Abstract
Background
In 2008–2009, the first multinational study was completed comparing closed-loop control (artificial pancreas) to state-of-the-art open-loop therapy in adults with type 1 diabetes mellitus (T1DM).
Methods
The design of the control algorithm was done entirely in silico, i.e., using computer simulation experiments with N = 300 synthetic “subjects” with T1DM instead of traditional animal trials. The clinical experiments recruited 20 adults with T1DM at the Universities of Virginia (11); Padova, Italy (6); and Montpellier, France (3). Open-loop and closed-loop admission was scheduled 3–4 weeks apart, continued for 22 h (14.5 h of which were in closed loop), and used a continuous glucose monitor and an insulin pump. The only difference between the two sessions was that insulin dosing was performed by the patient under a physician’s supervision during open loop, whereas insulin dosing was performed by a control algorithm during closed loop.
Results
In silico design resulted in rapid (less than 6 months compared to years of animal trials) and cost-effective system development, testing, and regulatory approvals in the United States, Italy, and France. In the clinic, compared to open-loop, closed-loop control reduced nocturnal hypoglycemia (blood glucose below 3.9 mmol/liter) from 23 to 5 episodes (p < .01) and increased the amount of time spent overnight within the target range (3.9 to 7.8 mmol/liter) from 64% to 78% (p = .03).
Conclusions
In silico experiments can be used as viable alternatives to animal trials for the preclinical testing of insulin treatment strategies. Compared to open-loop treatment under identical conditions, closed-loop control improves the overnight regulation of diabetes.
Keywords: closed-loop control, continuous glucose monitoring, type 1 diabetes mellitus
Introduction
The concept of an “artificial pancreas,” or an external closed-loop control system that regulates blood glucose levels in patients with diabetes, has gained momentum. The roots of this concept can be traced back to the 1970s, when exogenous regulation of blood glucose concentrations in people with diabetes became possible by using intravenous (IV) glucose measurement and IV infusion of glucose and insulin.1,2 Systems such as the Biostator™ were introduced and used in hospital settings to maintain normoglycemia. Subsequent work spanned a broader range of physiological modeling and control techniques, including adaptive and model-based algorithms.3 However, IV closed-loop glucose control remains unsuitable for outpatient use because of the need for continuous IV access and the cumbersome technology that needs to be employed. An alternative to extracorporeal IV control of blood glucose is an implantable intraperitoneal system using IV glucose sampling and intraperitoneal insulin delivery.4 Implanting these systems, however, is quite invasive. Thus, with the advent of minimally invasive subcutaneous (SC) continuous glucose monitors, which sample interstitial glucose through a micro needle implanted subcutaneously and then convert the values into blood glucose, increasing academic and industrial effort has been focused on the development of SC–SC closed-loop glucose control, using continuous glucose monitoring (CGM) coupled with an insulin pump and a control algorithm.5–7
The clinical utility of CGM for the optimization of glycemic control in type 1 diabetes mellitus (T1DM) has been demonstrated by a 2008 landmark study that showed a significant improvement in hemoglobin A1c after 6 months of CGM in adults with T1DM.8 The next logical step is the demonstration of the feasibility of SC–SC closed-loop control. To date, several studies have reported clinical results for closed-loop glucose control using SC CGM and insulin delivery.9–12 These studies used one of two algorithmic strategies known as proportional-integral-derivative10,11 or model-predictive control (MPC).12–15 Model-based approach was also used for the detection of meals16 or hypoglycemia17, as well as for the implementation of models that can “learn” the specifics of patients’ daily routine (e.g., timing of meals) and then optimize insulin response using this information.18,19
Two studies reported positive results from clinical trials of algorithm-based insulin delivery tested in children with T1DM12 in combination with glucagon assisting with prevention of hypoglycemia14 and brought closed-loop control to the forefront of mainstream medical journals. This article extends their findings with the following new elements: (1) regulatory Food and Drug Administration (FDA) approval of the control system based entirely on in silico experiments, i.e., by a series of computer simulations performed on virtual subjects; (2) clinical experiments performed in three independent centers in the United States, Italy, and France, which enhances the external validity of the results; and (3) automated (as opposed to manual) transfer of CGM data to the control system, which brings the artificial pancreas one step closer to routine clinical application. Detailed clinician impressions of system performance during this study have been previously presented in this journal.20,21 We now summarize these previous reports and present the overall statistical effect of closed-loop versus open-loop control performed under identical hospital conditions.
