Abstract
Background
Current continuous glucose monitoring (CGM) systems measure glucose levels in the interstitial fluid to estimate blood glucose concentration. A lag time has been observed between CGM system glucose readings and blood glucose levels when glucose levels are changing. Although this lag has been attributed to the time it takes glucose to equilibrate between blood and interstitial fluid compartments, it is unclear to what extent these inaccuracies reflect an intrinsic delay of the device itself.
Methods
Four Guardian® REAL-Time CGM systems (CGMSs) (Medtronic Diabetes, Minimed, CA) and eight glucose sensors were tested in glucose solutions prepared in Krebs bicarbonate buffers at 37 °C. Glucose readings obtained from CGMSs were compared with actual glucose concentrations during controlled changes in glucose concentration performed at four rates (30, 90, and 220 mg/dl/hr-1 and an instantaneous change of 110 mg/dl) using a linear gradient maker.
Results
Irrespective of the rate and direction of changes in glucose concentration, the readings obtained from CGMSs were significantly different from actual glucose levels. The faster the rise or fall in actual glucose concentration, the more pronounced the mismatch with CGMS glucose readings. Furthermore, the intrinsic lag times (8.3 to 40.1 min) were high enough to account for the lags reported in previous in vivo studies.
Conclusions
The lag intrinsic of the CGMS may make a significant contribution to the mismatch between CGM system readings and blood glucose concentrations.
Keywords: continuous glucose monitoring, intrinsic lag
Introduction
The introduction of continuous glucose monitoring (CGM) systems offers patients with diabetes an advantage over conventional self-monitoring of blood glucose by providing comprehensive blood glucose profiles without the need for numerous invasive finger-stick tests. These monitors use a glucose sensor inserted subcutaneously that estimates blood glucose from interstitial fluid glucose levels. The rationale for using such estimates is based on the observation that interstitial glucose concentrations match blood glucose levels under steady state conditions because glucose is capable of free diffusion across capillary epithelium from the blood to the interstitial compart-ment.1,2 It must be stressed, however, that although some investigators have reported no delay between blood glucose and interstitial fluid glucose levels when blood glucose levels are changing,3–8 others have reported a delay in interstitial glucose readings following blood glucose changes, regardless of whether blood glucose is rising or falling.9-11
These differences in blood and interstitial fluid glucose levels are believed to contribute to inaccuracies in glucose sensor readings.10–16 There is also evidence that the magnitude of the delay between CGM system readings and blood glucose readings may depend on the direction14,16 or rate17,18 of change in blood glucose level, with some proposing that the association between glucose in blood and interstitial fluid compartments may follow a pattern explained by a push-pull mechanism.12,16,19 However, others have argued against the existence of this phenomenon,4,7 with the delays between CGM system readings and blood glucose levels proposed to be caused by a number of factors including diffusion rate of glucose across capillary epithelium, tissue blood flow at the site of glucose sensor insertion, prevailing insulin levels, and the rate and direction of change in plasma glucose. For instance, Stout and colleagues20 demonstrated that a physiological lag could be mitigated by increasing local blood perfusion. It is also possible that the lag described above may not necessarily reflect biological factors but may be a product of the method of glucose sampling and experimental conditions such as the interstitial glucose measurement technique used and the methods adopted to investigate the relationship between blood and interstitial glucose concentrations.4
The possibility that the sensors themselves could contribute to the discrepancy between estimated and actual blood glucose values and contribute significantly to the assumed physiological lag has been raised by some investigators12,21,22 but not examined. That such a lag may be involved is supported by the 1980s work of Baker and Gough,23–25 who studied continuous biosensors developed in their laboratories and reported that an intrinsic lag was attributable to the glucose sensors themselves. However, the intrinsic lag of current commercially available glucose sensors remains to be evaluated. Intrinsic lag playing an important role is suggested by the recent observation that sensors worn concurrently show a time difference of 6.7 + 5.1 min, thus suggesting that sensor variation per se may account for some of the lag time between blood and interstitial fluid glucose concentrations.9 The issue of whether sensors themselves could contribute to the lag between glucose readings and glucose level has not been thoroughly examined before, thus, this study explores the potential contribution of an intrinsic lag of Medtronic CGM systems (CGMSs) to increases and decreases in glucose concentration performed at variable rates in vitro.
