Abstract
Background
A promising approach to treat diabetes is the development of an automated bihormonal pump administering glucagon and insulin. A physically and chemically stable glucagon formulation does not currently exist. Our goal is to develop a glucagon formulation that is stable as a clear ungelled solution, free of fibrils at a pH of 7 for at least 7 days at 37 °C.
Methods
Experimental glucagon formulations were studied for stability at 25 and 37 °C. Chemical degradation was quantified by reverse phase ultra-performance liquid chromatography. Physical changes were studied using light obscuration and visual observations.
Results
Glucagon content of Biodel glucagon and Lilly glucagon at pH 2 and pH 4, as measured by high-performance liquid chromatography at 25 °C, was 100% at 7 days compared to 87% and <7%, respectively. Light obscuration measurements indicated Lilly glucagon at pH 4 formed an opaque gel, while Biodel glucagon formulation remained a clear solution beyond 50 days at 37 °C. Visual observations confirmed these results.
Conclusions
Biodel glucagon is a stabilized formulation at physiological pH and remains chemically and physically stable beyond 7 days at 37 °C, suggesting its utility for use in a bihormonal pump.
Keywords: artificial pancreas, bihormonal pump, diabetic miniature swine, fibril formation, gelation, stabilized glucagon
Introduction
Pharmacologically, glucagon increases the concentration of glucose in the blood. The first six amino acids at the N-terminus of the glucagon molecule bind to specific receptors on liver cells. This leads to an increase in the production of cyclic adenosine monophosphate, which facilitates the catabolism of stored glycogen and increases hepatic gluconeogenesis and ketogenesis. The immediate pharmacologic result is an increase in blood glucose at the expense of stored hepatic glycogen. Glucagon is degraded in the liver and kidney at tissue receptor sites. The half life of glucagon in plasma is 3 to 6 minutes, similar to that of insulin.1,2
An advance in diabetes treatment is the development of the “artificial pancreas,” which uses a continuous glucose sensor coupled to a computer algorithm that controls a bihormonal pump.3–6 Insulin pumps have been used by patients with insulin-dependent diabetes for over a decade. These pumps are capable of providing a continuous flow of insulin to provide for the patients' basal insulin requirements.7 At meal times, the user can manually increase the insulin flow to cover an individual patient's requirements. Newer devices are being developed that combine continuous subcutaneous glucose monitoring with an algorithm on a microprocessor that controls the insulin pump and delivers insulin as needed to cover individual patient's requirements.8 However, should too much insulin be given, there is no automated way to prevent hypoglycemia. By adding a second pump to deliver glucagon to the patient that counteracts excessively low or rapidly falling blood glucose levels, an artificial pancreas capable of keeping a patient within ideal glucose levels can be achieved.9–14 This outcome is similar to how a normal pancreas functions in a nondiabetic individual. However, this application requires a glucagon product that is physically and chemically stable in solution for at least 3 and ideally 7 days at 30–37 °C. The current commercial formulations of glucagon are not capable of fulfilling this need.15
Commercial preparations of glucagon are intended for immediate use following reconstitution. They are sold as a two-part rescue kit and are available for intravenous, intramuscular, or subcutaneous administration. The kit contains 1 mg (1 U) of glucagon as a dry powder in a sterile vial and a liquid diluent. The diluent is injected into the powder vial and gently swirled to dissolve the glucagon, then the glucagon solution is pulled back into the same syringe, ready for injection. The pH of this solution is approximately 2. The recommended dose is typically 0.5–1 mg, and any leftover reconstituted glucagon is to be discarded because it is not stable in solution.16 Moreover, the currently available formulation is designed for “rescue” use, thus the acidic nature and pain of the injection is acceptable because it is a single dose, rarely used, and given to a patient while the patient is unconscious. For daily use in a bihormonal pump, the pH and isotonicity of the solution should be closer to physiological conditions.
