Abstract
This short review outlines the physiology of glucagon in vivo, with an emphasis on its neural control, the author’s area of interest. Glucagon is secreted from alpha cells, which are a minority of the pancreatic islet. Anatomically, they are down stream from the majority islet beta cells. Beta-cell secretory products restrain glucagon secretion. Activation of the autonomic nerves, which innervate the islet, increases glucagon secretion.
Glucagon is secreted into the portal vein and thus has its major physiologic action at the liver to break down glycogen. Glucagon thereby maintains hepatic glucose production during fasting and increases hepatic glucose production during stress, including the clinically important stress of hypoglycemia. Three different mechanisms proposed to stimulate glucagon secreted during hypoglycemia are discussed: (1) a stimulatory effect of low glucose directly on the alpha cell, (2) withdrawal of an inhibitory effect of adjacent beta cells, and (3) a stimulatory effect of autonomic activation.
In type 1 diabetes (T1DM), increased glucagon secretion contributes to the elevated ketones and acidosis present in diabetic ketoacidosis (DKA). It also contributes to the hyperglycemia seen with or without DKA. The glucagon response to insulin-induced hypoglycemia is impaired soon after the development of T1DM. The mediators of this impairment include loss of beta cells and loss of sympathetic nerves from the autoimmune diabetic islet.
Keywords: alpha cell, autonomic nervous system, glucagon, insulin-induced hypoglycemia, sympathetic nervous system, type 1 diabetes mellitus
Anatomy of the Islet
Islet Alpha Cells
In humans, glucagon is secreted from the islet alpha cells, which comprise only 10% of islet volume; 80% of islet volume is composed of beta cells, which secrete insulin. In rodents, alpha cells are located on the rim or mantle of the islet, but in humans, this arrangement is more complex.1 Nevertheless, in both species, alpha cells are located next to insulin-secreting beta cells, suggesting a local interaction.
Islet Vasculature
Pancreatic islets are supplied by individual arterioles, which divide into a tortuous capillary complex, resembling a renal glomerulus. In most species, these capillaries first supply the islet beta cells and then supply the islet alpha cells, exposing them to high concentrations of beta-cell secretory products, which tonically suppress glucagon secretion. Blood exiting the islet drains into the pancreatic vein, which, in turn, drains into the portal vein, exposing the liver to high concentrations of glucagon. Thus, not surprisingly, the liver is the site of glucagon’s major physiologic actions. Modest extraction of glucagon by the liver and major dilution of hepatic vein blood by systemic blood results in low concentrations of glucagon in peripheral plasma that, in the basal state, are difficult to measure reliably.
Islet Innervation
Sympathetic and parasympathetic nerves follow arterioles into the islet, but similar to other autonomic fibers, they do not form classical synapses. Rather, they release their neuro-transmitters, norepinephrine, and acetylcholine (ACh), respectively, near islet alpha and beta cells. Immunohistochemical staining reveals moderate density sympathetic2–5 and parasympathetic6–9 innervation of the islet, suggesting important neural control of glucagon secretion. Indeed, activation of sympathetic nerves elicits a robust glucagon response,10,11 which is largely retained in diabetic animals despite prevailing hyperglycemia. In contrast, activation of parasympathetic nerves elicits a more modest glucagon response.12 Both of these autonomic inputs to the islet are activated by central hypoglycemia13,14 and contribute to the glucagon response to hypoglycemia.15,16 This glucagon response is important in glucose counterregulation.17 It is long established that the glucagon response to hypoglycemia is defective in type 1 diabetes (T1DM).18 Evidence suggests that there are both functional and structural defects in the autonomic pathways to the islet in T1DM.
Glucagon Biosynthesis and Processing
Products of the Proglucagon Gene
Glucagon, like other polypeptide hormones, is encoded by a prepro gene. The preproglucagon gene has six exons, one of which encodes a glucagon precursor and two of which encode the precursors for glucagon-like peptide (GLP)-1 and GLP-2, respectively.19 Although glucagon raises blood sugar, the truncated form of GLP-1 is best known for its ability to stimulate insulin release,20 which has the opposite effect on blood sugar. Interestingly, GLP-1 and its analogs used to treat diabetes also inhibit glucagon secretion, which likely contributes to its glucose-lowering effect. Glucagon-like peptide-2 has neither of these effects but instead promotes the growth of intestinal epithelial cells.21 The primary structure of all three hormones is highly conserved across mammals, suggesting preservation of critical biological activity.