Methods
Continuous Glucose Monitoring Systems
Four Guardian® REAL-Time (RT) CGMSs (Medtronic Diabetes, Northridge, CA) were used in this investigation. The units incorporate a needle-type glucose sensor with an enzyme-based electrode designed to measure inter-stitial fluid glucose concentration for up to 3 days.26,27 Once calibrated, these CGMSs provide real-time glucose readings updated every 5 min and store all glucose data in memory that can later be downloaded for analysis.
Procedures
Prior to testing, all four CGMSs were calibrated simulta-neously as described by the manufacturer in a 144 mg/dl glucose solution prepared in a Krebs bicarbonate buffer solution kept at 37 °C. In order to investigate the response of these CGMSs to changes in glucose concentration, a gradient maker was used to generate different rates of linear changes in glucose concentrations. Before the beginning of a linear change in glucose concentration, the glucose sensors recorded continuous data for 1 h. Then the sensors recorded continuous glucose data while glucose levels were allowed to change at a rate determined by the speed of the pump. The rates of change in glucose concentration (30, 90, and 220 mg/dl/hr-1 and an instantaneous change of 110 mg/dl) were chosen to approximate those used in previous studies in humans in order to allow for comparisons between studies.3,8,28 Once the desired glucose concentration was reached, the pump was turned off and the sensors remained immersed in the solution for a further 60 min. At timed intervals, small samples (70 µl) were drawn from the solution and immediately frozen in liquid nitrogen before being stored at -80 °C until analyzed. The samples were later used for glucose assays, as described in Bergmeyer.29
Data Analysis
The CGMS data were analyzed using MiniMed Solutions CGMS Sensor Software version 3.0A (Medtronic MiniMed, Northridge, CA) and glucose buffer solution concentrations were determined as mentioned above. Intrinsic lag times were determined by calculating the amount of time required for CGMS glucose readings to match glucose levels when the levels had risen or fallen by ¼ (t1/4), ½ (t1/2), and ¾ (t3/4) of the absolute change in glucose concentration, as reported by Steil and colleagues.10 Furthermore, error grid analyses were used to determine whether clinically relevant errors in CGMS readings could be predicted on the basis of rate and direction of change in glucose concentration.30,31 The CGMSs and corresponding glucose solution concentrations were compared using two-way analysis of variance (ANOVA) with repeated measures and Fisher’s least significant difference (LSD) post hoc analysis. All data are expressed as mean ± standard deviation (SD).