Methods
In Silico Design of the Control System
For artificial pancreas design, we have developed a computer simulator environment based on a previously reported mathematical model of glucose metabolism.22 The computer simulation environment was equipped with a “population” of in silico images of N = 300 “subjects” with T1DM, separated in three age groups: N = 100 simulated “children” below the age of 11 years, N = 100 “adolescents” 12–18 years old, and N = 100 “adults” 21–78 years old. The simulated population has a wide range of intersubject variability approximating the variability observed between individuals in vivo.23 Three CGM devices—Navigator (Abbot Diabetes Care, Alameda, CA), Guardian RT (Medtronic, Northridge, CA), Dexcom STS, 7–day sensor (Dexcom, San Diego, CA)—and two insulin pumps—OmniPod Insulin Management System (Insulet Corp., Bedford, MA) and Deltec Cozmo (Smiths Medical MD, St. Paul, MN)—were simulated as well. With this technology, any open- or closed-loop control strategy and any meal and insulin delivery scenario can be pilot tested efficiently in silico prior to clinical application. This in silico algorithm design approach has been accepted by the FDA as a substitute for animal trials.23
Model-Predictive Control Algorithm
Using simulation experiments, we tested the robustness and the effectiveness of a new MPC algorithm with all 300 simulated “subjects” (i) under a nominal scenario corresponding exactly to the clinical protocol of the study and (ii) under deviations from the nominal scenario in the timing and amount of meals. An overview of the algorithm characteristics is as follows: First, data from an outpatient screening evaluation are used to tailor the algorithm for each person. These data include the patient’s individual body weight (kg), average total daily insulin use (U), typical carbohydrate ratio (gram carbohydrate/insulin unit), and typical insulin infusion basal rate. This allows the optimal choice of a single parameter (q) that specifies the aggressiveness of the MPC reaction to deviations from normoglycemia.15 Second, because, in this study, closed-loop control was always preceded by an open-loop day, meal profile data (timing and amount of meals) from the open-loop day were made available to the control algorithm, which permitted the MPC to anticipate meal disturbances. Third, upon switch-on, the algorithm acquires previous measures of SC glucose concentration and records of injected insulin, which allows its introduction to the current status of the patient. After initialization, the algorithm acquires CGM data every minute and suggests insulin boluses every 15 min. The 15 min actuation rate was set on the basis of previous results showing that, due to the “smoothing” inherent with SC insulin transport, blood insulin concentrations resulting from small, 15 min insulin boluses are indistinguishable from those resulting from a continuous basal rate.24 Full engineering details for the MPC algorithm have been published.15
Clinical Study Participants
Adults with T1DM were recruited at the Universities of Virginia, Charlottesville, Virginia (N = 11); Padova, Italy (N = 6); and Montpellier, France (N = 3). The average age of the participants was 41.1 (±10.4) years, the average duration of diabetes was 18.6 (±5.6) years, and the average hemoglobin A1c was 7.25% (±0.9%). There were 12 males and 8 females. Criteria for inclusion were 21 years of age or older, with T1DM for at least 2 years, use of insulin pump, and willingness to use lispro insulin for the duration of the study. Exclusion criteria were pregnancy, hematocrit <36% for females, hematocrit <38% for males, symptomatic coronary artery disease or history of a cerebrovascular event, use of a medication that significantly influences glucose metabolism (oral steroids), use of a device that may interfere electromagnetically with CGM (e.g., implantable defibrillator or electronic pacemaker), and allergy or adverse reaction to lispro insulin.
Clinical Procedure
Each patient had an outpatient screening evaluation, and two 22 h overnight hospital admissions separated by a 2- to 4-week waiting period. During the outpatient screening evaluation, the study physician reviewed the subject’s history and performed a physical examination. Routine vital signs were obtained, including height, weight, temperature, respiratory rate, and orthostatic measurement of blood pressure and pulse. Hematocrit/hemoglobin, comprehensive chemistry panel, and insulin antibody titers were determined. Each inpatient admission began at 15:00 and ended at 13:00 on the following day. Subjects ate dinners and lunches with carbohydrate content that was the same at admission 1 and admission 2 and had identical morning meals of Ensure Plus (Abbott Nutrition, Columbus, OH) containing 50 g carbohydrate, 11 g fat, and 13 g protein. Two days before each admission, two Freestyle Navigator CGM devices (Abbott Diabetes Care) continuous glucose monitors were applied to the patient to allow for stabilization of the sensors and for assessment of their performance. During admission 1, open-loop control was used, with the subjects’ usual insulin routine and their personal insulin pump. During admission 2, an OmniPod Insulin Management System (Insulet Corp.) was inserted and used for closed-loop control of blood glucose. Insulin lispro (Eli Lilly, Indianapolis, IN), chosen based on commercial assays available, was used during both inpatient admissions.
