vision that a glucagon analog that is sufficiently soluble and stable such that it can be formulated for medicinal use as a ready-to-use solution would constitute a transformation of how patients perceive and manage the treatment of emergency hypoglycemia.
Methods
Peptide Synthesis
Peptide syntheses were performed using 0.2 mmol 4-hydroxymethyl-phenylacetamidomethyl (PAM) resin or 4-methylbenzhydrylamine (MBHA) resin (Midwest Biotech Inc., Fishers, IN) on a modified Applied Biosystems 430A peptide synthesizer (Foster City, CA). Solid-phase peptide syntheses utilized in situ neutralization for tert-butyl-oxycarbonyl (Boc) chemistry as described by Kent.22 Completed peptidyl-resins were treated with hydrogen fluoride (HF)/p-cresol (10:0.5 v/v) at 0 °C for 1 hour. Hydrogen flouride was removed in vacuo and the deprotected peptide was precipitated and washed in diethyl ether. The peptide was dissolved in 20% acetonitrile/1% acetic acid and lyophilized. All peptides were prepared by Boc chemistry. The following side chain protecting groups were used for Boc-amino acids (Midwest Biotech Inc.): Arg(Tos), Asp(OcHex), Asn(Xan), Glu(OcHex), His(BOM), Lys(2-Cl-Z), Ser(Bzl), Thr(Bzl), Trp(CHO), Tyr(Br-Z). Peptide molecular weights were confirmed by electrospray ionization or matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry.
Peptide Purification
The impure peptide extracts obtained following treatment of the peptidyl resin with anhydrous HF were lyophilized. Once lyophilized, a sample of crude peptide was reconstituted in dilute acidic buffer and analyzed by analytical reverse-phase high-performance liquid chromatography (HPLC). Analytical HPLC was conducted in 0.1% trifluoroacetic acid (TFA) with an acetonitrile gradient on a Zorbax C8 column (Santa Clara, CA) (0.46 × 5 cm). After analytical analysis, the crude peptides were purified by semipreparative chromatography in 0.1% TFA with an acetonitrile gradient on a Vydac C4 or C18 column (Deerfield, IL) (2.2 × 25 cm) or a Luna C18 (Torrance, CA) column (2.2 × 25 cm). The purified peptides were analyzed by repeated HPLC in 0.1% TFA with an acetonitrile gradient on a Zorbax C8 column (0.46 × 5 cm) and secondly to assure maximal purity in 0.025 mol/liter ammonium bicarbonate buffer at pH 8 with an aceto-nitrile gradient on a Luna C18 column (0.46 × 25 cm).
Determination of Glucagon Peptide Solubility
Approximately 10.0 mg of glucagon and glucagonD28 (D28) were weighed into separate conical vials and each was dissolved in 8.0 ml of 10 mmol/liter tris(hydroxymethyl) aminomethane (Tris), pH 10.0. The pH of each solution was adjusted to 9.5 using 1.0 N hydrochloric acid (HCl) and a 1.0 ml aliquot was removed and equally divided into two Eppendorf tubes. Each 500 µl aliquot was stored overnight where one was at 25 °C, while the other was at 4 °C. The remaining 5.0 ml of the original solution was titrated to a lower pH (9.0, 8.5, 8.0, 7.5, and 7.0), where aliquots were taken and stored in the same fashion as described above. The following day, all samples were centrifuged and a 200 µl aliquot of the supernatant was removed and diluted 1:1 with 10 mmol/liter Tris, pH 10.0, prior to measurement of absorbance at 280 nm. Additionally, the pH of the remaining 300 µl was measured again for accuracy. The peptide concentration was determined using the calculated extinction coefficient at 280 nm for glucagon of 8480/mol/liter × cm Abs 0.1% (= 1 g/liter) 2.435.
Measure of Glucagon Peptide Stability
Longterm stability studies were designed to explore chemical and physical changes of glucagon analogs. Peptides were dissolved at 1 mg/ml in phosphate-buffered saline (PBS) and pH was adjusted to 7.4. The solutions were sterile filtered and peptide concentration was determined as mentioned in the section pertaining to solubility, after which the concentration was adjusted to exactly 1 mg/ml. The filtered stock solution was aliquoted to low protein retention Eppendorf tubes and incubated under various conditions. Additionally, a portion of the stock peptide solution was frozen to serve as a standard. Samples were studied at three temperatures: 4, 25, and 37 °C, and retained at these conditions for the duration of the experiment. The samples were analyzed at select time points by liquid chromatography coupled with mass spectrometry (LC-MS).