Results
Comparison of CGMS Readings and Actual Glucose Concentrations
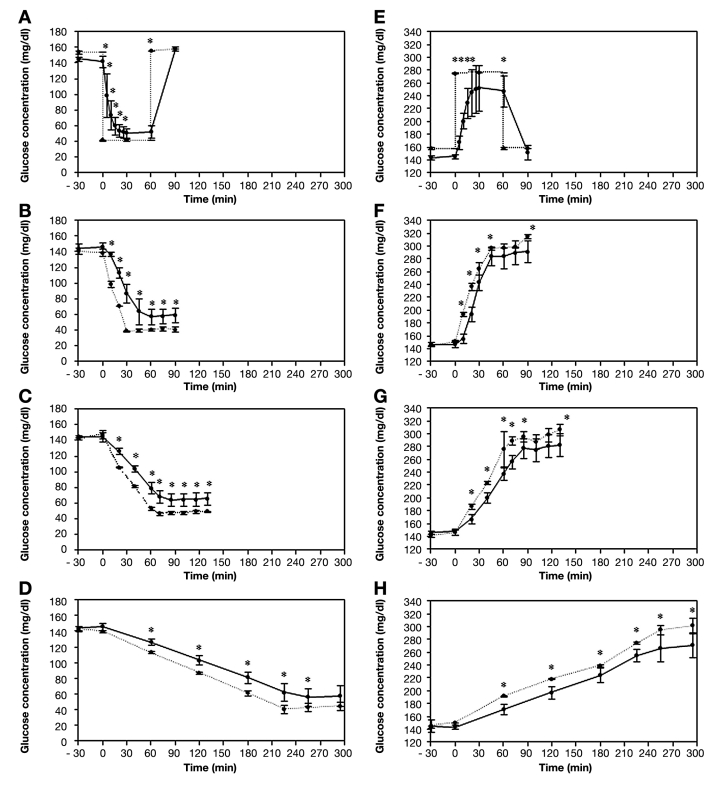
The relationship between CGMS readings and actual glucose concentrations in response to varying rates of change in glucose concentrations differed. First, sensor glucose readings overestimated glucose concentrations during falls in glucose levels and underestimated glucose concentrations during rises in glucose levels, irrespective of the rate of change in these levels. Second, when glucose reached stable levels after fast or moderate rates of fall, the differences between CGMS readings and glucose concentration in the buffer remained significant despite no further decline in glucose levels. This was also observed following increases in glucose concentration at all rates (Figure 1). The lag times ranged from 8.3 to 40.1 min, depending on the rate and direction of the change in glucose concentration (Table 1).
Figure 1.
CGMS readings (solid lines) and corresponding glucose buffer solution concentrations (dashed lines) in response to (A) an instantaneous, (B) rapid (220 mg/dl/hr-1), (C) moderate (90 mg/dl/hr-1), and (D) slow (30 mg/dl/hr-1) rate of fall in glucose concentration and (E) an instantaneous, (F) rapid (220 mg/dl/hr-1), (G) moderate (90 mg/dl/hr-1), and (H) slow (30 mg/dl/hr-1) rise in glucose concentration. Results are expressed as mean ± SD. *Significant difference between CGMS and glucose buffer concentrations (p ≤ .01).
Table 1.
Intrinsic Lag Times Calculated at Various Rates of Change in Glucose Concentration and for Magnitude of Change in Glucose Levels
| Rate (mg/dl/hr-1) | Lag time | ||
|---|---|---|---|
| Glucose falling (min) | Glucose rising (min) | ||
| Rapid (220) | t1/4 | 12.7 | 9.3 |
| t1/2 | 15.1 | 8.3 | |
| t3/4 | 16.4 | 7.4 | |
| Moderate (90) | t1/4 | 8.3 | 5.4 |
| t1/2 | 13.6 | 10.6 | |
| t3/4 | 18.9 | 15.8 | |
| Slow (30) | t1/4 | 30.0 | 30.2 |
| t1/2 | 40.1 | 34.7 | |
| t3/4 | 50.2 | 39.2 | |
Effect of Rate of Change in Glucose Concentration on Sensor Accuracy
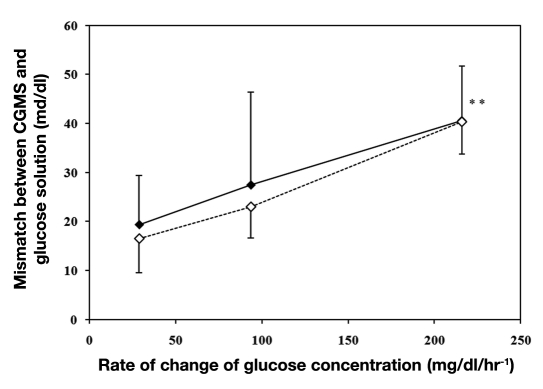
A plot of the average mismatch between sensor readings and glucose concentration against the rate of change in glucose concentration (Figure 2) shows that the mismatch between CGMSs and actual glucose readings increased at faster rates of change, irrespective of the direction of change, and was on average ∼40 mg/dl for faster rates and ∼20 mg/dl for slower rates.
