Abstract
Background
The objective of this study was to examine whether setting the low glucose alarm of a Guardian® REAL-Time continuous glucose monitoring system (CGMS) to 80 mg/dl for 3 days and providing instructions to users reduce the risk of hypoglycemia under free-living conditions in individuals with type 1 diabetes mellitus (T1DM).
Methods
Fourteen participants with T1DM aged 26.1 ± 6.0 years (mean ± standard deviation) were fitted with a CGMS and assigned for 3 days to either an alarm [low and high blood glucose (BG) alarms set at 80 and 200 mg/dl, respectively] or no alarm condition, with each treatment administered to all participants following a counterbalanced design. All participants were given detailed instructions on how to respond appropriately to low glucose alarms.
Results
The CGMS with alarm reduced the incidence of hypoglycemia (CGMS readings ≤65 mg/dl) by 44% as well as the time spent below this hypoglycemic threshold by 64% without increasing average BG levels. However, the CGMS with alarm had no effect on the incidence of symptomatic hypoglycemia.
Conclusions
Short-term use of the CGMS with alarm, together with appropriate instructions for users, reduces the incidence and duration of hypoglycemia, but only to a limited extent, in part because it overestimates BG in the low glucose range.
Keywords: continuous glucose monitoring, hypoglycemia, low glucose alarm
Introduction
Continuous glucose monitoring systems (CGMSs) that provide real- time (RT) glucose values and alarms for impending hypo- and hyperglycemia have the potential to improve diabetes therapy significantly. Clinical and home-based studies have shown that CGMSs improve glycemic control and detect hypoglycemic events when used in conjunction with conventional therapy.1–8 However, despite these potential benefits, the efficacy of CGMSs in preventing hypoglycemia remains unclear. This is vital to address, as preventing even mild hypoglycemia is clinically important because the blood glucose (BG) gap between the onset of mild hypoglycemia and more severe hypoglycemia associated with neuroglucopenic and autonomic responses is so narrow (15–30 mg/dl) that mild hypoglycemia can progress to severe hypoglycemia in a matter of minutes, particularly in individuals who are physically active. Moreover, severe hypoglycemia may remain undetected in hypoglycemia-unaware individuals and have severe effects on their health.
Unfortunately, only a few randomized controlled trials have examined whether a CGMS with an appropriately set low glucose alarm could prevent hypoglycemia while keeping the proportion of false alarms to a minimum in a free-living context. In this regard, it has been reported that setting the low alarm at 70 mg/dl is useful for reducing the duration of hypoglycemic excursions, but does not reduce the incidence of hypoglycemia.9 Another study reported a reduction in time spent below 55 mg/dl when the low glucose alarm is set to 100 mg/dl, but included no data on the incidence of hypoglycemia or false alarms.4 In a subsequent study, the same group reported a 21% reduction in time spent below the threshold for hypoglycemia with low glucose alarms set to 80 and 55 mg/dl, but again included no data on the incidence of hypoglycemia or false alarms.10
The reported limited efficacy of CGMSs in reducing the incidence and duration of hypoglycemia may be related to the performance of the CGMS in the hypoglycemic range. The accuracy of CGMSs has been examined in individuals subjected to controlled hypoglycemia in the laboratory11–13 and in a free-living context.14,15 Some studies have shown acceptable clinical accuracy of CGMSs at low BG levels,15 reporting only modest discrepancies between BG levels and CGMS readings13 irrespective of BG dynamics and also during falls in BG concentration.11,16–19 However, others have reported a delay in interstitial glucose readings following a BG change, regardless of whether BG is rising or falling.20–22 Evidence also shows that the magnitude of this delay may depend on the direction23,24 or rate25 of change in BG level, with evidence of poor accuracy of CGMSs in the low BG range where CGMS readings have been reported to both overestimate and underestimate BG levels.12,26–31
To warn users of impending hypoglycemia, it follows that it would be an appropriate strategy to account for inaccuracies of CGMS readings in the low BG range by setting the low glucose alarm above the threshold for hypoglycemia but below a level that would result in an unacceptable number of false alarms. Bode and colleagues9 suggested that the low glucose alarm setting for detection of hypoglycemia should be at a threshold of ∼80 mg/dl for a 51% false alarm rate. However, this had no effect on the incidence of hypoglycemia.9 One solution to this problem is to increase the alarm setting; however, this would result in a rise in the incidence of false alarms. Alternatively, because many studies that have examined the efficacy of setting a low glucose alarm to prevent hypoglycemia share the limitation of an absence of specific instructions for participants on how to respond to low glucose alarms,32 it is possible that providing such instructions may improve the capability of CGMSs to prevent hypoglycemia. Indeed, considering that the recommended CGMS alarm of 80 mg/dl9 would result in a BG concentration relatively close to the threshold for autonomic symptoms of hypoglycemia, it would be critical that immediate action be taken once the alarm is triggered. For this reason, this study examined whether setting the BG alarm at 80 mg/dl while providing participants with RT glucose readings and stringent guidelines favoring immediate action could reduce the incidence and duration of hypoglycemia in a population of individuals with type 1 diabetes mellitus (T1DM) in a free-living context. In addition, because the efficacy of setting the low BG alarm above the hypoglycemia threshold is limited by the associated increased rate of a false alarm, another novel aspect of this study is to examine the extent to which the false alarm rate limits the efficacy of CGMS.