Tissue-Specific Processing
The product of the proglucagon gene that predominates depends on the tissue from which it is released.19 In the alpha cells of the pancreatic islet, the biologically active form released is the 29 amino acid hormone glucagon. A major proglucagon fragment is also released from the islet alpha cells. Its sequence includes both GLP-1 and GLP-2, but these sequences are flanked by amino acids that render both products biologically inactive. In the L-cells of the small and large bowel, the reverse is true. Here, GLP-1 and GLP-2 are cleaved from the proglucagon sequence in their biologically active forms and released. The remaining sequence of the proglucagon molecule is also released, but it includes amino acids that flank glucagon and render it biologically inactive. The marked tissue difference in the secreted products of the proglucagon gene is due to different posttranslational processing mediated presumably by different processing enzymes contained within the two tissues.
Effects of Glucagon
Liver Action
The major site of glucagon’s physiologic action is the liver, for several reasons. First, the liver is exposed to glucagon concentrations that are two to three times higher than the levels to which other organs are exposed. Glucagon is secreted into the portal vein and partially extracted by the liver22 before it is diluted by the glucagon-poor blood of the systemic circulation. Second, the systemic levels of endogenous glucagon are usually below those needed to affect the glucagon receptors on adipose tissue to cause lipolysis. Further, glucagon has no appreciable effect on muscle glycogenolysis. Third, portal vein glucagon levels are high enough to activate the abundant hepatic glucagon receptors. These receptors are coupled to G protein S (Gs),which activates adenylate cyclase through its alpha subunit. The resultant increase of hepatic cyclic adenosine monophosphate levels activates protein kinase A, which, in turn, phosphorylates the enzymes needed to activate liver glycogenolysis.23
Glycogenolysis versus Gluconeogenesis
Even basal levels of portal vein glucagon are sufficient to mediate three-fourths of fasting glucose production, both in large experimental animals24 and humans.25 However, this degree of stimulation depends on insulin levels being low, as they are in the fasting state. Although glucagon increases hepatic gluconeogenic enzymes, the contribution of gluconeogenesis to basal glucose production is usually minor in large animals and humans. Only when fasting is quite prolonged is there a substantial mobilization of the precursors required for significant gluconeogenesis. Physiologic glucagon levels have no direct role in mobilizing gluconeogenic glycerol from adipose tissue26 and amino acids from muscle.27 That function is served by a substantial decrease of the systemic insulin levels.
Increases of endogenous glucagon above the fasting level also potently stimulate hepatic glucose production, largely by glycogenolysis. For example, glucagon increments as little as 10 pg/ml increase hepatic glucose production by 25%. Thus changes of glucagon secretion throughout its physiologic range control hepatic glucose production throughout its physiologic range.
Duration of Effect
The effect of glucagon on hepatic glucose production has been described as “evanescent,” which seems to imply a transient, rather than a sustained, effect. In fact, the hepatic glucose production response to a stepwise increase of glucagon is a rapid peak followed by a lower, but more sustained, plateau.28 Interestingly, this is the pattern of hepatic glucose production needed to achieve a rapid but sustained increase of plasma glucose. Indeed, part of the “evanescent” effect of glucagon is due to that increase of plasma glucose and its stimulation of insulin secretion, both of which inhibit hepatic glucose production.29 However, this classic feedback inhibition of a metabolic pathway by its end product explains only part of the evanescent effect; the majority appears to be a downregulation of the rapid intrahepatic glucagon-signaling pathway. Nonetheless, the fact that suppression of basal glucagon secretion markedly suppresses fasting glucose production30 reinforces the view that glucagon was chronically stimulating hepatic glucose production. Indeed, the speed with which inhibition of glucagon secretion lowers hepatic glucose production is similar to the speed with which it stimulates hepatic glucose production.31 Thus, although pulsatility in glucagon secretion can entrain hepatic glucose production, the amount of glucose released by the liver is similar, whether the glucagon is delivered in a pulsatile or constant fashion.
Glucagon Response to Hypoglycemia
Mechanism
Three different mechanisms have been proposed to mediate the alpha-cell response to hypoglycemia: (1) a direct effect of hypoglycemia to stimulate the pancreatic alpha cell, (2) release from suppression by the islet beta cell, and (3) autonomic stimulation of the islet alpha cell.
Direct Effect of Low Glucose
Although low glucose in vitro increases glucagon secretion from the isolated perfused pancreas,32 exposing isolated alpha cells to low glucose fails to directly stimulate glucagon secretion.33 Thus the effect of low glucose directly on the islet alpha cell does not appear to be a major mediator of the glucagon response to hypoglycemia.
Inhibitory Effect of the Islet Beta Cell
If islet beta cells tonically inhibit glucagon secretion from neighboring alpha cells, then the direct effect of low glucose to inhibit the beta cell could contribute to the glucagon response to hypoglycemia. Such an inhibitory action of endogenous insulin was originally proposed by Samols and colleagues34 and first strongly supported by the data of Weir and associates.35 There is other evidence that gamma amino butyric acid (GABA)36 and zinc,37 secreted from the islet beta cell, play similar inhibitory roles. Indeed, destruction of islet beta cells prevents low glucose media from stimulating glucagon secretion in vitro.38 Thus the hypothesis that a “switch-off” of inhibitory beta-cell factors help stimulate glucagon secretion during hypoglycemia is supported by a variety of in vitro data.