Figure 2.
Average mismatch between CGMS and glucose solution concentrations during increases (♦ and solid line) and decreases (♢ and dashed line) in glucose concentrations at slow (30 mg/dl/hr-1), moderate (90 mg/dl/hr-1), and rapid (220 mg/dl/hr-1) rates of change in glucose concentration. Results are expressed as mean ± SD. (∗)Significantly different to moderate and slow rates (p ≤ .05).
Error Grid Analysis
Error grid analysis was performed on all data points during decreases and increases in glucose concentration to assess the clinical significance of the in vitro inaccuracies of the CGMSs. For all paired data points, 82% of values fell within the clinically acceptable zones A and B of the Clarke error grid, and 18% fell within zone D when glucose concentration was falling. However, 100% of values fell within zones A and B of the Clarke error grid during a rise in glucose concentration.
Discussion
The efficacy of CGMSs in estimating blood glucose levels is limited by the presence of a time lag between CGMS readings and changing blood glucose levels. This study examines whether this lag could be attributed, at least in part, to the CGMS itself, and it shows that under conditions when glucose concentrations are changing at physiological rates in vitro, an intrinsic lag time limits the accuracy of glucose readings obtained by the Guardian RT CGMS. This lag between CGMS readings and actual glucose levels occurs irrespective of the direction and rate of change in glucose levels. When glucose levels are decreasing, glucose readings from CGMSs overestimate true glucose by approximately 20 to 40 mg/dl, but underestimate glucose concentration to a similar extent when glucose levels are increasing. Furthermore, when glucose readings stabilize following a fall or a rise in glucose concentration, glucose readings from CGMSs overestimate and underestimate true glucose concentration, respectively. In the context of using CGMSs to achieve glycemic targets, prevent hypoglycemia, and close the loop between blood glucose monitoring and insulin delivery, these are important findings because they indicate that future research aimed at improving the accuracy of CGMSs should target not only the physiological lag between interstitial and blood glucose levels, but also the lag intrinsic to the CGMSs themselves.
It is important to note that the mismatch between CGMSs and glucose levels in the testing solution is not due to a progressive deterioration in the calibration of the unit. When the sensors were subjected to a fall or rise in glucose concentration following initialization and calibration in a 144 mg/dl solution (Figures 1A and 1E), they provided accurate readings of the 144 mg/dl solution when reimmersed into the original solution, indicating that the sensors maintained their calibration state following a change in glucose concentration.
Our results indicate that the rate of change in glucose concentration affects the mismatch between CGMS readings and actual glucose concentrations (Figure 2). In general, when glucose is decreasing or increasing at a fast rate, the mismatch of any glucose concentration is more pronounced than at slow and moderate rates. This observation is in agreement with in vitro tests performed by others,25 although that study used glucose biosensors manufactured with 1980s technology. The mismatch is more pronounced as the rate of change increases, which suggests that the ability of the CGMSs to estimate true glucose accurately becomes increasingly limited under these conditions. Unfortunately, the effect of the rate of change in glucose concentration on the difference between CGMS readings and actual glucose levels does not seem to be predictable in a way that the mismatch could be anticipated based solely on the rate of change in actual glucose concentration. This is partly due to the large variability in the mismatches measured at these rates. The mismatch between glucose readings from CGMSs and true glucose concentration is of concern, particularly in the hypoglycemic range, where CGMS time lag causes CGMS glucose readings to deviate from actual glucose levels by more than 40 mg/dl in response to rapid rates of decline in glucose concentration.
Our findings also identify the limitations of using error grid analysis to evaluate the efficacy of CGM systems. Indeed, this analysis revealed that 100% of CGMS readings fell within the clinically acceptable zones A and B when glucose was rising, irrespective of the rate, whereas 82% of CGMS readings fell within zones A and B when glucose was decreasing. These apparently acceptable results tend to obscure the fact that CGMS readings overestimate blood glucose concentration during a fall in blood glucose level to such an extent that this could result in failure of the CGMS to accurately detect and prevent hypoglycemia.