Measure of the Maximum Solubility of GlucagonCex Analogs
Saturated solutions of the glucagon analogs were prepared in PBS and serial dilutions were made until the point where no visible precipitate could be visualized and a linear correlation of ultraviolet absorbance was observed. Concentration was calculated based on the absorbance measurement at 280 nm. The absorbance readings were repeated the following day, as well as the day after.
Biological Activity
Each peptide analog was tested for its ability to stimulate cAMP production through the glucagon and glucagon-like peptide-1 (GLP-1) receptors. Human embryonic kidney cell line 293 (HEK293) cells were cotransfected with the glucagon receptor (GCGR) or GLP-1 receptor cDNAs and a luciferase reporter gene linked to a cAMP response element. Cells were serum-deprived for 16 h by culturing in Dulbecco’s Modified Eagle Medium (Invitrogen, Carlsbad, CA) supplemented with 0.25% fetal bovine serum (HyClone, Logan, UT). Serial dilutions of glucagon and GLP-1 analogs were added to 96-well poly-D-lysine-coated plates (BD Biosciences, San Jose, CA) containing cotransfected HEK293 cells, and plates were incubated for 5 h at 37 °C, 5% CO2. Following incubation, an equivalent volume (100 µl) of LucLite luminescence substrate reagent (PerkinElmer Inc., Wellesley, MA) was added to each well and the plate was shaken for 3 min at 800 rpm. The plate was incubated for 10 min in the dark and light output was quantified on a MicroBeta-1450 liquid scintillation counter (PerkinElmer, Wellesley, MA). Effective concentrations (EC50) were calculated by Origin software (OriginLab Corporation, Northampton, MA).
Results
The synthesis of the glucagon peptide by solid phase synthesis using Boc-amino acid-mediated synthesis and HF-cleavage provided high quality peptides that could be purified by HPLC to greater than 95% homogeneity when assessed by analytical HPLC in two systems and by mass spectrometry (MS) analysis. The peptides were stored as their TFA salts prior to study in various buffers of differing pH values. The extension of the glucagon sequence to include the C-terminal extension (Cex) peptide sequence of exendin was observed to enhance the quality of the crude peptide obtained and in most instances facilitated the purification by preparative HPLC.
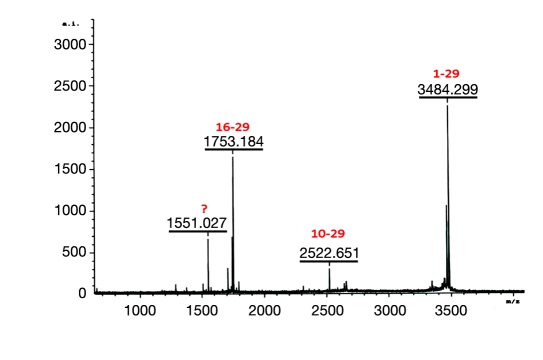
Variable levels of peptide bond hydrolysis are commonly observed for peptides containing aspartic acid (Asp) and asparagine (Asn) when stored at extremes of pH. Glucagon contains an Asp at positions 9, 15, and 21 and an Asn at position 28. Aspartic acid and Asn are susceptible to forming a five-member ring resulting in cleavage of the amide backbone as described by Kirsch.10 When we incubated glucagon in acidic solution (0.01 N HCl) we identified two cleavage products, fragment 10-29 and 16-29. These fragments are indicative of cleavage at the Asp9 and Asp15 residues and were abundant after one month at 37 °C (data not shown). Our interest was in less aggressive temperatures and so we explored whether extended duration at ambient temperature was capable of similarly cleaving glucagon at 25 °C. As shown in Figure 1, the characteristic cleavage peptides 16-29 and 10-29 were observed by MS analysis after 6 weeks at room temperature when incubating glucagon in 0.01 N HCl. While MS analysis is not quantitative, the larger size of the 16-29 signal is consistent with other more quantitative measures that demonstrate that the Asp15-Ser16 peptide bond is of considerably greater susceptibility to acid-mediated cleavage.