In vivo, the evidence for a beta-cell role is mixed. Older data show that in humans without diabetes, prior partial suppression of endogenous insulin secretion before hypoglycemia did not impair the glucagon response to hypoglycemia.39 Similarly, patients with T1DM, who presumably have markedly reduced endogenous insulin secretion, can exhibit a normal glucagon response to hypoglycemia, at least very early after diabetes onset.39 Conversely, more recent data suggest that prior inhibition of islet beta cells impairs40—and prior stimulation of islet beta cells augments41—the glucagon response to hypoglycemia in humans without diabetes.
Stimulatory Effect of Autonomic Activation
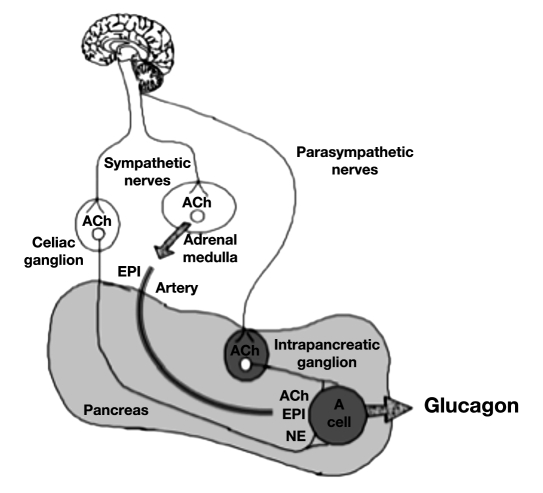
There are three autonomic inputs to the islet alpha cell: sympathetic nerves, parasympathetic nerves, and the circulating hormone epinephrine, which is also under neural control (Figure 1). All three autonomic inputs stimulate glucagon secretion when activated,10–12,42,43 and all three are activated during hypoglycemic stress.13,14,44–47 Indeed, pharmacological blockade of the ganglionic neurotransmission involved in all three autonomic pathways markedly impairs the glucagon response to insulin-induced hypoglycemia (IIH) in all species tested,48–50 including primates.51 In addition, the alternative experimental approach of surgical disruption of all three autonomic pathways produces a similar impairment of the glucagon response to IIH.15,50 These data suggest that the autonomic activation that accompanies marked hypoglycemia in vivo is a major mediator of the glucagon response to IIH.
Figure 1.
Three autonomic inputs to the islet alpha cell. Preganglionic sympathetic nerves travel in the spinal cord releasing ACh from their terminals within the celiac ganglion. The released ACh activates postganglionic sympathetic neuronal cell bodies whose fibers innervate the islet alpha cells and release norepinephrine. Preganglionic parasympathetic nerves travel in the vagus releasing ACh from their terminals within intrapancreatic parasympathetic ganglia. The released ACh activates postganglionic parasympathetic neuronal cell bodies, whose fibers innervate the islet alpha cells and release ACh. Preganglionic sympathetic nerves travel in the spinal cord releasing ACh from their terminals within the adrenal medulla. The released ACh activates the chromaffin cells to release of the sympathetic neurohormone epinephrine, which reaches the alpha cell via the arterial circulation. EPI, epinephrine; NE, norepinephrine.
The results of these studies, ablating the autonomic nervous system’s input to the islet, are similar to those from studies that inhibit the central nervous system pathways that trigger the autonomic activation. Thus, lesioning the ventromedial hypothalamus (VMH) significantly impairs both autonomic activation and the glucagon response to IIH.52 Direct application of urocortin-153 or insulin54 to the VMH also impairs the glucagon response to IIH. Finally, preventing central, but not peripheral, hypoglycemia impairs the glucagon response to IIH55 as does microdialysis of glucose into the VMH.56 These studies demonstrate the necessity of central activation, presumably of the three autonomic pathways, for the glucagon response to hypoglycemia in animals.
Two studies suggest a similar autonomic mediation in humans without diabetes. First, repeated hypoglycemia reduces both autonomic and glucagon responses to IIH57 in humans. Second, ganglionic blockade in humans markedly reduces the glucagon response to IIH.58
Glucagon Secretion in Type 1 Diabetes
During Diabetic Ketoacidosis
The basal level of glucagon circulating in the systemic plasma of patients with T1DM can be markedly elevated if they present with diabetic ketoacidosis (DKA).59 This elevated glucagon level contributes both to their marked hyperketonemia as well as their hyperglycemia.60 This elevation of basal glucagon levels occurs despite their hyperglycemia, which, in subjects without diabetes, suppresses glucagon secretion. Two factors likely contribute to this stimulation of the islet alpha cell. The first is activation of the sympathetic nervous system secondary to the volume depletion usually present in patients with DKA. The second is their severe beta-cell loss, which removes a tonic restraint on the alpha cell.
