Abstract
Aims
While there has been much debate about the clinical importance of glycemic variation (GV), little attention has been directed to the properties of data sets from which it is measured. The purpose of this study is to assess the minimum frequency of glucose measurements from which GV can be consistently and meaningfully measured.
Methods
Forty-eight 72 h continuous glucose monitoring traces from children with type 1 diabetes were assessed. Measures of GV included standard deviation (SD), mean amplitude of glycemic excursion (MAGE), and continuous overlapping net glycemic action (CONGA1–4). Measures of GV calculated using 5 min sampling were designated as the 100% or “best estimate” value. Calculations were then repeated for each patient using glucose values spaced at increasing intervals. For each of the specified sampling frequencies, the ratio (%) of the between-subject SD based on the reduced subset of data to the estimate of the SD based on the full 5 min sampling data set was calculated.
Results
As the interval between observations increased, so did the variability of the estimators of GV. Standard deviation exhibited the least systematic change at all measurement intervals, and MAGE exhibited the greatest systematic change.
Conclusions
In patients with type 1 diabetes, GV as measured by SD or CONGA4, becomes unreliable if observations are more than 2–4 h apart, and estimates of MAGE become unreliable if glucose measurements are more than 1 h apart. MAGE is more unstable and prone to random measurement error than either SD or CONGA. The frequency of glycemic measurements is thus pivotal when selecting a parameter for measurement of GV.
Keywords: continuous glucose monitoring, continuous overlapping net glycemic action, glucose profiles, glycemic variability, mean amplitude of glycemic excursion, standard deviation, statistical experimental design
Introduction
There is currently debate as to the clinical significance of glycemic variation (GV) in determining risk of cellular damage and microvascular pathology.1,2 The debate has been complicated due to use of multiple measures of disease outcome, differing measures of GV, and different experimental designs and the nature of the data sets from which GV has been estimated. The range of data sets reported varies from 5 min continuous glucose monitoring (CGM) described by Monnier and colleagues3 to 7-point glucose measures used in the Diabetes Control and Complications Trial described by Kilpatrick and associates4 and 70 measurements over 4 weeks (an average of 2.5 measures per day) reported by Bragd and coworkers.5 While there has been some discussion as to the optimal metric of GV, there has been virtually no discussion as to what constitutes a minimal data set from which GV can be meaningfully assessed. The purpose of this study is to assess the minimum frequency of glucose measurements from which GV can be consistently and meaningfully measured.
Methods
Forty-eight CGM traces were chosen from our clinical research data set of primary-school-aged patients with type 1 diabetes receiving twice daily free-mixed insulin regimens (ages 4.3 to 10.3 years, mean duration of diabetes 3.5 years, mean hemoglobin A1c 8.1%). Seventy-two-hour CGM traces were obtained using the Minimed® CGMS (Northridge, CA) system on each patient. Each CGMS trace was calibrated by a minimum of four capillary blood glucose measurements per 24 h. In order for a data set to be included, a calibration had to be performed at least once every 8 h. Data cleaning entailed confirmation of calibration frequency, identification of errors with paired sensor values, and review of missing data points and was performed using Stata™ statistical software.
The measures of glycemic variability examined included the standard deviation (SD) of all glucose values in the series for a given patient; continuous overlapping net glycemic action (CONGAn) at n = 1, 2, and 4 h;6 and mean amplitude of glycemic excursion (MAGE) as proposed by Service and colleagues.7 All five measures of glycemic variability were calculated for each patient using all data (5 min sampling). These values were designated as the 100% or “best estimate” value. The calculations were then repeated for each patient, using glucose values spaced at 10, 15, 20, 30, 60, …, 240 min. The corresponding values obtained using 10–240 min sampling for each subject were expressed as a percentage of the “best estimate” value calculated using the 5 min sampling. The SD of the ratio of the estimate based on the reduced subset of data to the estimate based on the full 5 min sampling data set (expressed as a percentage) was then calculated for each of the specified sampling frequencies. The SD of these percentages, which can be regarded as a percentage of error in the estimate of the glycemic variability relative to the “best estimate,” was plotted against the sampling frequency