Figure 1.
Matrix-assisted laser desorption/ionization of glucagon after 1 month at 25 °C. Glucagon incubated at 1 mg/ml in 0.01 N HCl, pH 2.3. Native glucagon 1-29 (m/z 3484.299), fragment 10-29 (m/z 2522.651), and fragment 16-29 (m/z 17.53.184).
The search for more stable analogs began with attempts to increase solubility at physiological pH. To achieve enhanced solubility, glucagon analogs were designed to alter the isoelectric point (pI) of the molecule. A general rule for achieving solubility is that the pH of the peptide solution should reside at least one, and preferably two units, above or below the pI of the peptide. The theoretical pI of glucagon is just below 7, and the hormone displays poor solubility in the pH range from 4–8. The charge distribution for glucagon at physiological pH is shown here, where the positively charged residues are colored blue and the negatively charged residues red. The peptide is
HSQGTFTSDYSKYLDSRRAQDFVQWLMNT
and is nearly balanced, with the N-terminal histidine providing some additional cationic nature. Given the chemical liability of Asp(D) and Asn(N), it was deemed preferable to modify these amino acids, since the change in pI could be achieved while simultaneously eliminating a point of chemical destruction. Unfortunately, the two points of greatest interest, Asp9 and 15, where we previously observed peptide bond cleavage, were not amenable to chemical substitution without appreciable loss in biopotency (results to be reported elsewhere). Consequently, we focused upon the next most likely site for chemical degradation, Asn28.
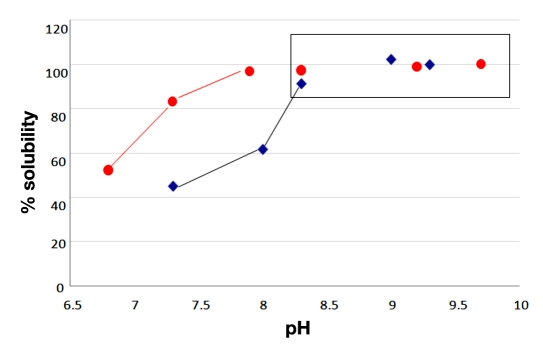
An Asp residue was selectively substituted in place of the native Asn in position 28. The substitution of a negatively charged amino acid in the C-terminal portion of the glucagon sequence yields a lower pI. The calculated pI of the peptide decreases from 6.7 to 5.4, more than a full unit. The solubility of the respective peptide analogs was determined relative to the native peptide. The results are reported in Figure 2, and it is interesting to note how the transition point to solubility at 1 mg/ml is similarly shifted approximately one pH unit lower in the D28 analog. The D28 analog is representative of the single-site deamidation of the amide side chain of Asn to the carboxylic acid side, and it provides a sizable increase in the solubility of the peptide. Most notably, the peptide exhibits nearly twice the solubility of the native hormone at a physiological pH of 7.4.
Figure 2.
Solubility of synthetic glucagon (blue) vs. synthetic D28 after 24 hours. Each analog was 1 mg/ml in pH adjusted 10 mM Tris base. Ultraviolet absorption measured at 280 nm after 24 hours.