Glucagon is a highly conserved polypeptide consisting of a single chain of 29 amino acids, with a molecular weight of 3485 Da, made in the alpha cells of the pancreas.17 It has a helical conformation in the crystalline state, while, in dilute aqueous solutions, it has a random coil conformation with 15% alpha helix at the C-terminal end.18 Glucagon is soluble in aqueous solutions at pH less than 3 or greater than 9 and has low solubility in the pH range of 4 to 8 due to its isoelectric point of 7.1.1
The stability of the glucagon molecule is dependent on temperature, conformation, and interactions in solution or solid state. Degradation can occur through a variety of pathways. It readily forms a gel in acidic aqueous conditions (pH 3–5) and precipitates within an hour of preparation in neutral aqueous solutions at the therapeutic concentration of 1 mg/ml.1 The gel may be a clear gel that is easily resolublized or an opaque gel that appears to be aggregated gelatinous particles that does not readily disperse. Fibrils may also be formed and can be promoted by salt or an increase in temperature.19–24 In addition, there is rapid chemical degradation at room temperature and especially at physiological temperatures of 37 °C. These physical and chemical changes in the molecule restrict the usefulness of glucagon in a bihormonal pump. It is therefore our goal to develop a glucagon formulation that is chemically and physically stable as a clear solution, free of fibrils at a neutral pH for at least 7 days when kept at 37 °C for extended use in a pump device.
Our approach was to immobilize the glucagon in solution to prevent it from interacting with itself. This was accomplished by using a combination of a reducing sugar, a lysolecithin and an alcohol to form micelles and minimize glucagon–glucagon interactions. We tested numerous iterations of this approach and show the most successful ones in this article.
The various glucagon formulations were tested for chemical stablity using ultra-performance liquid chromatography (UPLC) and physical stability using visual observations, light microscopy, and light obscuration.25
Methods
Materials
Biodel glucagon was formulated with pure crystalline glucagon from Celtek (Nashville, TN). The formulation consists of lysolecithin, reducing sugar, alcohol, and phosphate buffer to maintain the pH at 7. The concept is to immobilize the glucagon in a micelle. For comparative purposes, commercially available glucagon for injection (rDNA origin) from Lilly, provided as a two-part formulation of lyophilized powder (1 mg glucagon and 49 mg lactose) and a diluent (12 mg/ml glycerin in water for injection) was used in these studies.16 Lilly glucagon was reconstituted with the provided diluent. Biodel glucagon was compared to Lilly glucagon at pH 2 (commercial formulation) and Lilly glucagon after a moderate pH adjustment with 1 N NaOH to pH 4 to make the pH less acidic for pump use.
Experimental Methods
Chemical Stability Confirmation of Test Formulation
Ultra-performance liquid chromatography method: An AcquityTM UPLC (Waters) equipped with an ultraviolet detector was used with an Acquity UPLC® ethylene-bridged hybrid C18 1.7 μ m, 2.1 × 100 mm (Waters) column. The mobile phase consisted of two parts: (A) 0.15 M sodium phosphate, pH 2.6 (pH adjusted by phosphoric acid and filtered via 0.45 μ m membrane), and (B) 100% acetonitrile. The linear gradient starts as 80% A and ends as 65% A, where it runs an isocratic elution for 8–14 min post injection, then back to 80% A at 16 min via a linear gradient. The column temperature is maintained at 35 °C and sample chamber temperature at 5 °C. The flow rate is 0.4 ml/min, using 214 nm ultraviolet detection, with an injection volume of 3 μ l.
Physical Stability: Light Obscuration and Light Microscopy
-
1
Light obscuration measurement analysis method using a heavy duty light meter (Extech, model 407026) equipped with personal computer and sample chamber (Design Spring) and a light type of tungsten/sodium: A glucagon sample (2 ml) is pipetted into a polystyrene cuvette (1 × 1 cm) and placed in line with a linear light-emitting diode array with a narrow beam exit angle. The light emits a fan-shaped light beam directed against the side of the square 1 × 1 cm cuvette. The exit beam passes through a low-pass sharp cut-off optical filter to minimize ambient light leakage. Output beam intensity is periodically monitored and recorded. Optical opacity or scattering changes are observed by a reduction in the transmitted light output.