Research Design and Methods
Participants
Seven male and seven female participants aged 26.1 ± 6.0 (mean ± standard deviation) years, with a duration of T1DM of 9.0 ± 6.1 years, and glycated hemoglobin of 7.9 ± 1.8% volunteered for this study. None of the participants were hypoglycemia unaware and all self- monitored their BG at least three times per day as part of their usual treatment. This investigation was approved by the University of Western Australia Human Research Ethics Committee, and written informed consent was obtained from the participants.
Study Design
Participants were initially required to attend a familiari-zation session where the study procedures were described and were then given instructions on how to use their Guardian® REAL-Time CGMS (Medtronic, MiniMed, Northridge, CA). During this session, the personal glucose meter of each participant was validated and replaced if inaccurate.
All participants completed two randomly assigned monitoring periods, including a control treatment and an alarm treatment, both administered following a counter-balanced study design where half the participants were exposed to the alarm treatment first and the other half to the control condition. Each treatment required participants to wear a Guardian RT CGMS for 72 hours. Prior to data collection, the Guardian RT CGMS was initialized and calibrated as per the manufacturer’s instructions using BG values obtained from personal glucose meters. Thereafter, participants were instructed to calibrate their CGMS at least three times per day as per the manufacturer’s instructions for optimal use of the Guardian RT CGMS.
Other than performing necessary calibrations, participants in the control group had limited interaction with their CGMS and received no feedback from it. In contrast, participants in the alarm treatment were able to view their RT glucose readings at their discretion and had high and low glucose alarms set to alert them when readings rose above 200 mg/dl or fell below 80 mg/dl. The high threshold was chosen to encourage good metabolic control, as average BG levels below 200 mg/dl should yield glycated hemoglobin of ≤8.5%.33 The low threshold was set 15 mg/dl above hypoglycemic levels to warn users of impending hypoglycemia; defined here operationally as a CGMS reading of ≤65 mg/dl, thus encompassing both asymptomatic and symptomatic hypoglycemia. This hypoglycemic threshold was chosen on the grounds that it is used commonly because it is within the BG range that usually elicits a mild autonomic response to a low BG level in hypoglycemia-aware individuals.34 Participants in this treatment were given detailed instructions on how to view their current glucose level and how to respond to high and low glucose alarms. In response to low glucose alarms, participants were required to act promptly and perform a BG test to confirm the reading such that no treatment decisions were made on the basis of CGMS readings alone in accordance with the manufacturer’s instructions. If the value obtained was ≤80 mg/dl, participants were instructed to consume two portions (∼30 grams) of a high glycemic index carbohydrate if the low alarm occurred either when insulin was expected to be peaking or following exercise or an increase in activity or if the glucose values displayed on the CGMS were falling sharply prior to the low alarm. If the low alarm occurred at any other time, participants were instructed to consume one portion of carbohydrates (∼15 grams).