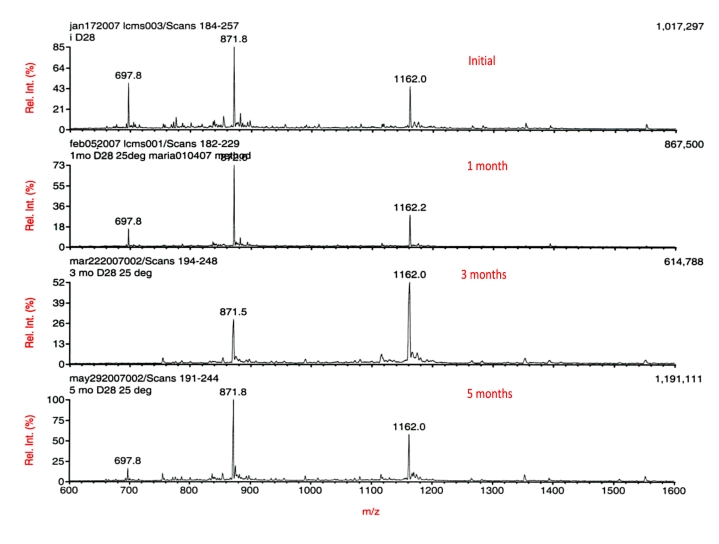
Having achieved enhanced solubility at physiological pH, it was possible to begin a series of chemical stability studies in aqueous formulations at or near pH 7. The D28 analog was incubated at three different temperatures (4, 25, and 37 °C) in PBS at a concentration of 1 mg/ml. Aliquots at various incubation times were analyzed by LC-MS. The results are reported in Figure 3. The D28 displayed high stability through many months of incubation. Most importantly, there did not appear to be any evidence of aspartyl-bond cleavage in the PBS over a period of five months at 25 °C. While MS spectral analysis is not quantitative and total counts can vary when the same sample is assessed at different times separated by months, it is encouraging to note that the total counts at the end of the study appeared comparable to that at the start and the variation is no more than two-fold observed at the intermediate time points. Most importantly, the character of the mass spectrum was not changed, indicating that glucagon is much more stable over an extended period at physiological pH than in the dilute acid commonly used for medicinal purposes.
Figure 3.
LC-MS of D28 at various time points at 25 °C. Peptide concentration equal to ∼1 mg/ml in PBS, pH 7.4.
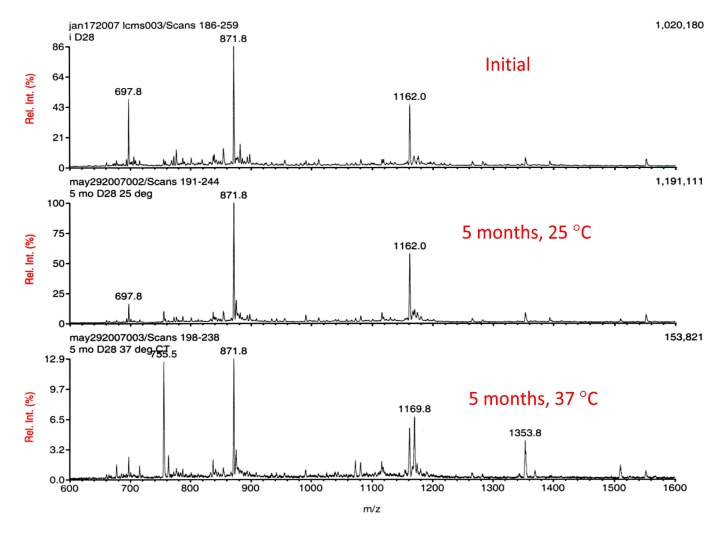
To further examine the depth of the stability enhancement achieved at physiological pH, D28 incubated in PBS at 37 °C was similarly studied. The results are shown in Figure 4 and there are two striking differences. The D28incubated at 37 °C exhibited sizable degradation, wherein the total counts decreased to a mere fraction of that at the start (1,020,180 to 153,821). Additionally, the integrity of the parent (main peak) was severely compromised. A second, later-eluting peak not observed in the 25 °C sample was detected. This larger molecular entity is consistent with the formation of the methionine sulfoxide derivative of D28. The results demonstrate that glucagon is temperature sensitive, and that there is a noticeable decrease in the stability as temperature is elevated from 25 to 37 °C, and the sulfoxide derivative is a likely degradation product. The majority of the material is not observed by MS analysis and assumed to be high-molecular-weight aggregates.
Figure 4.
LC-MS of D28 at initial time point (top), at 25 °C (middle), and at 37 °C (bottom), after five months. Peptide concentration equal to ∼1 mg/ml in PBS, pH 7.4.
To further explore the prospect of using enhanced solubility at physiological pH as a basis to achieve enhanced stability, we studied an alteration to peptide sequence that had provided enhanced solubility in a hormone structurally related to glucagon. This alternative approach entailed the addition of the Cex sequence of exendin-4 to the C terminus of glucagon. We have previously reported on the preliminary crystal structure of this particular glucagon analog.20 Cex is a nine-amino acid sequence that derives from exendin-4, a structural analog of GLP-1.21 This nine-residue sequence comprises the C-terminal portion of the exendin-4 peptide, and we have observed it to contribute to a dramatic increase in aqueous solubility of core GLP-1 peptides. Comparison of the sequences of glucagon (1-29), GLP-1 (7-37), and exendin-4 (1-39) are provided below.