and the relationship fitted with a least-squares linear regression line using log–log regression [this corresponds to a power function relationship, percentage error in estimate of GV = a*(sampling frequency)b, where b is the slope of the log–log plot.] Only a subset of the possible spacings provided data sets in which measurements were available at exactly 1, 2, or 4 h intervals as required by the definition of CONGA1–CONGA4. The MAGE was calculated by a new automated algorithm designed by Peter A. Baghurst to locate all the peaks and nadirs in each CGM data set (and its subsets) according to the rules defined by Service and colleagues7 with two minor modifications: the SD required to determine whether a glycemic excursion was eligible to be included in MAGE was estimated from each subject’s entire 72 h CGM data set—and not recalculated for each 24 h period—and only the magnitudes of upward excursions were averaged.8
Results
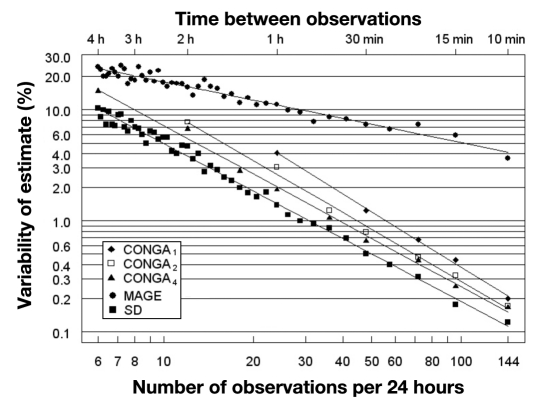
As the interval between consecutive observations increases, so does the variability of all the estimators of glycemic variability (Figure 1). Table 1 shows the coefficients of variation of the various measures of GV as the interval of glucose data points increases from 1 to 4 h, utilizing the values from the fitted lines in Figure 1. For SD, the variability of estimates (expressed as a percentage) is 10.2% of the estimate based on 288 glucose values per day when the observations are 4 h apart. For CONGA4, the percentage error is 15.1% with 4 h spacing, falling to 5.5% with 2 h spacing, and CONGA2 shows a larger percentage random error than CONGA4 (7.7% at 2 h spacing of glucose measurements), while CONGA1 is subject to more random error than either CONGA4 or CONGA2 [at the maximum spacing that can be used to generate all three measures (1 h), the percentage errors for CONGA4, CONGA2, and CONGA1 were 2, 2.6, and 4.1%, respectively]. A dramatically larger random error was observed for MAGE. In order for the percentage error for MAGE to remain less than 10% (relative to the “best” estimate based on all data at 5 min sampling), glucose values must be measured at least once per hour. The percentage error in the estimate of MAGE increases rapidly to 23.5% as the interval between glucose data points increases from 1 to 4 h.
Figure 1.
Pooled data from CGMS traces of 48 patients with type 1 diabetes plotting error of estimate of GV against the interval of glycemic data points. A plot of the variability of estimates of the MAGE, CONGA1,2,4, and SD as observations are progressively omitted from a full 72 h CGM data set containing measurements made at 5 min intervals. The variability is expressed as a coefficient of variation relative to the estimate obtained from the full CGM data set.
Table 1.
Percentage Error in Measures of Glycemic Variability According to the Spacing between Glucose Measurements
| Measure of GV | Spacing between successive glucose values | ||
|---|---|---|---|
| 1 h | 2 h | 4 h | |
| SD | 1.4 | 3.8 | 10.2 |
| CONGA4 | 2.0 | 5.5 | 15.1 |
| CONGA2 | 2.6 | 7.7 | not applicable |
| CONGA1 | 4.1 | not applicable | not applicable |
| MAGE | 11.0 | 16.1 | 23.5 |
Discussion
In patients with type 1 diabetes, the GV as measured by SD or CONGA4, becomes unreliable if observations are more than 2–4 hours apart, and estimates of MAGE become unreliable if glucose measurements are more than 1 h apart. The MAGE is more unstable and prone to measurement error than either SD or CONGA. MAGE, by definition, is based on “distances” between peaks and nadirs. When using CGMS data and ignoring every second observation, half the maxima and half the minima will be lost by chance alone, so that the graphical pattern of excursions is likely to remain nearly the same, but excursions will be slightly smaller in magnitude. As the interval between glucose measure-ments is further increased, the pattern of peaks and nadirs in glucose may change significantly, and therefore MAGE is inevitably more unreliable as the interval between glucose values increases. At 4 h sampling (six observations per day) the percentage error for MAGE is 2.5-fold larger than the percentage error for SD. As sampling frequency increases (reading left to right in Figure 1), the percentage error in MAGE is reduced much more slowly than for SD or CONGA1–CONGA4. The slope of the log–log relationship in Figure 1 is -0.56 for MAGE, which is close to the theoretically derived value (-0.5) when dealing with independent Gaussian-distributed observations. In contrast, the slope of the log– log relationship for SD and CONGA4 is -0.72, which is significantly steeper. This may be explained by the fact that sequential CGM glucose measurements show a highly significant positive autocorrelation, and as sampling frequency decreases, the autocorrelation fades to zero, resulting in a more rapid change in the percentage error of the estimate.