Four days after this initial 72-hour monitoring period, participants completed the other condition. Testing sessions were performed on the same days of consecutive weeks to replicate routine activity and to reduce the potential effect of environmental factors on behavior. Because both treatments occurred within a 10-day period, female participants were monitored during the follicular phase of their menstrual cycle on both occasions.
Data Analyses
Continuous glucose monitoring system data were uploaded and analyzed using Medtronic–MiniMed Solutions software. The accuracy of the Guardian RT was determined by using BG values entered for calibration as reference values. In order to assess the accuracy of CGMS readings compared to concomitant BG values used for calibration without introducing a bias as a result of the calibration procedure, CGMS and BG readings were always collected before calibrating the CGMS. Accuracy was evaluated using error grid analysis35,36 and by determining the mean absolute difference between CGMS readings and BG readings. In addition, performance of the hypoglycemia alarm was evaluated by determining alarm sensitivity (ability to identify hypoglycemic events correctly) and specificity (ability to identify the absence of hypoglycemic events correctly) at a number of possible alarm boundaries.6,37 False alarms were defined as low glucose alarms that occurred when BG values were >65 mg/dl.6 Finally, results were analyzed using paired t tests to compare BG and corresponding sensor glucose readings in the hypo-glycemic range as well as the effect of the two treatments on the incidence and duration of hypoglycemia.
Results
Effect of CGMS on Frequency and Duration of Hypoglycemia
The CGMS with alarm set at 80 mg/dl significantly reduced the time spent below hypoglycemic thresholds defined operationally as CGMS readings ≤65 mg/dl by 64% (p ≤ 0.05) and decreased the incidence of hypo-glycemic episodes by 44% (p ≤ 0.05), but without affecting average BG level (Table 1). Furthermore, total time spent below the low alarm threshold (80 mg/dl) relative to the duration of sensor wear was reduced significantly by more than 52% in the alarm condition compared with the control condition (p ≤ 0.05). However, the alarm treatment did not affect the incidence of symptomatic hypoglycemia (Table 1).
Table 1.
Summary of CGMS Dataa
| Alarm | Control | |
|---|---|---|
| Duration of sensor wear per participant (hours) | 70.7 ± 5.3 (71.5–74.0) | 70.0 ± 7.0 (71.0–74.0) |
| Average sensor glucose (mg/dl) | 173 ± 40 (139–194) | 176 ± 49 (137–221) |
| Average number of low glucose alarms per patient | 9.0 ± 8.4 (3.0–17.0) | Not applicable |
| Incidence of symptomatic hypoglycemia per patient | 1.3 ± 1.4 (0–2.0) | 1.5 ± 1.6 (0–3.0) |
| Duration of sensor glucose ≤80 mg/dl (min) | 155 ± 156 (40–310) | 307 ± 378 (75–305)b |
| Relative time spent below hypoglycemic threshold (%) | ||
| CGMS ≤65 mg/dl | 0.8 ± 1.2 (0–1.7) | 2.2 ± 3.1 (0–2.6)b |
| Number of episodes of CGMS hypoglycemia | ||
| CGMS ≤65 mg/dl | 0.9 ± 1.5 (0–2.0) | 1.6 ± 1.7 (0–2.0)b |
| Lowest sensor glucose reading (mg/dl) | 67 ± 16 (52–76) | 63 ± 14 (54–74) |
All results expressed as mean ± standard deviation (interquartile range) for each 3-day treatment period.
Significant difference between alarm and control treatments.