At the beginning of admission 2, one of the two CGM devices was designated as primary, and the closed-loop control algorithm used the data of that system, unless a problem was detected. At 17:00, the MPC was initiated in a data-collection mode, automatically receiving CGM data every minute. Administration of the predinner insulin bolus was overseen by the attending physician. MPC closed-loop control began at 21:30 and continued until 12:00 the next day for a total of 14.5 h.
Per FDA restrictions, the algorithm did not automatically control the insulin pump. Instead, the algorithm suggested insulin boluses every 15 min which, if accepted, were programmed into the insulin pump by the attending physician. This was done for safety reasons, allowing the physician to override insulin delivery suggestions at any time. Reference blood glucose (using a YSI Life Sciences or a Beckman glucose analyzer) was sampled every 30 min. The protocol required switching to more frequent 15 min reference blood glucose sampling if hypoglycemia occurred or was imminent. Fast-acting carbohydrate (glucose tablets or fruit juice) was given when reference blood glucose fell below 3.9 mmol/liter, regardless of the CGM readings.
Statistical Analysis
The primary outcomes of the study included the number of hypoglycemic events below 3.9 mmol/liter and the percentage of time within the range of 3.9 to 7.8 mmol/liter overnight (21:30 until 08:00). Both of these variables were measured by reference blood glucose (YSI), regardless of CGM. We tested directional hypotheses: closed-loop superior to open-loop control, which implied the reporting of one-sided significance levels. Because the primary outcomes were not normally distributed random variables, we used nonparametric Wilcoxon matched pairs test to compare open-loop versus closed-loop control. Secondary outcomes included time with target of 3.9–10 mmol/liter, the magnitude of postbreakfast glucose excursions, as well as outcomes based on CGM data.
Institutional and Regulatory Approvals
The three studies were approved by the Institutional Review Boards of their respective institutions. In addition, the study at the University of Virginia received an investigation device exemption from the FDA, which was based on extensive computer simulation experiments. Information was exchanged among the three studies only as de-identified data and was governed by established interinstitutional contracts.25
Results
In Silico Testing of the Control System
The entire clinical protocol for the (then upcoming) clinical experiments was run in silico 2700 times, which took 3 months. The control algorithm was tested using the three populations of simulated “adults,” “adolescents,” and “children,” subjected to a number of scenarios, including nominal conditions emulating exactly the clinical protocol, as well as deviations from nominal conditions designed to assess the robustness of control. These deviations included initiation of the control at nearly hypoglycemic or nearly hyperglycemic blood glucose levels (4.4 and 10 mmol/liter, respectively), meals that are delayed or arriving early, and various degrees of CGM error. In order to focus on the control algorithm, these results were done under conditions of “perfectly working” CGM devices. It was therefore expected that malfunctioning CGM sensors would cause inferior control performance in vivo. Table 1 presents the outcomes of some of these tests for N = 100 simulated adults—a population that corresponds to the participants in the clinical studies.
Table 1.
Adults: Safety and Effectiveness Endpoints From In Silico Testinga
| Nominal scenario matching the clinical protocol | Control initiated at near hypoglycemia (4.4 mmol/liter) | Control initiated at near hyperglycemia (10 mmol/liter) | |
|---|---|---|---|
| Mean blood glucose (mmol/liter) | 6.77 | 6.65 | 7.19 |
| Percentage of time within 3.9–10 mmol/liter | 97% | 96% | 91% |
| Percentage of time <3.9 mmol/liter | 1% | 3% | 1% |
These in silico results are comparable to the clinical results presented in Table 2.