During Insulin Treatment
In insulin-treated T1DM patients with stabilized and moderate hyperglycemia, fasting glucagon levels are usually within normal range. However, since hyperglycemia normally suppresses glucagon secretion, an effect dependent on the high levels of insulin within the islet,61 these patients actually have a relative basal hyper-glucagonemia. Subjects with type 2 diabetes, who have much less beta-cell loss, still have a relative basal hyper-glucagonemia.
During Insulin-Induced Hypoglycemia
Despite this relative basal hyperglucagonemia, the glucagon response to IIH is impaired within the first year of T1DM and lost entirely several years later.62 In contrast, the glucagon response to arginine can be normal in T1DM,18,63,64 although there are reports of moderate impairments in patients when insulin is infused to achieve euglycemia.63,65,66
Beta-Cell Factors
The mechanism for the rapid loss of the glucagon response to IIH is likely multifactorial. One factor is the loss of islet beta-cell function since, in subjects with T1DM, retention of the glucagon response to IIH seems related to the retention of C-peptide secretion.67 Further, type 2diabetes patients have no impairment of their glucagon response to IIH until they lose enough islet beta cells to become dependent on insulin treatment.68 It has been proposed in the switch-off hypothesis that factors secreted from the islet beta cell (e.g., insulin,35,69 zinc,37 GABA36) tonically restrain basal glucagon secretion. The hypothesis posits that hypoglycemia switches off this restraint by inhibiting islet beta-cell secretion. Indeed, reproducing this tonic restraint experimentally in diabetic animals and then releasing it, concomitant with hypoglycemia, can elicit a glucagon response.70
Neural Factors
However, the magnitude of the glucagon response to IIH is dependent on the recognition of hypoglycemia by the brain, not the islet. For example, preventing central glucopenia, while allowing peripheral hypoglycemia, abolishes the glucagon response to IIH.55 This central glucopenia activates the autonomic nervous system, and blocking this autonomic activation markedly impairs the majority of the glucagon response to IIH in experimental animals48–50,71 and humans.58 Interestingly, data from three animal models of autoimmune diabetes4,5,72 and preliminary data from humans with T1DM73 reveal a significant loss of islet sympathetic nerves, one of the autonomic inputs that is activated by hypoglycemia13 and which helps mediate the glucagon response to hypoglycemia.15 Thus this early sympathetic islet neuro-pathy may partly explain the impaired glucagon response to IIH seen in T1DM.
Another factor to consider is the exogenous insulin that produces the hypoglycemia. Early studies in C-peptide-negative T1DM patients clearly showed that exogenous insulin can suppress glucagon responses to other secretagogues.63 Since insulin infusions in subjects withoutdiabetes do not produce a net inhibition of these glucagon responses,63 the alpha cell in the insulin-deficient islet may be supersensitive to the inhibitory effects of exogenous insulin.
Other types of chronic neuropathy, such as diabetic autonomic neuropathy, can impair the epinephrine response to IIH,74 which, with an impaired glucagon response to IIH, renders patients with T1DM vulnerable to frequent episodes of hypoglycemia. However, even in subjects without diabetes, repeated episodes of hypo-glycemia can impair the epinephrine response to IIH,57 a phenomenon called hypoglycemia-associated autonomic failure. This type of autonomic impairment also occurs during intensive insulin therapy in T1DM.75 Hypoglycemia-associated autonomic failure is not a classical neuropathy, but rather a desensitization of the central neural pathways that initiate the autonomic response to hypoglycemia. The cortisol response to prior hypoglycemia helps mediate this central desensitization,76 as does upregulation of brain glucose transporters.77 Thus intensive insulin therapy in recent-onset T1DM patients may lead to impaired glucagon responses not only due to their loss of islet sympathetic nerves but also secondary to an induced autonomic dysfunction.