Evaluation of the Accuracy of CGMS and Performance in the Hypoglycemic Range
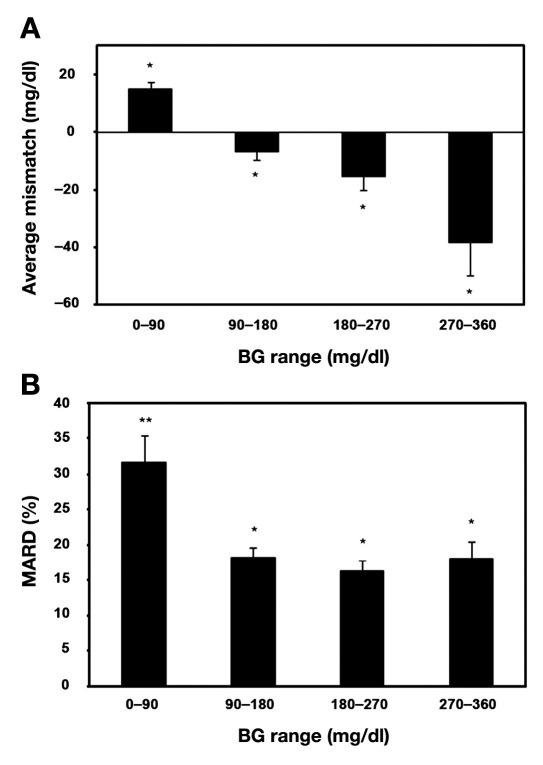
The mean absolute difference, defined as the average of the magnitude of absolute differences between CGMS readings and BG meter readings, was 31 ± 31 mg/dl for all CGMS and glucose meter readings. Altogether, 91 and 96% of CGMS values fell within the clinically acceptable zones A and B of the Clarke and consensus error grids, respectively. The average mismatch between CGMS and BG readings (CGMS reading minus BG reading) over a range of glucose concentrations was 14.8 ± 2.5 mg/dl (mean ± standard error of the mean) for BG levels of 0–90 mg/dl, –6.7 ± 3.0 mg/dl for BG levels of 90–180 mg/dl, –15.6 ± 4.7 mg/dl for BG levels of 180–270 mg/dl, and –38.3 ± 11.6 mg/dl for BG levels of 270–360 mg/dl; the mismatch was significant for all BG ranges (p ≤ 0.05; Figure 1A). When expressed as a percentage, the mean absolute relative difference was significantly greater in the 0- to 90-mg/dl BG range compared with those above 90 mg/dl (Figure 1B).
Figure 1.
Average mismatch between CGMS and BG readings (CGMS readings minus BG readings) over a range of glucose concentrations (A) and mean absolute relative difference (MARD) between CGMS and BG readings expressed as a percentage over a range of BG concentrations (B). All results expressed as mean ± standard error of the mean. *Significant difference in mismatch (p ≤ 0.05). **Significant difference in mismatch compared with other groups (p ≤ 0.05).
Evaluation of the Low Glucose Alarm
The ability of the CGMS to discriminate between true and falsely identified hypoglycemic events was evaluated as described elsewhere.37 Analysis showed that the CGMS low alarm set at 80 mg/dl is able to detect hypoglycemia (BG ≤65 mg/dl) with a sensitivity of 76% and a false alarm rate of 62%. Increasing the alarm level to 90 mg/dl would result in a favorable increase in the sensitivity of the CGMS to detect BG levels ≤65 mg/dl (88%); however, this would also increase the false alarm rate (67%).
Discussion
This study shows for the first time that the short-term availability of continuous glucose readings, together with a low glucose alarm set to 80 mg/dl and specific instructions for responding to low alarms, significantly reduces the incidence and duration of hypoglycemia by 44 and 64%, respectively, without having any effect on average BG levels. The inability of the CGMSs tested here to prevent all hypoglycemia is most likely due to its ∼15-mg/dl overestimation of BG level in the hypoglycemic range.
Our findings support those of others who have shown that use of a low glucose alarm reduces time spent below hypoglycemic thresholds, but differ from previous studies in that our protocol results in a marked reduction in the incidence of hypoglycemia. As mentioned previously, use of an alarm has been reported by others to reduce the average duration of hypoglycemic episodes by between only 21 and 47%,4,9,10,38,39 with no other study reporting a fall in the incidence of hypoglycemia. The findings of these studies are consistent with those of McGarraugh and Bergenstal,40 who reported that many hypoglycemic events detected by a CGMS and verified with BG tests are not treated adequately, leading to an extended episode of hypoglycemia. Also, it was noted that most studies assessing the capabilities of a CGMS with RT glucose information and glucose alarms do not specify what the patient should do with the information made available to them.32 In our study, patients were given specific instructions favoring immediate action in response to low glucose alarms, which may explain why the relative fall in the duration of hypoglycemia was so pronounced and why the incidence of hypoglycemia was reduced markedly with the alarm treatment. It is important to stress, however, that the effect of providing instructions per se on the incidence and duration of hypoglycemia was not examined here and remains to be determined. Finally, the fact that the lesser incidence and the duration of hypoglycemia in the alarm treatment were not accompanied by an increase in average BG level is not surprising given that less than 3.5% of the time was spent below the hypoglycemic threshold.