H S Q G T F T S D Y S K Y L D S R R A Q D F V Q W L M N T
H A E G T F T S D V S S Y L E G D A A K E F I A W L V K G R G
H G E G T F T S D L S K Q M E E E A V R L F I E W L K N G G P S S G A P P P S
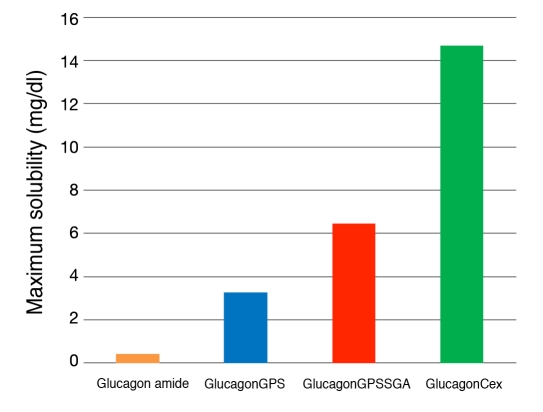
We prepared an analog of glucagon that extended the C terminus from the native threonine-29 (Thr29) through the added Cex. To maintain the register of the linear peptide and the conformational tryptophan-cage (Trp-cage) of the exendin-4, an additional glycine was added between glucagon Thr29 and the nine-residue Cex peptide of exendin-4 to generate a peptide of 39 amino acids that terminated as an amide. Additionally, three other glucagon peptides were made to establish the nature of any structural contribution to enhanced solubility. These additional peptides were glucagon T29-amide, glucagon T29GPS-amide, and glucagon T29GPSSGA-amide. The maximum solubility of this series of glucagon analogs was determined in PBS, at pH 7.4 and reported in Figure 5.
Figure 5.
Maximum solubility of glucagonCex and truncated analogs in PBS at pH 7.4.
The maximum solubility of the truncated analogs was determined to be glucagon amide (0.4 mg/ml), glucagonGPS (3.3 mg/ml), and glucagonGPSSGA (6.4 mg/ml), and glucagonCex (14.7 mg/ml). The results are striking and demonstrate the constructive contribution that the Cex sequence can make to solubility. Even shortened elements of Cex provided enhanced solubility, despite the fact that there is no apparent change in pI relative to the comparative glucagon amide analog.
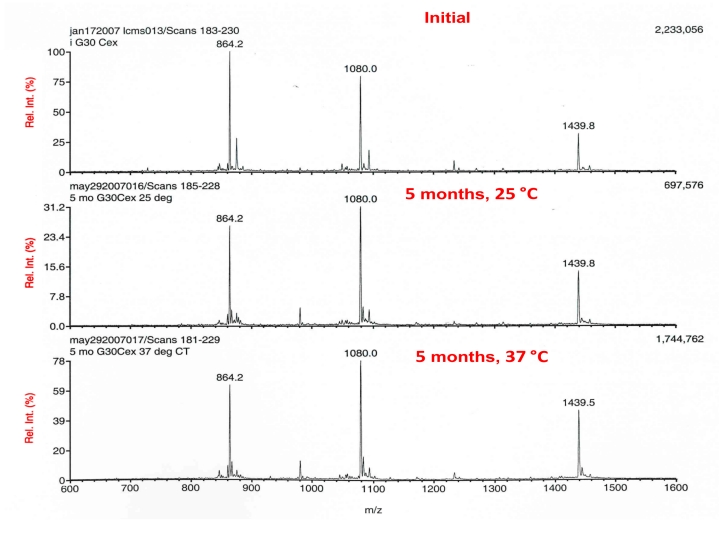
Given the enhanced solubility of the glucagonCex, it was imperative that its stability in PBS over an extended time be studied, in similar fashion to the D28 analog. The results of this study are presented in Figure 6. The MS analysis of glucagonCex incubated at 25 and 37 °C for 5 months relative to an initial standard are shown. The peptide demonstrated amazingly high chemical stability at both temperatures studied, as witnessed by mass spectral fingerprints that are highly similar. Interestingly, the higher-temperature incubation sample demonstrated ion intensity appreciably greater than the lower-temperature sample and consistent with the initial analysis. This stands in stark contrast to the integrity of D28 after similar treatment and is worthy of additional study.