An uncritical use of GV metrics and glycemic data sets has thus far been used as a basis for the debate as to the significance of GV in microvascular and cellular damage in diabetes.9 A meta-analysis concluded that, while there is evidence of an association between GV and microvascular complications in type 2 diabetes, this has not been found consistently in type 1 patients.10 This conclusion may simply be a reflection of the different parameters used to characterize glycemic variability. Only three out of eight studies of type 1 diabetes patients reviewed by Nalysnyk and associates10 measured glucose at least once every 2 h. The validity of comparing 7-point glucose profiles with CGM when using MAGE4 must be questioned in view of the findings presented in Figure 1. Our analyses imply that 7-point glucose profiles (or 5-point glucose profiles in the case of the Diabetes Control and Complications Trial11) are expected to have an unacceptably large level of random error. However, the present study did not directly compare CGM with glucose profiles. It is possible that 5- or 7-point glucose profiles, as conventionally employed, may behave differently than five or seven glucose values obtained at constant intervals throughout the day.
This study is the first to demonstrate the importance of the frequency of glucose measurements in determining the reliability of measures of GV. It appears that glucose measurements must be no more than 2–4 hours apart in order for GV to be consistently assessed by SD, CONGA4, CONGA2, or hourly in the case of MAGE. This calls into question the validity of some of the previous studies that have investigated the pathophysiological significance of GV in type 1 diabetes. Further studies are needed to compare the consistency of measures of GV using CGM and self-monitoring of blood glucose simultaneously on the same subject subjected to the same protocol and to evaluate the reliability of results based on the use of 5-, 7-, or 8-point glucose profiles vis a vis CGM. Despite this caveat, we conclude that there is a major advantage to the use of either SD (also designated SDT)12 or CONGA16 in preference to MAGE.7
Abbreviations
- (CGM)
continuous glucose monitoring
- (CONGA)
continuous overlapping net glycemic action
- (MAGE)
mean amplitude of glycemic excursion
- (SD)
standard deviation
References
- 1.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295(14):1707–1708. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 2.Kilpatrick ES, Rigby AS, Atkin SL. For debate—after Ceriello Glucose variability and diabetes complication risk: we need to know the answer. Diabet Med. doi: 10.1111/j.1464-5491.2010.02929.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 4.Kilpatrick ES, Rigby AS, Atkin SL. Effect of glucose variability on the long-term risk of microvascular complications in type 1 diabetes. Diabetes Care. 2009;32:1901–1903. doi: 10.2337/dc09-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bragd J, Adamson U, Bäcklund LB, Lins PE, Moberg E, Oskarsson P. Can glycaemic variability, as calculated from blood glucose self-monitoring, predict the development of complications in type 1 diabetes over a decade? Diabetes Metab. 2008;34((6 Pt 1)):612–616. doi: 10.1016/j.diabet.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell CM, Donath SM, Vidmar SI, Werther GA, Cameron FJ. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7(2):253–263. doi: 10.1089/dia.2005.7.253. [DOI] [PubMed] [Google Scholar]
- 7.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 8.Baghurst P. Calculating the mean amplitude of glycemic excursion (MAGE) from continuous glucose monitoring data: an automated algorithm. Diabetes Technol Ther. 2010 doi: 10.1089/dia.2010.0090. (Forthcoming.) [DOI] [PubMed] [Google Scholar]
- 9.Cameron FJ, Donath SM, Baghurst PA. Measuring glycaemic variation. Curr Diabetes Rev. 2010;6(1):17–26. doi: 10.2174/157339910790442592. [DOI] [PubMed] [Google Scholar]
- 10.Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. 2010;12(4):288–298. doi: 10.1111/j.1463-1326.2009.01160.x. [DOI] [PubMed] [Google Scholar]
- 11.Siegelaar SE, Kilpatrick ES, Rigby AS, Atkin SL, Hoekstra JB, Devries JH. Glucose variability does not contribute to the development of peripheral and autonomic neuropathy in type 1 diabetes: data from the DCCT. Diabetologia. 2009;52(10):2229–2232. doi: 10.1007/s00125-009-1473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodbard D. New approaches to display of self-monitoring of blood glucose data. J Diabetes Sci Technol. 2009;3(5):1121–1127. doi: 10.1177/193229680900300515. [DOI] [PMC free article] [PubMed] [Google Scholar]