Abbreviations
- (ACh)
acetylcholine
- (DKA)
diabetic ketoacidosis
- (GABA)
gamma aminobutyric acid
- (GLP)
glucagon-like peptide
- (IIH)
insulin-induced hypoglycemia
- (VMH)
ventromedial hypothalamus
- (T1DM)
type 1 diabetes mellitus
References
- 1.Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G, Berney T. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59(5):1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding WG, Kimura H, Fujimura M, Fujimiya M. Neuropeptide Y and peptide YY immunoreactivities in the pancreas of various vertebrates. Peptides. 1997;18(10):1523–1529. doi: 10.1016/s0196-9781(97)00237-4. [DOI] [PubMed] [Google Scholar]
- 3.Lindsay TH, Halvorson KG, Peters CM, Ghilardi JR, Kuskowski MA, Wong GY, Mantyh PW. A quantitative analysis of the sensory and sympathetic innervation of the mouse pancreas. Neuroscience. 2006;137(4):1417–1426. doi: 10.1016/j.neuroscience.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 4.Mei Q, Mundinger TO, Lernmark A, Taborsky GJ., Jr Early, selective, and marked loss of sympathetic nerves from the islets of BioBreeder diabetic rats. Diabetes. 2002;51(10):2997–3002. doi: 10.2337/diabetes.51.10.2997. [DOI] [PubMed] [Google Scholar]
- 5.Taborsky GJ, Jr, Mei Q, Hackney DJ, Figlewicz DP, LeBoeuf R, Mundinger TO. Loss of islet sympathetic nerves and impairment of glucagon secretion in the NOD mouse: relationship to invasive insulitis. Diabetologia. 2009;52(12):2602–2611. doi: 10.1007/s00125-009-1494-5. [DOI] [PubMed] [Google Scholar]
- 6.Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol. 1997;378(4):454–467. [PubMed] [Google Scholar]
- 7.Schäfer MK, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neuroscience. 1998;84(2):361–376. doi: 10.1016/s0306-4522(97)80196-0. [DOI] [PubMed] [Google Scholar]
- 8.Rossi J, Santamäki P, Airaksinen MS, Herzig KH. Parasympathetic innervation and function of endocrine pancreas requires the glial cell line-derived factor family receptor alpha2 (GFRalpha2) Diabetes. 2005;54(5):1324–1330. doi: 10.2337/diabetes.54.5.1324. [DOI] [PubMed] [Google Scholar]
- 9.Mei Q, Bornfeldt K, Hackney D, Mundinger T, Taborsky GJ., Jr Neural selectivity in the loss of autonomic nerves from the islets of animal models of type 1 diabetes: implications for mechanism. Diabetes. 2010;59(Suppl 1):A210. [Google Scholar]
- 10.Marliss EB, Girardier L, Seydoux J, Wollheim CB, Kanazawa Y, Orci L, Renoldv AE, Porte D., Jr Glucagon release induced by pancreatic nerve stimulation in the dog. J Clin Invest. 1973;52(5):1246–1259. doi: 10.1172/JCI107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahrén B, Veith RC, Taborsky GJ., Jr Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1) Effects on basal release of insulin and glucagon. Endocrinology. 1987;121(1):323–331. doi: 10.1210/endo-121-1-323. [DOI] [PubMed] [Google Scholar]
- 12.Ahrén B, Taborsky GJ., Jr The mechanism of vagal nerve stimulation of glucagon and insulin secretion in the dog. Endocrinology. 1986;118(4):1551–1557. doi: 10.1210/endo-118-4-1551. [DOI] [PubMed] [Google Scholar]
- 13.Havel PJ, Veith RC, Dunning BE, Taborsky GJ., Jr Pancreatic noradrenergic nerves are activated by neuroglucopenia but not hypotension or hypoxia in the dog. Evidence for stress-specific and regionally selective activation of the sympathetic nervous system. J Clin Invest. 1988;82(5):1538–1545. doi: 10.1172/JCI113763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz TW, Holst JJ, Fahrenkrug J, Jensen SL, Nielsen OV, Rehfeld JF, De Muckadell OB, Stadil F. Vagal, cholinergic regulation of pancreatic polypeptide secretion. J Clin Invest. 1978;61(3):781–789. doi: 10.1172/JCI108992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havel PJ, Mundinger TO, Taborsky GJ., Jr Pancreatic sympathetic nerves contribute to increased glucagon secretion during severe hypoglycemia in dogs. Am J Physiol. 1996;270((1 Pt 1)):E20–E26. doi: 10.1152/ajpendo.1996.270.1.E20. [DOI] [PubMed] [Google Scholar]
- 16.Patel DG. Role of parasympathetic nervous system in glucagon response to insulin-induced hypoglycemia in normal and diabetic rats. Metabolism. 1984;33(12):1123–1127. doi: 10.1016/0026-0495(84)90098-2. [DOI] [PubMed] [Google Scholar]
- 17.Gerich J, Davis J, Lorenzi M, Rizza R, Bohannon N, Karam J, Lewis S, Kaplan R, Schultz T, Cryer P. Hormonal mechanisms of recovery from insulin-induced hypoglycemia in man. Am J Physiol. 1979;236(4):E380–E385. doi: 10.1152/ajpendo.1979.236.4.E380. [DOI] [PubMed] [Google Scholar]