Figure 6.
LC-MS of glucagonCex at initial time point (top), at 25 °C (middle), and at 37 °C (bottom), after 5 months. Peptide concentration equal to ∼1 mg/ml in PBS, pH 7.4.
A final point for consideration in this initial search of a glucagon agonist of preferred properties is the issue of biological potency. A clinical candidate will need to maintain high potency and selectivity for glucagon action relative to other related receptors in the glucagon family. Of particular interest is whether the application of Cex derived from a GLP-1 agonist might significantly alter receptor selectivity. Consequently, the D28 and Cex glucagon analogs were studied for in vitro potency in engineered cells that overexpress the glucagon and GLP-1 receptors linked to a luciferase reporter. Each of the two analogs is appreciably active at the glucagon receptor and show low activity at the GLP-1 receptor. Table 1 provides a summary of the potencies of the associated compounds at both the glucagon and GLP-1 receptors. The D28 analog is more than 70% the potency of native glucagon and demonstrates very similar, if not enhanced, selectivity relative to the native hormone. The Cex analog is slightly less potent than the D28 and native hormone. It maintains sizable selectivity in excess of 30-fold, but is less selective than the two comparator peptides.
Table 1.
Biological Activity of Glucagon Analogs as Determined Via in Vitro Analysis in HEK293 Cells Overexpressing the Glucagon or GLP-1 Receptors Linked to a Luciferase Reporter of cAMP Production. NA: no detectable activity below 10 nmol/liter.
| Compound | Glucagon receptor EC50 (nmol/liter) | % Activity at glucagon receptor | GLP-1 receptor EC50 (nmol/liter) | % Activity at GLP-1 receptor |
|---|---|---|---|---|
| Glucagon | 0.06 ± 0.04 (n = 47) | 100% | 3.74 ± 1.83 (n = 5) | 0.7% |
| GLP-1 | NA | NA | 0.03 ± 0.01 (n = 8) | 100% |
| D28 | 0.09 ± 0.08 (n = 12) | 72% | 7.99 ± 3.14 (n = 5) | 0.3% |
| GlucagonCex | 0.12 ± 0.03 (n = 4) | 56% | 1.63 ± 0.57 (n = 6) | 1.6 % |
Discussion
Glucagon is a life-saving therapeutic of indispensable importance in the treatment of severe hypoglycemia. The relative therapeutic respect it receives pales in comparison to insulin, and in more recent years, to the structurally related peptide GLP-1. It is our belief that glucagon represents an untapped resource for achieving enhanced glycemic control in insulin-dependent diabetes, where the fear of hypoglycemia constitutes a barrier to improved therapy. Glucagon is a peptide that was designed for physiological action and not pharmaceutical use. It is poorly soluble at physiological pH and of low stability at extremes of pH. Consequently, glucagon has been provided for more than 50 years as a solid requiring reconstitution at the time of administration, which in most instances constitutes an emergency.
Advances in structure-based drug optimization, coupled with advances in commercial-scale synthesis of peptides, renders optimization of glucagon something we believe is doable and highly desirable. It is our vision to develop a solution-stable formulation of glucagon that is as portable as insulin, such that each patient with insulin-treated diabetes might have the immediate ability to increase glucose to whatever degree they deem desirable. We characterize this by analogy to current insulin therapy as the difference between driving a boat and a car. The immediate availability of glucagon in adjustable doses should provide motivated and well-informed patients with insulin-dependent diabetes the opportunity to control their blood glucose much like a professional driver controls a high performance race car. While to some, this might seem excessively complex, it was not that long ago that pump-administered insulin was similarly viewed. It certainly seems plausible to expect that advances in closing the loop to pump-delivered insulin will demand improved versions of glucagon.