-
2
Light microscopy with microscopic analysis: 20 μ l of Congo red aqueous solution (0.25g/ml) was added to a 50 μ l glucagon sample, gently mixed, and incubated ∼30 min at room temperature. A drop of the dyed sample was placed on standard glass slide and cover slipped. Images were taken at 400 × with a high-resolution camera (CoolSNAP EZ, Photometrics, Tucson, AZ) on a Nikon Microscope (Model LH-M100C-1).
Results
Chemical Degradation
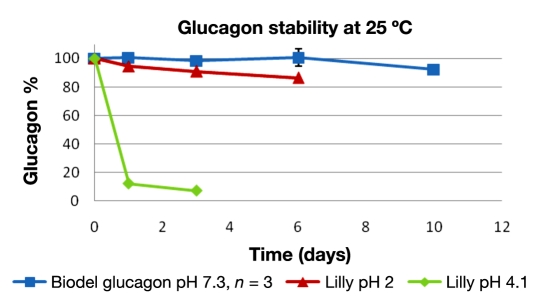
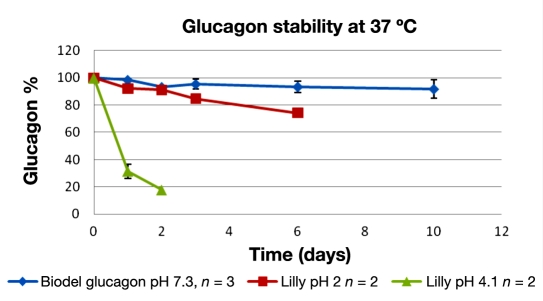
The chemical degradation of glucagon was quantitated by UPLC over a 10-day period. Only clear solutions of glucagon were used for analysis; no gels or suspensions were placed in high-performance liquid chromatography sample vials. The results from accelerated stability temperatures, 25 °C, are shown in percentage of original glucagon quantity in Figure 1, and results from 37 °C are shown in Figure 2.
Figure 1.
Glucagon ± standard deviation versus time at 25 °C. Biodel glucagon (blue) versus Lilly glucagon at pH 2 (red) and 4 (green).
Figure 2.
Glucagon ± standard deviation versus time at 37 °C. Biodel glucagon (blue) versus Lilly at pH 2 (red) and 4.1 (green).
Samples that gelled were not quantifiable, thus Lilly at pH 4 has no data past 3 days and Lilly pH 2 after 6 days at 25 °C and 37 °C The Biodel glucagon did not gel over the 10-day study.
Physical Degradation
-
1
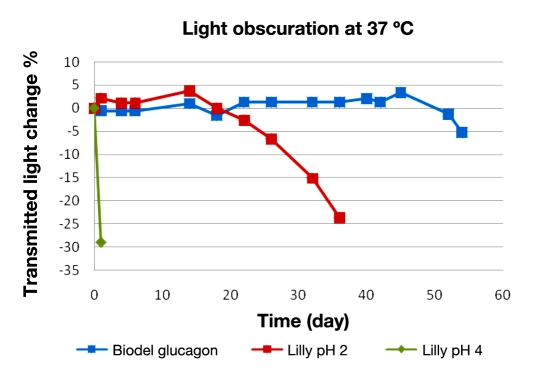
Light obscuration: Results on glucagon gelation are shown in Figure 3. The drop in transmitted light is indicitave of an opaque gel formation, or suspended particles. The Lilly glucagon at pH 4 immediately shows a decrease in transmitted light, indicating a precipitated gel. The pH 2 formulation does not have a reduction in transmitted light for ∼20 days, since the transmitted light is not sensitive to clear gel formation. The Biodel glucagon formulation resists opaque and clear gel formation for a considerably longer period of time, out to 50 days at 37 °C. This is a marked improvement over the commercially available glucagon formulation.