- 18.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182(108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- 19.Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261(25):11880–11889. [PubMed] [Google Scholar]
- 20.Mojsov S, Weir GC, Habener JF. Insulinotropin:glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987;79(2):616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA. 1996;93(15):7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herold KC, Jaspan JB. Hepatic glucagon clearance during insulin induced hypoglycemia. Horm Metab Res. 1986;18(7):431–435. doi: 10.1055/s-2007-1012339. [DOI] [PubMed] [Google Scholar]
- 23.Exton JH. Glucagon signal-transduction mechanisms. In: Jefferson LS, Cherrington AD, Goodman HM, editors. Handbook of physiology. New York: Oxford University Press; 2001. pp. 435–450. [Google Scholar]
- 24.Cherrington AD, Liljenquist JE, Shulman GI, Williams PE, Lacy WW. Importance of hypoglycemia-induced glucose production during isolated glucagon deficiency. Am J Physiol. 1979;236(3):E263–E271. doi: 10.1152/ajpendo.1979.236.3.E263. [DOI] [PubMed] [Google Scholar]
- 25.Liljenquist JE, Mueller GL, Cherrington AD, Keller U, Chiasson JL, Perry JM, Lacy WW, Rabinowitz D. Evidence for an important role of glucagon in the regulation of hepatic glucose production in normal man. J Clin Invest. 1977;59(2):369–374. doi: 10.1172/JCI108649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes. 1989;38(12):1595–1601. doi: 10.2337/diab.38.12.1595. [DOI] [PubMed] [Google Scholar]
- 27.Kettelhut IC, Wing SS, Goldberg AL. Endocrine regulation of protein breakdown in skeletal muscle. Diabetes Metab Rev. 1988;4(8):751–772. doi: 10.1002/dmr.5610040805. [DOI] [PubMed] [Google Scholar]
- 28.Wada M, Connolly CC, Tarumi C, Neal DW, Cherrington AD. Hepatic denervation does not significantly change the response of the liver to glucagon in conscious dogs. Am J Physiol. 1995;268((2 Pt 1)):E194–E203. doi: 10.1152/ajpendo.1995.268.2.E194. [DOI] [PubMed] [Google Scholar]
- 29.DeFronzo RA, Ferrannini E, Hendler R, Wahren J, Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci USA. 1978;75(10):5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherrington AD, Chiasson JL, Liljenquist JE, Jennings AS, Keller U, Lacy WW. The role of insulin and glucagon in the regulation of basal glucose production in the postabsorptive dog. J Clin Invest. 1976;58(6):1407–1418. doi: 10.1172/JCI108596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobbins RL, Davis SN, Neal D, Caumo A, Cobelli C, Cherrington AD. Rates of glucagon activation and deactivation of hepatic glucose production in conscious dogs. Metabolism. 1998;47(2):135–142. doi: 10.1016/s0026-0495(98)90209-8. [DOI] [PubMed] [Google Scholar]
- 32.Gerich JE, Charles MA, Grodsky GM. Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. J Clin Invest. 1974;54(4):833–841. doi: 10.1172/JCI107823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54(6):1808–1815. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- 34.Samols E, Tyler JM, Marks V. Glucagon-insulin interrelationships. In: Lefebvre PJ, Unger RH, editors. Glucagon: molecular physiology, clinical and therapeutic implications. Oxford: Pergamon Press; 1972. pp. 151–173. [Google Scholar]
- 35.Weir GC, Knowlton SD, Atkins RF, McKennan KX, Martin DB. Glucagon secretion from the perfused pancreas of streptozotocin-treated rats. Diabetes. 1976;25(4):275–282. doi: 10.2337/diab.25.4.275. [DOI] [PubMed] [Google Scholar]
- 36.Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P, Braun M. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes. 2004;53(4):1038–1045. doi: 10.2337/diabetes.53.4.1038. [DOI] [PubMed] [Google Scholar]
- 37.Zhou H, Zhang T, Harmon JS, Bryan J, Roberston RP. Zinc, not insulin, regulates the rat alpha-cell response to hypoglycemia in vivo. Diabetes. 2007;56(4):1107–1112. doi: 10.2337/db06-1454. [DOI] [PubMed] [Google Scholar]
- 38.Tominaga M, Maruyama H, Vasko MR, Baetens D, Orci L, Unger RH. Morphologic and functional changes in sympathetic nerve relationships with pancreatic alpha-cells after destruction of beta-cells in rats. Diabetes. 1987;36(3):365–373. doi: 10.2337/diab.36.3.365. [DOI] [PubMed] [Google Scholar]