Illustrative Clinical Example
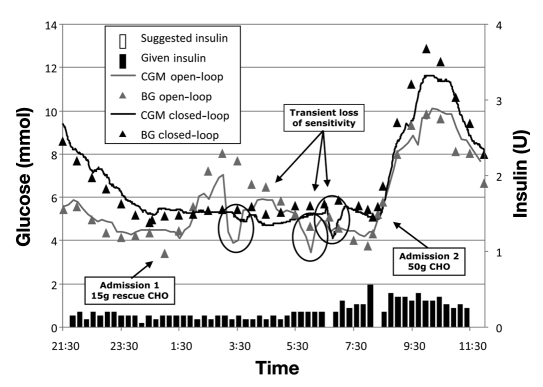
To illustrate the design of the study and the operation of the tested SC MPC, Figure 1 presents data on University of Virginia subject 104, which is representative of events occurring during closed-loop control.
Figure 1.
Illustration of open-loop versus closed-loop control in one study participant. The grey curve represents primary CGM data. The grey squares represent reference blood glucose data during admission 1 (open-loop control). The black curve shows primary CGM data, and the black triangles show reference blood glucose data during admission 2 (closed-loop control). The bars on the x axis display insulin delivery prior to initiation of closed-loop control before 21:30 and display insulin boluses suggested by the control algorithm during closed-loop control after 21:30. Despite much higher glucose excursions after dinner immediately prior to initiation of closed-loop control, the control algorithm brings the subject within target and avoids any nocturnal hypoglycemia afterward. At 06:00 during closed-loop control, the sensor lost sensitivity for approximately 30 min; nevertheless, the control algorithm (which uses only sensor but not reference blood glucose data) was robust, canceling one insulin bolus until the sensor stabilized. BG, blood glucose; CHO, carbohydrate.
Summary Data
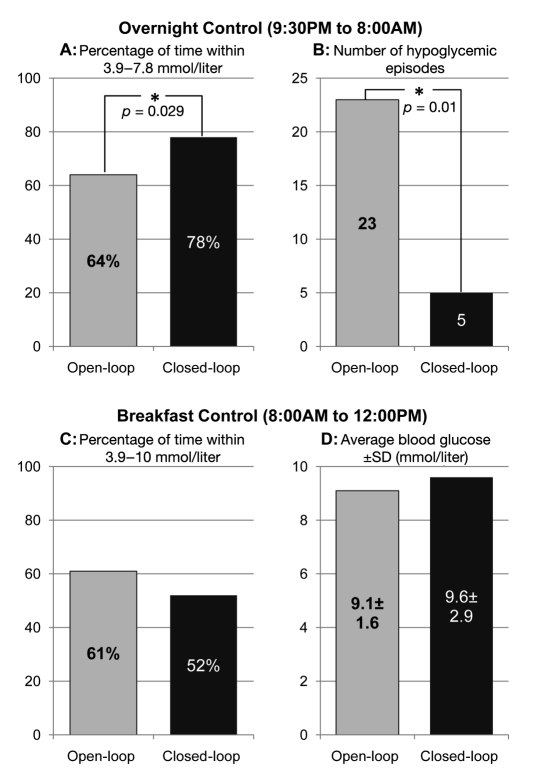
Figure 2 summarizes the course of glucose control overnight (upper panels) and following breakfast (lower panels) and during open-loop (gray bars) and closed-loop control (black bars).
Figure 2.
Summary outcome data. Key parameters of glucose control overnight (upper panels) and following breakfast (lower panels) during open-loop (gray bars) and closed-loop control (black bars). Panel A: percentage of time within the target range (3.9–7.8 mmol/liter) overnight was 64% on open-loop control and 78% on closed-loop control. With N = 20 matched pairs, a Wilcoxon nonparametric test was significant, p = .029, for a directional hypothesis: closed-loop > open-loop. Panel B: the number of hypoglycemic episodes overnight decreased from 23 on open-loop control to 5 on closed-loop control. With N = 20 matched pairs, Wilcoxon nonparametric test was significant, p = .01. Panel C: percentage of time within the target range (3.9–10 mmol/liter) after breakfast was not significantly different between open- and closed-loop control. Panel D: the mean of the maximal blood glucose values following breakfast was not different between open- and closed-loop control. SD, standard deviation.