The work we present in this article is the first step in the discovery of glucagon analogs optimized for medicinal use. Our results demonstrate the poor stability of glucagon when stored for extended periods in acidic solution. It is very clear from the work we present that D28 and Cex glucagon analogs are far more stable at physiological pH than the native amino acid sequence. The degradation of the native sequence leading to peptide bond cleavage following the two aspartic acid residues is virtually non-existent when studied at physiological pH. The chemical basis for this difference is the greatly reduced propensity for formation of the five-member aspartimide ring that is intermediate to the acid-facilitated bond cleavage at pH values above the pKa of the aspartic acid.
The unparalleled increase in solubility of the Cex series of glucagon analogs is striking in that it is achieved without a change in the number of charged amino acids. The most plausible rationale for this behavior might be the relative propensity of the peptide to form trimers. The Trp-cage nature of the Cex extension peptide provides a surface for hiding the hydrophobic face of the native C terminus and, in so doing, minimizes a structural element that diminishes aqueous solubility. The shortened analogs, while not nearly as soluble as the full-length Cex, still represent sizable increases in solubility that are more than sufficient to further optimize pH in a range where concentration remains therapeutically relevant. The D28 is the simplest of altered forms and as such represents the least risk for immunological recognition that might lead to antibody formation. In this setting, binding antibodies that change pharmacokinetics without altering absolute effectiveness could be detrimental, given the importance of speed to onset of action in the treatment of severe hypoglycemia.
These two analogs proved to be highly potent and of sufficient selectively to constitute attractive drug leads. Nonetheless, there is appreciably more to be accomplished before a drug candidate can be selected. Clearly, the nature of the in vivo pharmacodynamics is of utmost importance, with the total response and consistency of speed to glucose elevation being of paramount importance. The chemical and physical stability studies need to be extended to longer duration times of a single year at ambient and elevated temperatures, and for 2 years at 4 °C. Finally, the question of immunogenicity needs to be addressed. A point of note in this regard is the difficulty that glucagon has represented in raising high-affinity antibodies. As such, one might expect that soluble analogs that are devoid of physical phase-transformation at point of injection will minimize the risk associated with changes to native sequence.
Abbreviations
- (Asn)
asparagine
- (Asp)
aspartic acid
- (Boc)
tert-butyloxycarbonyl
- (cAMP)
cyclic adenosine monophosphate
- (Cex)
C-terminal extension (GPSSGAPPPS)
- (D28)
glucagonD28
- (EC50)
effective concentration
- (GCGR)
glucagon receptor
- (GLP-1)
glucagon-like peptide-1
- (glucagonCex)
glucagonGPSSGAPPPS
- (HCl)
hydrochloric acid
- (HEK293)
human embryonic kidney cell line 293
- (HF)
hydrogen fluoride
- (HPLC)
high-performance liquid chromatography
- (LC-MS)
liquid chromatography coupled with mass spectrometry
- (MS)
mass spectrometry
- (PBS)
phosphate-buffered saline
- (pI)
isoeclectric point
- (T1DM)
type 1 diabetes mellitus
- (Thr)
threonine
- (Trp)
tryptophan
- (Tris)
tris (hydroxymethyl)aminomethane
- (TFA)
trifluoroacetic acid
References
- 1.Kimball CP, Murlin JR. Aqueous extracts of pancreas. III. Some precipitation reactions of insulin. J Biol Chem. 1923;58:337–348. [Google Scholar]
- 2.Staub A, Sinn L, Behrens OK. Purification and crystallization of glucagon. J Biol Chem. 1955;214(2):619–632. [PubMed] [Google Scholar]
- 3.Unger RH, Orci L. Physiology and pathophysiology of glucagon. Physiol Rev. 1976;56(4):778–826. doi: 10.1152/physrev.1976.56.4.778. [DOI] [PubMed] [Google Scholar]