- 39.Bolli G, De Feo P, Perriello G, De Cosmo S, Compagnucci P, Santeusanio F, Brunetti P, Unger R. Mechanisms of glucagon secretion during insulin-induced hypoglycemia in man. Role of the beta cell and arterial hyperinsulinemia. J Clin Invest. 1984;73(4):917–922. doi: 10.1172/JCI111315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raju B, Cryer PE. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Diabetes. 2005;54(3):757–764. doi: 10.2337/diabetes.54.3.757. [DOI] [PubMed] [Google Scholar]
- 41.Banarer S, McGregor VP, Cryer PE. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes. 2002;51(4):958–965. doi: 10.2337/diabetes.51.4.958. [DOI] [PubMed] [Google Scholar]
- 42.Bloom SR, Edwards AV. Pancreatic endocrine responses to stimulation of the peripheral ends of the vagus nerves in conscious calves. J Physiol. 1981;315:31–41. doi: 10.1113/jphysiol.1981.sp013730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerich JE, Karam JH, Forsham PH. Stimulation of glucagon secretion by epinephrine in man. J Clin Endocrinol Metab. 1973;37(3):479–481. doi: 10.1210/jcem-37-3-479. [DOI] [PubMed] [Google Scholar]
- 44.Dunning BE, Scott MF, Neal DW, Cherrington AD. Direct quantification of norepinephrine spillover and hormone output from the pancreas of the conscious dog. Am J Physiol. 1997;272((5 Pt 1)):E746–E755. doi: 10.1152/ajpendo.1997.272.5.E746. [DOI] [PubMed] [Google Scholar]
- 45.Taborsky GJ, Jr, Paquette TL, Pfeifer MA, Gingerich RL. Pento-barbital suppresses basal and reflexive pancreatic polypeptide release in dogs. Am J Physiol. 1985;249((6 Pt 1)):E577–E583. doi: 10.1152/ajpendo.1985.249.6.E577. [DOI] [PubMed] [Google Scholar]
- 46.Cannon WB, McIver MA, Bliss SW. Studies on the condition of activity in endocrine glands. XIII. A sympathetic and adrenal mechanism for mobilizing sugar in hypoglycemia. Am J Physiol. 1924;69:46–66. [Google Scholar]
- 47.Garber AJ, Cryer PE, Santiago JV, Haymond MW, Pagliara AS, Kipnis DM. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest. 1976;58(1):7–15. doi: 10.1172/JCI108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Havel PJ, Akpan JO, Curry DL, Stern JS, Gingerich RL, Ahren B. Autonomic control of pancreatic polypeptide and glucagon secretion during neuroglucopenia and hypoglycemia in mice. Am J Physiol. 1993;265((1 Pt 2)):R246–R254. doi: 10.1152/ajpregu.1993.265.1.R246. [DOI] [PubMed] [Google Scholar]
- 49.Havel PJ, Parry SJ, Stern JS, Akpan JO, Gingerich RL, Taborsky GJ, Jr, Curry DL. Redundant parasympathetic and sympathoadrenal mediation of increased glucagon secretion during insulin-induced hypoglycemia in conscious rats. Metabolism. 1994;43(7):860–866. doi: 10.1016/0026-0495(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 50.Havel PJ, Veith RC, Dunning BE, Taborsky GJ., Jr Role for autonomic nervous system to increase pancreatic glucagon secretion during marked insulin-induced hypoglycemia in dogs. Diabetes. 1991;40(9):1107–1114. doi: 10.2337/diab.40.9.1107. [DOI] [PubMed] [Google Scholar]
- 51.Havel PJ, Valverde C. Autonomic mediation of glucagon secretion during insulin-induced hypoglycemia in rhesus monkeys. Diabetes. 1996;45(7):960–966. doi: 10.2337/diab.45.7.960. [DOI] [PubMed] [Google Scholar]
- 52.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest. 1994 Apr;93(4):1677–1682. doi: 10.1172/JCI117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCrimmon RJ, Song Z, Cheng H, McNay EC, Weikart-Yeckel C, Fan X, Routh VH, Sherwin RS. Corticotrophin-releasing factor receptors within the ventromedial hypothalamus regulate hypoglycemia-induced hormonal counterregulation. J Clin Invest. 2006;116(6):1723–1730. doi: 10.1172/JCI27775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paranjape SA, Chan O, Zhu W, Horblitt AM, McNay EC, Cresswell JA, Bogan JS, McCrimmon RJ, Sherwin RS. Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Diabetes. 2010;59(6):1521–1527. doi: 10.2337/db10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biggers DW, Myers SR, Neal D, Stinson R, Cooper NB, Jaspan JB, Williams PE, Cherrington AD, Fizzell RT. Role of brain in counterregulation of insulin-induced hypoglycemia in dogs. Diabetes. 1989;38(1):7–16. doi: 10.2337/diab.38.1.7. [DOI] [PubMed] [Google Scholar]
- 56.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks conterregulation during systemic hypoglycemia in awake rats. J Clin Invest. 1997;99(2):361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40(2):223–226. doi: 10.2337/diab.40.2.223. [DOI] [PubMed] [Google Scholar]