-
2
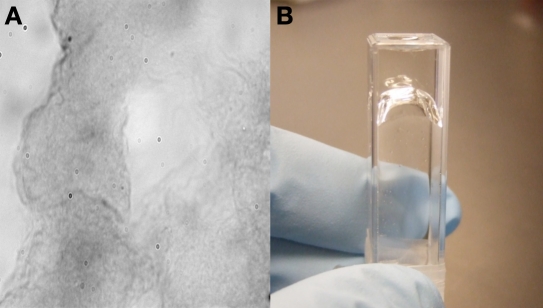
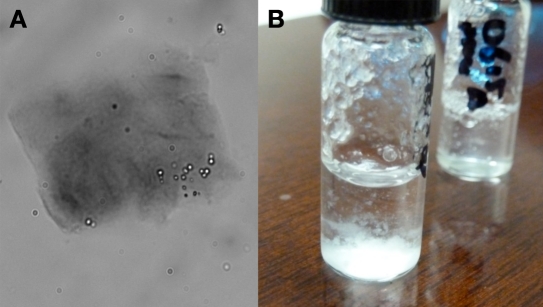
Microscopic images: Example images of gels stained with Congo red are shown in Figures 4 and 5. Figure 4 is a 400× image of a clear gel. Showing more contrast and darker staining, Figure 5 is representative of a “cloudy” or aggregated gel.
Figure 3.
Percentage of change in transmitted light over time (days). Biodel glucagon (blue) versus Lilly glucagon at pH 2 (red) and 4 (green).
Figure 4.
Glucagon test formulation showing a clear gel after being incubated 61 days at 25 °C. (A) A 400 × image of a clear gel stained with Congo red. (B) An inverted cuvette containing a clear gel.
Figure 5.
(A) Example of an opaque gel as imaged at 400 × following staining with Congo red. This sample is from Lilly glucagon pH 4, after three days at 37 °C. (B) A visualization of an opaque gel.
A summary of visual observations of gelation and chemical stability is shown in Table 1. The chemical stability is defined as the time in days the formulation remains within 90% of the original glucagon concentation after incubation at 37 °C. The physical instability in days is the first day post incubation at 37 °C that the sample shows sign of gelation or precipitation. The gel may be clear or opaque. In the case of a clear gel, it is a visual observation of the increased viscosity of the solution. Opaque gels can be quantitated by a reduction in transmitted light.
Table 1.
Summary of Gelation and Chemical Stability in Days
| Time (days) | |||
|---|---|---|---|
| Lilly pH 2 | Lilly pH 4 | Biodel glucagon | |
| Chemical stability (90% of original glucagon content stored at 37 °C) | 2 | 1 | 11 |
| First gelation or turbidity (visual or decreased transmitted light at 37 °C) | 22 | 1 | 52 |
In both comparisons of chemical and physical changes of the glucagon, the Biodel glucagon exceeds the stability of the commercial formulation glucagon.
Discussion
A healthy pancreas maintains exquisite control of systemic blood glucose in a very narrow range, under widely varying conditions. It achieves this feat, in part, by sensing glucose levels and modulating its release of insulin, amylin, C-peptide, and glucagon accordingly. In the 1920s, a diagnosis of a diseased pancreas in the form of type 1 diabetes was a death sentence. The discovery and clinical use of first animal-derived insulin, later recombinant human insulin, and later still the analog insulins have gone a long way in controlling this disease and making it manageable. Despite these improvements, managing insulin-dependent diabetes is still difficult, time consuming, and far less than ideal. The glycemic variability in well-controlled patients who use insulin pumps is an order of magnitude greater than healthy individuals, as assessed by continuous glucose monitoring (CGM).
Currently, a number of investigative teams around the world are combining the use of CGM devices and pumps that administer insulin and glucagon with micro-processors. The microprocessor receives information from the CGM device and, employing an algorithm, controls the administration of insulin and glucagon to maintain euglycemia. Such an approach holds out the promise of far greater glycemic control and marked improvement in the patients' quality of life, not only from the benefits attendant to less glycemic variability, but also because the device would automatically and continuously make adjustments, freeing the patient of this never-ending and burdensome task.
The various components necessary to make an artificial pancreas a reality are pumps, CGM, microprocessor and algorithm, ultra-rapid-acting insulin, and stable glucagon.