Average Glycemia and Time within Target Range
The average blood glucose concentration overnight (21:30–08:00) was not different in open and closed loop: 6.78 ± 1.52 versus 6.89 ± 1.21 mmol/liter. However, the percentage of time spent within the narrow target range of 3.9–7.8 mmol/liter overnight increased from 64% in open loop to 78% in closed loop (Figure 2, panel A). Nonparametric Wilcoxon test showed that this increase was statistically significant: Z = 1.9, p = .029 (one-tail directional hypothesis).
Hypoglycemic Events
Overnight on open-loop, there were 1.15 hypoglycemic episodes (reference blood glucose below 3.9 mmol/liter) per subject, range 0–4 episodes. On closed-loop control, hypoglycemia was reduced to 0.25 episodes per subject, range 0–1. Thus, we observed a nearly five-fold reduction of nocturnal hypoglycemic episodes requiring a rescue with 15 g carbohydrate (Figure 2, panel B)—from 23 episodes on open-loop control to 5 episodes on closed-loop control. This effect was significant: Z = 2.5, p < .01 (Wilcoxon test, directional hypothesis).
Secondary outcomes are summarized in Table 2 and are presented using both YSI and CGM data. As seen, YSI and CGM results were quite close throughout the study and were close to the results suggested by the preclinical in silico experiments (e.g., average blood glucose of 6.77 mmol/liter and 1–3% hypoglycemia in Table 1). A significant difference between open- and closed-loop control was marked by the percentage of time in hypoglycemia (below 3.9 mmol/liter), p = .01; there was also a trend toward lower variation of blood glucose (standard deviation) on closed loop (p = .055).
Table 2.
Overnight (21:30–08:00) Characteristics Based on YSI and Continuous Glucose Monitoring Data
| Open loop | Closed loop | |||
|---|---|---|---|---|
| YSI | CGM | YSI | CGM | |
| Average blood glucose (mmol/liter) | 6.78 | 6.66 | 6.89 | 6.75 |
| Standard deviation of blood glucose (mmol/liter)a | 2.0 | 1.83 | 1.57 | 1.35 |
| Percentage of time within 3.9–10 mmol/liter | 80.6 | 81.1 | 89.0 | 90.2 |
| Hypoglycemia (percentage of time below 3.9 mmol/liter)b | 8.44 | 9.1 | 2.0 | 2.3 |
Trend.
Significant difference.
After breakfast, on closed loop, the participants spent 9% less time below 10 mmol/liter and had 0.4 mmol/liter higher average blood glucose (Figure 2, panels C and D). These differences were not statistically significant.
Other Events
The attending physician decided to override the insulin suggestions of the closed-loop control algorithm on four occasions, resulting in 2.5 h (2%) loss of closed-loop control time in all 20 patients. The primary reason for overriding the algorithm was near-hypoglycemia indicated by reference blood glucose but not reflected by sensor data. At most admissions, the sensor experienced episodes of transient loss of sensitivity overnight (see Figure 1). All but two of these episodes recovered when the subject changed position and did not require switching to the backup sensor. One experiment at the University of Virginia (patient 107) had to be rescheduled because the patient was hypoglycemic at the time of initiation of closed-loop control, followed by adhesive failure of the insulin pump, kinking of the pump cannula, and termination of insulin delivery. This was the only insulin pump failure observed during the study.
Discussion
This work contributes two elements toward the quest for closed-loop control of T1DM. First, the regulatory approval of the clinical trials was based entirely on in silico experiments performed in a computer-simulation environment.23 Second, the control algorithm was tested at three centers in three different countries, which added external validity to the data. Fully automated CGM data transfer is another characteristic distinguishing this study from other reports.12 Key features of this MPC algorithm are therefore automated SC glucose monitoring, SC insulin delivery, and personalization for each study participant using routinely available characteristics.
An important feature of this study is its repeated- measures design: each participant was tested twice under identical conditions in a tightly controlled hospital setting. The only variable that differed between admissions 1 and 2 was, therefore, the control strategy: patient-directed open-loop control at admission 1 and algorithm-suggested closed-loop control at admission 2. This permitted an objective assessment of the performance of MPC. The major advantage of closed-loop control was the nearly five-fold reduction in the number of nocturnal hypoglycemic episodes, plus a greater percentage of time that blood glucose spent within the narrow target range of 3.9–7.8 mmol/liter overnight. On the other hand, the performance of the MPC algorithm before and after breakfast was generally inferior to the open-loop control.