- 58.Havel PJ, Ahren B. Activation of autonomic nerves and the adrenal medulla contributes to increased glucagon secretion during moderate insulin-induced hypoglycemia in women. Diabetes. 1997;46(5):801–807. doi: 10.2337/diab.46.5.801. [DOI] [PubMed] [Google Scholar]
- 59.Müller WA, Faloona GR, Unger RH. Hyperglucogonemia in diabetic ketoacidosis. Its prevalence and significance. Am J Med. 1973;54(1):52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- 60.Unger RH, Orci L. Role of glucagon in diabetes. Arch Intern Med. 1977;137(4):482–491. [PubMed] [Google Scholar]
- 61.Greenbaum CJ, Havel PJ, Taborsky GJ, Jr, Klaff LJ. Intra-islet insulin permits glucose to directly suppress pancreatic A cell function. J Clin Invest. 1991;88(3):767–773. doi: 10.1172/JCI115375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolli G, De Feo P, Compagnucci P, Cartechini MG, Angeletti G, Santeusanio F, Brunetti P, Gerich JE. Abnormal glucose counter-regulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes. 1983;32(2):134–141. doi: 10.2337/diab.32.2.134. [DOI] [PubMed] [Google Scholar]
- 63.Asplin CM, Paquette TL, Palmer JP. In vivo inhibition of glucagon secretion by paracrine beta cell activity in man. J Clin Invest. 1981;68(1):314–318. doi: 10.1172/JCI110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caprio S, Tamborlane WV, Zych K, Gerow K, Sherwin RS. Loss of potentiating effect of hypoglycemia on the glucagon response to hyperaminoacidemia in IDDM. Diabetes. 1993;42(4):550–555. doi: 10.2337/diab.42.4.550. [DOI] [PubMed] [Google Scholar]
- 65.Wiethop BV, Cryer PE. Glycemic actions of alanine and terbutaline in IDDM. Diabetes Care. 1993;16(8):1124–1130. doi: 10.2337/diacare.16.8.1124. [DOI] [PubMed] [Google Scholar]
- 66.Rossetti P, Porcellati F, Busciantella Ricci N, Candeloro P, Cioli P, Nair KS, Santeusanio F, Bolli GB, Fanelli CG. Effect of oral amino acids on counterregulatory responses and cognitive function during insulin-induced hypoglycemia in nondiabetic and type 1 diabetic people. Diabetes. 2008;57(7):1905–1917. doi: 10.2337/db08-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukuda M, Tanaka A, Tahara Y, Ikegami H, Yamamoto Y, Kumahara Y, Shima K. Correlation between minimal secretory capacity of pancreatic beta-cells and stability of diabetic control. Diabetes. 1988;37(1):81–88. doi: 10.2337/diab.37.1.81. [DOI] [PubMed] [Google Scholar]
- 68.Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51(3):724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 69.Cooperberg B, Cryer PE. Insulin reciprocally regulates glucagon secretion in humans. Diabetes. 2010 doi: 10.2337/db10-0728. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou H, Tran PO, Yang S, Zhang T, LeRoy E, Oseid E, Robertson RP. Regulation of alpha-cell function by the beta-cell during hypoglycemia in Wistar rats: the “switch-off” hypothesis. Diabetes. 2004;53(6):1482–1487. doi: 10.2337/diabetes.53.6.1482. [DOI] [PubMed] [Google Scholar]
- 71.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA. 2004;101(29):10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mei Q, Bornfeldt KE, Taborsky GJ., Jr Loss of islet sympathetic nerves in a virally induced mouse model of autoimmune diabetes: implications for mechanisms. Diabetes. 2009;58(Suppl 1):A35. [Google Scholar]
- 73.Mei Q, Foulis AK, Fligner C, Hull R, Gilliam L, Taborsky GJ., Jr Selective loss of sympathetic nerves from the islet in human type 1 diabetes: a potential mechanism for impaired glucagon responses to hypoglycemia. Diabetes. 2006a;55:A15. [Google Scholar]
- 74.Hoeldtke RD, Boden G. Epinephrine secretion, hypoglycemia unawareness, and diabetic autonomic neuropathy. Ann Intern Med. 1994;120(6):512–517. doi: 10.7326/0003-4819-120-6-199403150-00011. [DOI] [PubMed] [Google Scholar]
- 75.Cryer PE. Hypoglycemia begets hypoglycemia in IDDM. Diabetes. 1993;42(12):1691–1693. doi: 10.2337/diab.42.12.1691. [DOI] [PubMed] [Google Scholar]
- 76.Davis SN, Shavers C, Costa F, Mosqueda-Garcia R. Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. J Clin Invest. 1996;98(3):680–691. doi: 10.1172/JCI118839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCall AL, Fixman LB, Fleming N, Tornheim K, Chick W, Ruderman NB. Chronic hypoglycemia increases brain glucose transport. Am J Physiol. 1986;251((4 Pt 1)):E442–E447. doi: 10.1152/ajpendo.1986.251.4.E442. [DOI] [PubMed] [Google Scholar]