- 4.Hoare SR. Mechanism of peptide and nonpeptide ligand binding to Class B G-protein-coupled receptors. Drug Discov Today. 2005;10(6):417–427. doi: 10.1016/S1359-6446(05)03370-2. [DOI] [PubMed] [Google Scholar]
- 5.Pohl SL, Birnbaumer L, Rodbell M. Glucagon-sensitive adenyl cyclase in plasma membrane of hepatic parenchymal cells. Science. 1969;164(879):566–567. doi: 10.1126/science.164.3879.566. [DOI] [PubMed] [Google Scholar]
- 6.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182(108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- 7.Cryer PE. Banting Lecture. Hypoglycemia: the limiting factor in the management of IDDM. Diabetes. 1994;43(11):1378–1389. doi: 10.2337/diab.43.11.1378. [DOI] [PubMed] [Google Scholar]
- 8.Bromer WW, Sinn LG, Staub A, Behrens OK. The amino acid sequence of glucagon. Diabetes. 1957;6(3):234–238. doi: 10.2337/diab.6.3.234. [DOI] [PubMed] [Google Scholar]
- 9.Joshi AB, Rus E, Kirsch LE. The degradation pathways of glucagon in acidic solutions. Int J Pharm. 2000;203((1-2)):115–125. doi: 10.1016/s0378-5173(00)00438-5. [DOI] [PubMed] [Google Scholar]
- 10.Joshi AB, Kirsch LE. The estimation of glutaminyl deamidation and aspartyl cleavage rates in glucagon. Int J Pharm. 2004;273((1-2)):213–219. doi: 10.1016/j.ijpharm.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Panijpan B, Gratzer WB. Conformational nature of monomeric glucagon. Eur J Biochem. 1974;45(2):547–553. doi: 10.1111/j.1432-1033.1974.tb03580.x. [DOI] [PubMed] [Google Scholar]
- 12.Korn AP, Ottensmeyer FP. A model for the three-dimensional structure of glucagon. J Theor Biol. 1983;105(3):403–425. doi: 10.1016/0022-5193(83)90184-4. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki K, Dockerill S, Adamiak DA, Tickle IJ, Blundell T. X-ray analysis of glucagon and its relationship to receptor binding. Nature. 1975;257(5529):751–757. doi: 10.1038/257751a0. [DOI] [PubMed] [Google Scholar]
- 14.Gratzer WB, Creeth JM, Beaven GH. Presence ot trimers in glucagon solution. Eur J Biochem. 1972;31(3):505–509. doi: 10.1111/j.1432-1033.1972.tb02558.x. [DOI] [PubMed] [Google Scholar]
- 15.Gratzer WB, Beaven GH, Rattle HW, Bradbury EM. A conformational study of glucagon. Eur J Biochem. 1968;3(3):276–283. doi: 10.1111/j.1432-1033.1968.tb19527.x. [DOI] [PubMed] [Google Scholar]
- 16.Beaven GH, Gratzer WB, Davies HG. Formation and structure of gels and fibrils from glucagon. Eur J Biochem. 1969;11(1):37–42. doi: 10.1111/j.1432-1033.1969.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 17.Onoue S, Ohshima K, Debari K, Koh K, Shioda S, Iwasa S, Kashimoto K, Yajima T. Mishandling of the therapeutic peptide glucagon generates cytotoxic amyloidogenic fibrils. Pharm Res. 2004;21(7):1274–1283. doi: 10.1023/b:pham.0000033016.36825.2c. [DOI] [PubMed] [Google Scholar]
- 18.Mojsov S, Merrifield RB. Solid-phase synthesis of crystalline glucagon. Biochemistry. 1981;20(10):2950–2956. doi: 10.1021/bi00513a036. [DOI] [PubMed] [Google Scholar]
- 19.Gysin B, Johnson DG, Trivedi D, Hruby VJ. Synthesis of two glucagon antagonists: receptor binding, adenylate cyclase, and effects on blood plasma glucose levels. J Med Chem. 1987;30(8):1409–1415. doi: 10.1021/jm00391a024. [DOI] [PubMed] [Google Scholar]
- 20.Li P, Rogers T, Smiley D, DiMarchi RD, Zhang F. Design, synthesis and crystallization of a novel glucagon analog as a therapeutic agent. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007;63((Pt 7)):599–601. doi: 10.1107/S1744309107028655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neidigh JW, Fesinmeyer RM, Prickett KS, Andersen NH. Exendin-4 and glucagon-like-peptide-1: NMR structural comparisons in the solution and micelle-associated states. Biochemistry. 2001;40(44):13188–13200. doi: 10.1021/bi010902s. [DOI] [PubMed] [Google Scholar]
- 22.Schnölzer M, Alewood P, Jones A, Alewood D, Kent SB. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Rapid, high yield assembly of difficult sequences. Int J Pept Protein Res. 1992;40((3-4)):180–193. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]