A weakness of the study was that the order of open-loop versus closed-loop conditions was not randomized. Typically, such a randomization is required in order to avoid “learning” effects. In this study, we need to differentiate the effect of “algorithm learning,” i.e., the control algorithm “learning” about the subject and his/her meal profile from open-loop data, and the effect of “human learning,” which could potentially contaminate the results. The idea of algorithm learning is that, over time, a profile of a person’s characteristics and daily regiment can be estimated and then used for control. In order to test algorithm learning, in this first study we supplied the algorithm with information about meals from the open-loop trial. In order to do so, we departed from the gold-standard randomized-order trials and had open loop always first. However, this was a pilot study testing new technology and not necessarily aiming for perfection in study design. Our subsequent studies (now ongoing) employ randomized order. Human (patient, personnel) learning was possible in this trial, but during closed loop, the patient and the attending personnel had little influence on insulin dosing, which was entirely done by the closed-loop control algorithm. Thus the influence of the order of open- and closed-loop control experiments should be minimal.
In terms of technology advancement, two comments are important. First, the control algorithm used insulin boluses administered every 15 min instead of the continuous basal rate. This was done because, in preparation for this study, we found that, due to SC insulin transport and “smoothing” of the insulin boluses during their transit from SC space to the circulation, 15 min boluses result in blood insulin concentrations that are indistinguishable from those generated by continuous insulin administration.24 Other practical advantages of such a discrete insulin delivery include more precise insulin dosing and optimization of pump battery life. Second, although the overall CGM performance was satisfactory, the CGM devices suffered from transient loss of sensitivity, particularly overnight. Although the exact definition of such events is not possible, on approximately 15 occasions across all subjects, the CGM readings experienced rapid drops, which did not correspond to reference blood glucose changes. For example, in Figure 1, three such drops were observed—two during admission 1 and one during admission 2. Such events may have been caused by increased pressure on the sensor during sleep that recovered after the patient repositioned.
Conclusions
A system using personalized MPC to control blood glucose in T1DM has been developed entirely in silico and then tested successfully in the clinic. We anticipate that the routine clinical application of such a technology is in the future. Nevertheless, the merging of contemporary CGM and insulin pumps with models of human metabolism and MPC algorithms may result in viable closed-loop glucose control based entirely on SC glucose sensing and insulin delivery.
Acknowledgments
The authors thank Dr. Laurissa Kashmer for her assistance with the execution of the clinical protocol; Pamela Mendosa, RN, for the patient recruitment and the logistic support of this study; Jeff Hawley for the technical assistance with this paper; and our students, Alice Chan and Colleen Hughes, for their assistance with the design of the control algorithm and with the clinical studies. Continuous glucose monitors were provided by Abbott Diabetes Care, Alameda, CA. Insulin pumps were provided by Insulet Corp., Boston, MA.
Abbreviations
- (CGM)
continuous glucose monitoring
- (FDA)
Food and Drug Administration
- (IV)
intravenous
- (MPC)
model-predictive control
- (SC)
subcutaneous
- (T1DM)
type 1 diabetes mellitus
References
- 1.Albisser AM, Leibel BS, Ewart TG, Davidovac Z, Botz CK, Zingg W. An artificial endocrine pancreas. Diabetes. 1974;23(5):389–396. doi: 10.2337/diab.23.5.389. [DOI] [PubMed] [Google Scholar]
- 2.Santiago JV, Clemens AH, Clarke WL, Kipnis DM. Closed-loop and open-loop devices for blood glucose control in normal and diabetic subjects. Diabetes. 1979;28(1):71–84. doi: 10.2337/diab.28.1.71. [DOI] [PubMed] [Google Scholar]
- 3.Parker RS, Doyle FJ, 3rd, Peppas NA. A model-based algorithm for blood glucose control in type I diabetic patients. IEEE Trans Biomed Eng. 1999;46(2):148–157. doi: 10.1109/10.740877. [DOI] [PubMed] [Google Scholar]
- 4.Renard E. Implantable closed-loop glucose-sensing and insulin delivery: the future for insulin pump therapy. Curr Opin Pharmacol. 2002;2(6):708–716. doi: 10.1016/s1471-4892(02)00216-3. [DOI] [PubMed] [Google Scholar]