Existing insulin pumps with relatively minor modifications are already capable of accurately pumping either glucagon or insulin. Newer CGM devices are in various stages of development that, with redundancy, should supply sufficient reliability and accuracy to allow patient-independent control of the artificial pancreas. One way that this can be achieved is to employ two separate devices, each using a different principle by which it measures glucose. When the two devices provide reasonably similar measurement of glucose, the artificial pancreas functions automatically; when the two devices provide discrepant information, the device alerts the patient to intervene. Microprocessors exist that are so very reliable that we land jumbo jets with them. Some existing algorithms “learn” and others are rapidly being developed. Ultra-rapid acting insulins are in late stages of development, which, because of their quicker absorption, should allow for more accurate control algorithms with resultant better glycemic control. The final component necessary to make an artificial pancreas a reality is a stabilized glucagon; the Biodel formulation reported here has the potential to be this component. Its large animal toxicological profile as well as its performance in clinical trials has yet to be studied.
Conclusions
-
1
An appropriate formulation for pump use must remain free of solids (particles, gels, and fibrils) for at least 5 days at temperatures above 30 °C for safety considerations and in order to keep the small diameter tubes that deliver glucagon to the patient clear from debris The Biodel formulation, at a neutral pH, achieves these goals and goes well beyond, making it preferable over the commercial formulations for pump use.
-
2
In addition to fewer problems with physical changes in stability, the chemical stability of Biodel glucagon, as demonstrated by UPLC peak measurement, remains within 90% of the original glucagon content for more than 10 days at 37 °C.
-
3
Biodel glucagon formulation has prolonged stability over commercially prepared glucagon (Lilly, pH 2) and a pH adjusted version (pH 4) of the commercial formulation examined for possible pump use in these studies.
-
4
There is no single measurement that quantifies the transformation of glucagon into gels (clear and opaque), suspensions, and increases in nano (molecular) size Therefore, a combination of visual, light obscuration, and light microscopy may be used to understand the physical nature of the glucagon stability.
-
5
It is important to consider both the physical and chemical stability of glucagon before using in a bihormonal pump device.
Abbreviations
- (CGM)
continuous glucose monitoring
- (UPLC)
ultra-performance liquid chromatography
References
- 1.Joshi AB, Rus E, Kirsh LE. The degradation pathways of glucagon in acidic solutions. Int J Pharm. 2000;203((1-2)):115–125. doi: 10.1016/s0378-5173(00)00438-5. [DOI] [PubMed] [Google Scholar]
- 2.Paolisso G, Scheen AJ, Albert A, Lefebvre PJ. Effects of pulsatile delivery of insulin and glucagon in humans. Am J Physiol. 1989;257((5 Pt 1)):E686–E696. doi: 10.1152/ajpendo.1989.257.5.E686. [DOI] [PubMed] [Google Scholar]
- 3.Van Bon AC, Kohinor MJ, Hoekstra JB, von Basum G, deVries JH. Patients' perception and future acceptance of an artificial pancreas. J Diabetes Sci Technol. 2010;4(3):596–602. doi: 10.1177/193229681000400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosman B, Dassau E, Zisser HC, Jovanovic L, Doyle FJ., 3rd Zone model predictive control: a strategy to minimize hyper-and hypoglycemic events. J Diabetes Sci Technol. 2010;4(4):961–975. doi: 10.1177/193229681000400428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauseth R, Wang Y, Dassau E, Kircher R, Jr, Matheson D, Zisser H, Jovanovic L, Doyle FJ., 3rd Proposed clinical application for tuning fuzzy logic controller of artificial pancreas utilizing a personalization factor. J Diabetes Sci Technol. 2010;4(4):913–922. doi: 10.1177/193229681000400422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinkos A, Arreaza-Rubin G, Heetderks WJ, Irony I, Joffe HV, Schneider B, Zimliki CL. FDA's proactive role in the development of an artificial pancreas for the treatment of diabetes mellitus. Drug Discov Today Technol. 2007;4(1):25–28. doi: 10.1016/j.ddtec.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Albisser AM, Jackman WS, Ferguson R, Bahoric A, Goriya Y. A portable precision pumping system for chronic, programmed insulin infusion. Med Prog Technol. 1978;5(4):187–193. [PubMed] [Google Scholar]