Interestingly, when glucose concentration is changing, the lag time attributable to the CGMS itself is of a magnitude comparable to or greater than those reported to occur between CGMS glucose readings of human subjects and actual blood glucose levels. Indeed, the calculated lag times here range between 8 and 40 min during falls and rises in glucose concentration at slow, moderate, and fast rates (Table 1). These lag times are comparable with the 4 to 27 min lag times reported in studies using CGM systems in human subjects.9–11,22 It is important to note, however, that these comparisons are made difficult by the fact that the method used to calculate lag times varies to some degree across studies.
Clearly our results suggest that the well documented lag times between CGM system glucose and blood glucose readings when blood glucose levels are changing may be explained to a large extent by the inertia of the CGMS itself rather than by a physiological lag. However, this does not necessarily imply that a physio-logical lag does not make a significant contribution to the mismatch between blood glucose levels and interstitial fluid glucose levels because there is evidence that physiological differences exist.22,32 Although the relative contribution of physiological and intrinsic lags remains to be established, one should not assume that their effects are necessarily additive. Potentially there are conditions when the intrinsic lag of the CGM systems might even contribute to a better match between interstitial fluid glucose readings and estimates of blood glucose concentration. For instance, this might be the case when an insulin-mediated fall in interstitial glucose concentration precedes a fall in blood glucose levels when insulin causes a decrement in interstitial fluid glucose prior to a matching decrement in blood glucose levels.14 Under these conditions, it is possible that an intrinsic delay in sensor readings of interstitial fluid glucose concentrations could more closely approximate changes in blood glucose levels, particularly if the intrinsic lag matches the physiological lag. This may explain, in part, why some studies have reported no lag in vivo. 3–8
Conclusions
This study shows that the accuracy of readings obtained by the Guardian RT CGMS is limited by an intrinsic lag, the magnitude of which is affected by the rate of change in glucose concentration. Such an intrinsic lag of implantable glucose sensors could have an important impact on closed-loop systems, not only in controlling postprandial blood glucose levels but also in preventing hypoglycemia. However, it is uncertain how much this lag contributes to the error of CGMS estimates of blood glucose level in situ because the lag may even be beneficial by providing more accurate estimates of blood glucose levels under some conditions. The extent to which the performance of other CGM system models is affected by such a lag also remains to be determined. It is clear, however, that any research aimed at predicting or decreasing the lag between CGM glucose readings and blood glucose level readings must take into consideration both the intrinsic lag of the device and the physiological lag.
Acknowledgments
The authors thank Medtronic Diabetes, which supplied the Guardian units and glucose sensors for this study.
Abbreviations
- (CGM)
continuous glucose monitoring
- (CGMS)
MimiMed Guardian® REAL-Time continuous glucose monitoring system
- (RT)
REAL-Time
- (SD)
standard deviation
References