- 5.Bellazzi R, Nucci G, Cobelli C. The subcutaneous route to insulin-dependent diabetes therapy. IEEE Eng Med Biol Mag. 2001;20(1):54–64. doi: 10.1109/51.897828. [DOI] [PubMed] [Google Scholar]
- 6.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23(1):1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 7.Klonoff DC. The artificial pancreas: how sweet engineering will solve bitter problems. J Diabetes Sci Technol. 2007;1(1):72–81. doi: 10.1177/193229680700100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O’Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–176. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 9.Hovorka R, Chassin LJ, Wilinska ME, Canonico V, Akwi JA, Federici MO, Massi-Benedetti M, Hutzli I, Zaugg C, Kaufmann H, Both M, Vering T, Schaller HC, Schaupp L, Bodenlenz M, Pieber TR. Closing the loop: the adicol experience. Diabetes Technol Ther. 2004;6(3):307–318. doi: 10.1089/152091504774197990. [DOI] [PubMed] [Google Scholar]
- 10.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):3344–3350. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 11.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 12.Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AM, Nodale M, De Palma A, Wilinska ME, Acerini CL, Dunger DB. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375(9716):743–751. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
- 13.Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Orsini Federici M, Pieber TR, Schaller HC, Schaupp L, Vering T, Wilinska ME. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25(4):905–920. doi: 10.1088/0967-3334/25/4/010. [DOI] [PubMed] [Google Scholar]
- 14.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Science Trans Med. 2010;2(27):27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magni L, Raimondo DM, Bossi L, Man CD, De Nicolao G, Kovatchev B, Cobelli C. Model predictive control of type 1 diabetes: an in silico trial. J Diabetes Sci Technol. 2007;1(6):804–812. doi: 10.1177/193229680700100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dassau E, Bequette BW, Buckingham BA, Doyle FJ., 3rd Detection of a meal using continuous glucose monitoring: implications for an artificial beta-cell. Diabetes Care. 2008;31(2):295–300. doi: 10.2337/dc07-1293. [DOI] [PubMed] [Google Scholar]
- 17.Buckingham B, Cobry E, Clinton P, Gage V, Caswell K, Kunselman E, Cameron F, Chase HP. Preventing hypoglycemia using predictive alarm algorithms and insulin pump suspension. Diabetes Technol Ther. 2009;11(2):93–97. doi: 10.1089/dia.2008.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owens C, Zisser H, Jovanovic L, Srinivasan B, Bonvin D, Doyle FJ., 3rd Run-to-run control of blood glucose concentrations for people with type 1 diabetes mellitus. IEEE Trans Biomed Eng. 2006;53(6):996–1005. doi: 10.1109/TBME.2006.872818. [DOI] [PubMed] [Google Scholar]
- 19.Palerm CC, Zisser H, Bevier WC, Jovanovic L, Doyle FJ., 3rd Prandial insulin dosing using run-to-run control: application of clinical data and medical expertise to define a suitable performance metric. Diabetes Care. 2007;30(5):1131–1136. doi: 10.2337/dc06-2115. [DOI] [PubMed] [Google Scholar]
- 20.Clarke WL, Anderson S, Breton M, Patek S, Kashmer L, Kovatchev B. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: the Virginia experience. J Diabetes Sci Technol. 2009;3(5):1031–1038. doi: 10.1177/193229680900300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruttomesso D, Farret A, Costa S, Marescotti MC, Vettore M, Avogaro A, Tiengo A, Dalla Man C, Place J, Facchinetti A, Guerra S, Magni L, De Nicolao G, Cobelli C, Renard E, Maran A. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: preliminary studies in Padova and Montpellier. J Diabetes Sci Technol. 2009;3(5):1014–1021. doi: 10.1177/193229680900300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalla Man C, Rizza RA, Cobelli C. Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng. 2007;54(10):1740–1749. doi: 10.1109/TBME.2007.893506. [DOI] [PubMed] [Google Scholar]
- 23.Kovatchev BP, Breton M, Man CD, Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol. 2009;3(1):44–55. doi: 10.1177/193229680900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan A, Breton MD, Kovatchev BP. Effects of pulsatile subcutaneous injections of insulin lispro on plasma insulin concentration levels. J Diabetes Sci Technol. 2008;2(5):844–852. doi: 10.1901/jaba.2008.2-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke W, Kovatchev B. Statistical tools to analyze continuous glucose monitor data. Diabetes Technol Ther. 2009;11(Suppl 1):S45–S54. doi: 10.1089/dia.2008.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]