- 8.Dassau E, Cameron F, Lee H, Bequette BW, Zisser H, Jovanovic L, Chase HP, Wilson DM, Buckingham BA, Doyle FJ., 3rd Real-time hypoglycemia prediction suite using continuous glucose monitoring: a safety net for the artificial pancreas. Diabetes Care. 2010;33(6):1249–1254. doi: 10.2337/dc09-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2(27):27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Bon AC, Hermanides J, Koops R, Hoekstra JB, DeVries JH. Postprandial glycemic excursions with the use of a closed-loop platform in subjects with type 1 diabetes: a pilot study. J Diabetes Sci Technol. 2010;4(4):923–928. doi: 10.1177/193229681000400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Rmileh A, Garcia-Gabin W, Zambrano D. A robust sliding mode controller with internal model for closed-loop artificial pancreas. Med Biol Eng Comput. 2010 doi: 10.1007/s11517-010-0665-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Anhalt H, Bohannon NJ. Insulin patch pumps: their development and future in closed-loop systems. Diabetes Technol Ther. 2010;12(Suppl 1):S51–S58. doi: 10.1089/dia.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilinska ME, Budiman ES, Taub MB, Elleri D, Allen JM, Acerini CL, Dunger DB, Hovorka R. Overnight closed-loop insulin delivery with model predictive control: assessment of hypoglycemia and hyperglycemia risk using simulation studies. J Diabetes Sci Technol. 2009;3(5):1109–1120. doi: 10.1177/193229680900300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H, Buckingham BA, Wilson DM, Bequette BW. A closed-loop artificial pancreas using model predictive control and a sliding meal size estimator. J Diabetes Sci Technol. 2009;3(5):1082–1090. doi: 10.1177/193229680900300511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valla V. Therapeutics of diabetes mellitus: focus on insulin analogues and insulin pumps. Exp Diabetes Res. 2010;2010:178372. doi: 10.1155/2010/178372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glucagon for injection. Lilly glucagon package insert.
- 17.Sasaki K, Dockerill S, Adamiak DA, Tickle IJ, Blundell T. X-ray analysis of glucagon and its relationship to receptor binding. Nature. 1975;257(5529):751–757. doi: 10.1038/257751a0. [DOI] [PubMed] [Google Scholar]
- 18.Gratzer WB, Beaven GH, Rattle HW, Bradbury EM. A conformational study of glucagon. Eur J Biochem. 1968;3(3):276–283. doi: 10.1111/j.1432-1033.1968.tb19527.x. [DOI] [PubMed] [Google Scholar]
- 19.Beaven GH, Gratzer WB, Davies HG. Formation and structure of gels and fibrils from glucagon. Eur J Biochem. 1969;11(1):37–42. doi: 10.1111/j.1432-1033.1969.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson ML, Formisano S, Edelhoch H. Self-association of glucagon as measured by the optical properties of rhodamine 6G. J Biol Chem. 1978;253(5):1353–1356. [PubMed] [Google Scholar]
- 21.Johnson RE, Hruby VJ, Rupley JA. Thermodynamics of glucagon aggregation. Biochemistry. 1979;18(7):1176–1179. doi: 10.1021/bi00574a009. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen JS, Dikov D, Flinkv JL, Hjuler HA, Christiansen G, Otzen DE. The changing face of glucagon fibrillation: structural polymorphism and conformational imprinting. J Mol Biol. 2006;355(3):501–523. doi: 10.1016/j.jmb.2005.09.100. [DOI] [PubMed] [Google Scholar]
- 23.Blundell TL. Conformation and molecular biology of polypeptide hormones II. Glucagon. Trens Biochem Sci. 1979;4:80–83. [Google Scholar]
- 24.Onoue S, Ohshima K, Debari K, Koh K, Shioda S, Iwasa S, Kashimoto K, Yajima T. Mishandling of the therapeutic peptide glucagon generates cytotoxic amyloidogenic fibrils. Pharm Res. 2004;21(7):1274–1283. doi: 10.1023/b:pham.0000033016.36825.2c. [DOI] [PubMed] [Google Scholar]
- 25.EL-Khatib FH, Jiang J, Gerrity RG, Damiano ER. Pharmaco- dynamics and stability of subcutaneously infused glucagon in a type 1 diabetic swine model in vivo. Diabetes Technol Ther. 2007;9(2):135–144. doi: 10.1089/dia.2006.0006. [DOI] [PubMed] [Google Scholar]