- 1.Koschinsky T, Jungheim K, Heinemann L. Glucose sensors and the alternate site testing-like phenomenon: relationship between rapid blood glucose changes and glucose sensor signals. Diabetes Technol Ther. 2003;5(5):829–842. doi: 10.1089/152091503322527030. [DOI] [PubMed] [Google Scholar]
- 2.Lönnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987;253((2 Pt 1)):E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- 3.Caplin NJ, O’Leary P, Bulsara M, Davis EA, Jones TW. Subcutaneous glucose sensor values closely parallel blood glucose during insulin-induced hypoglycaemia. Diabet Med. 2003;20(3):238–241. doi: 10.1046/j.1464-5491.2003.00837.x. [DOI] [PubMed] [Google Scholar]
- 4.Rebrin K, Steil GM, van Antwerp WP, Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am J Physiol. 1999;277(3):E561–E571. doi: 10.1152/ajpendo.1999.277.3.E561. [DOI] [PubMed] [Google Scholar]
- 5.Thennadil SN, Rennert JL, Wenzel BJ, Hazen KH, Ruchti TL, Block MB. Comparison of glucose concentration in interstitial fluid, and capillary and venous blood during rapid changes in blood glucose levels. Diabetes Technol Ther. 2001;3(3):357–365. doi: 10.1089/15209150152607132. [DOI] [PubMed] [Google Scholar]
- 6.Wentholt IM, Vollebregt MA, Hart AA, Hoekstra JB, DeVries JH. Comparison of a needle-type and a microdialysis continuous glucose monitor in type 1 diabetic patients. Diabetes Care. 2005;28(12):2871–2876. doi: 10.2337/diacare.28.12.2871. [DOI] [PubMed] [Google Scholar]
- 7.Wentholt IM, Hart AA, Hoekstra JB, Devries JH. Relationship between interstitial and blood glucose in type 1 diabetes patients: delay and the push-pull phenomenon revisited. Diabetes Technol Ther. 2007;9(2):169–175. doi: 10.1089/dia.2006.0007. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm B, Forst S, Weber MM, Larbig M, Pfützner A, Forst T. Evaluation of CGMS during rapid blood glucose changes in patients with type 1 diabetes. Diabetes Technol Ther. 2006;8(2):146–155. doi: 10.1089/dia.2006.8.146. [DOI] [PubMed] [Google Scholar]
- 9.Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003;52(11):2790–2794. doi: 10.2337/diabetes.52.11.2790. [DOI] [PubMed] [Google Scholar]
- 10.Steil GM, Rebrin K, Mastrototaro J, Bernaba B, Saad MF. Determination of plasma glucose during rapid glucose excursions with a subcutaneous glucose sensor. Diabetes Technol Ther. 2003;5(1):27–31. doi: 10.1089/152091503763816436. [DOI] [PubMed] [Google Scholar]
- 11.Steil GM, Rebrin K, Hariri F, Jinagonda S, Tadros S, Darwin C, Saad MF. Interstitial fluid glucose dynamics during insulin-induced hypoglycaemia. Diabetologia. 2005;48(9):1833–1840. doi: 10.1007/s00125-005-1852-x. [DOI] [PubMed] [Google Scholar]
- 12.Aussedat B, Dupire-Angel M, Gifford R, Klein JC, Wilson GS, Reach G. Interstitial glucose concentration and glycemia: implications for continuous subcutaneous glucose monitoring. Am J Physiol Endocrinol. Metab. 2000;278(4):E716–E728. doi: 10.1152/ajpendo.2000.278.4.E716. [DOI] [PubMed] [Google Scholar]
- 13.Kulcu E, Tamada JA, Reach G, Potts RO, Lesho MJ. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 2003;26(8):2405–2409. doi: 10.2337/diacare.26.8.2405. [DOI] [PubMed] [Google Scholar]
- 14.Sternberg F, Meyerhoff C, Mennel FJ, Mayer H, Bischof F, Pfeiffer EF. Does fall in tissue glucose precede fall in blood glucose? Diabetologia. 1996;39(5):609–612. doi: 10.1007/BF00403309. [DOI] [PubMed] [Google Scholar]
- 15.Tamada JA, Garg S, Jovanovic L, Pitzer KR, Fermi S, Potts RO. Noninvasive glucose monitoring: comprehensive clinical results. Cygnus Research Team. J Am Med Assoc. 1999;282(19):1839–1844. doi: 10.1001/jama.282.19.1839. [DOI] [PubMed] [Google Scholar]
- 16.Thomé-Duret V, Reach G, Gangnerau MN, Lemonnier F, Klein JC, Zhang Y, Hu Y, Wilson GS. Use of a subcutaneous glucose sensor to detect decreases in glucose concentration prior to observation in blood. Anal Chem. 1996;68(21):3822–3826. doi: 10.1021/ac960069i. [DOI] [PubMed] [Google Scholar]
- 17.Jansson PA, Fowelin J, Smith U, Lönnroth P. Characterization by microdialysis of intracellular glucose level in subcutaneous tissue in humans. Am J Physiol. 1988;255((2 Pt 1)):E218–E220. doi: 10.1152/ajpendo.1988.255.2.E218. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein RL, Schwartz SL, Brazg RL, Bugler JR, Peyser TA, McGarraugh GV. Accuracy of the 5-day FreeStyle Navigator Continuous Glucose Monitoring System: comparison with frequent laboratory reference measurements. Diabetes Care. 2007;30(5):1125–1130. doi: 10.2337/dc06-1602. [DOI] [PubMed] [Google Scholar]
- 19.Monsod TP, Flanagan DE, Rife F, Saenz R, Caprio S, Sherwin RS, Tamborlane WV. Do sensor glucose levels accurately predict plasma glucose concentrations during hypoglycemia and hyperinsulinemia? Diabetes Care. 2002;25(5):889–893. doi: 10.2337/diacare.25.5.889. [DOI] [PubMed] [Google Scholar]
- 20.Stout PJ, Racchini JR, Hilgers ME. A novel approach to mitigating the physiological lag between blood and interstitial fluid glucose measurements. Diabetes Technol Ther. 2004;6(5):635–644. doi: 10.1089/dia.2004.6.635. [DOI] [PubMed] [Google Scholar]
- 21.Choleau C, Dokladal P, Klein JC, Ward WK, Wilson GS, Reach G. Prevention of hypoglycemia using risk assessment with a continuous glucose monitoring system. Diabetes. 2002;51(11):3263–3273. doi: 10.2337/diabetes.51.11.3263. [DOI] [PubMed] [Google Scholar]
- 22.Regittnig W, Ellmerer M, Fauler G, Sendlhofer G, Trajanoski Z, Leis HJ, Schaupp L, Wach P, Pieber TR. Assessment of transcapillary glucose exchange in human skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2003;285(2):E241–E251. doi: 10.1152/ajpendo.00351.2002. [DOI] [PubMed] [Google Scholar]
- 23.Baker DA, Gough DA. Dynamic concentration challenges for biosensor characterization. Biosens Bioelectron. 1993;8((9-10)):433–441. doi: 10.1016/0956-5663(93)80028-n. [DOI] [PubMed] [Google Scholar]
- 24.Baker DA, Gough DA. Comments on the relationship between time lag and dynamic delay in diffusion-reaction systems. J Phys Chem. 1994;98(50):13432–13433. [Google Scholar]
- 25.Baker DA, Gough DA. Dynamic delay and maximal dynamic error in continuous biosensors. Anal Chem. 1996;68(8):1292–1297. doi: 10.1021/ac960030d. [DOI] [PubMed] [Google Scholar]
- 26.Mastrototaro J. The MiniMed Continuous Glucose Monitoring System (CGMS) J Pediatr Endocrinol Metab. 1999;12(Suppl 3):751–758. [PubMed] [Google Scholar]
- 27.Mastrototaro JJ. The MiniMed continuous glucose monitoring system. Diabetes Technol Ther. 2000;2(Suppl 1):S13–S18. doi: 10.1089/15209150050214078. [DOI] [PubMed] [Google Scholar]
- 28.Eckert B, Ryding E, Agardh CD. The cerebral vascular response to a rapid decrease in blood glucose to values above normal in poorly controlled type 1 (insulin-dependent) diabetes mellitus. Diabetes Res Clin Pract. 1995;27(3):221–227. doi: 10.1016/0168-8227(95)01052-f. [DOI] [PubMed] [Google Scholar]
- 29.Bergmeyer HU. Methods of Enzymatic Analysis. 2nd ed. New York: Academic Press; 1974. [Google Scholar]
- 30.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 31.Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143–1148. doi: 10.2337/diacare.23.8.1143. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt FJ, Sluiter WJ, Schoonen AJ. Glucose concentration in subcutaneous extracellular space. Diabetes Care. 1993;16(12):695–700. doi: 10.2337/diacare.16.5.695. [DOI] [PubMed] [Google Scholar]