Our results showing that the use of a CGMS for 3 days can reduce the incidence and duration of hypoglycemic episodes in diabetic individuals is clinically highly relevant, as most individuals with T1DM are more likely to use a CGMS occasionally over successive days when at a high risk of hypoglycemia rather than continuously over weeks and months due to the high cost associated with using these devices. Also, triggering of the low BG level alarm informing the diabetes patient that an episode of hypoglycemia is imminent is particularly important during the day because BG can fall rapidly to reach severe hypoglycemic levels, whereas during the night nocturnal seizures can occur after several hours of low BG levels.41 Despite the potential benefits associated with the protocol described here, the extent to which the broader population of individuals with T1DM would benefit from using CGMSs in this way remains to be determined, as success of the measures adopted in this study to prevent hypoglycemia depends on some members of the population accepting and actually adhering to a few rather stringent guidelines. Also, the extent to which other devices from Medtronic and other manufacturers would perform compared to the Guardian RT CGMS tested in this study remains to be determined. Nevertheless, what this study suggests is that the risk of hypoglycemia should fall significantly if the principles adopted here were to be implemented irrespective of the CGMS tested.
The observation that use of the low glucose alarm, together with some simple instructions, did not decrease the incidence of symptomatic hypoglycemia despite reducing the risk of hypoglycemia determined from a BG level assay, was expected to some extent, due to the inaccuracies of the CGMS at low BG levels. If no mismatches existed between CGMS readings and BG levels, the incidence of symptomatic hypoglycemia and hypoglycemia determined based on BG readings would be expected to be lower for the alarm treatment. However, because CGMS readings in the low glucose range in this study overestimated BG levels by an average of 14.8 mg/dl, it follows that a CGMS alarm triggered at 80 mg/dl would, on average, correspond to a BG level of 65 mg/dl, which, as stated earlier, is within the BG range that would normally elicit a mild symptomatic response to hypoglycemia in hypoglycemia-aware individuals.42 As a result and as observed in our study, the incidence of symptomatic hypoglycemia would be expected to be comparable for both treatments. Also, it must be stressed that the highly subjective nature of the participants’ records of their symptomatic hypoglycemia further increases the difficulty of detecting significant effects of the alarm treatment.
Importantly, the mismatch between the CGMS reading and the BG level also implies that this and other studies9,10,39 underestimate the true frequency and durationof hypoglycemic events, thus overestimating the performance of a CGMS. However, it is important to mention that McGowan and colleagues12 reported thata CGMS may also overestimate the frequency and durationof hypoglycemia. Indeed, in their study, the CGMS underestimated BG in the low BG range, as determined using reference BG values, suggesting that asymptomatic hypoglycemic events detected by a CGMS may not have been true hypoglycemia.12 Irrespective of whether CGMSoverestimates or underestimates BG levels in the hypo-glycemic range, the susceptibility of a CGMS to error in the low BG range, as well as the considerable variation in the definition and assessment of hypoglycemia, suggests that studies showing that CGMSs are useful for detecting hypoglycemia2,7,43–48 should be interpreted cautiously.
One way to prevent hypoglycemia despite the CGMS overestimation of BG level observed in this study may be simply to increase the low glucose alarm. However, we show in agreement with others that increasing the alarm level results in a favorable increase in the sensitivity of the CGMS to detect hypoglycemia, but at the cost of an undesirable increase in already elevated false alarm rates.9 Because a high rate of false alarms is likely to reduce the compliance of the user,49 this would be considered unacceptable in a free-living setting. However, the improved sensitivity to detect hypoglycemia with an alarm set at levels above 80 mg/dl may be appropriate at times of increased hypoglycemic risk, such as following exercise or in cases of hypoglycemia unawareness.
Conclusions
In conclusion, this study shows that short-term use of the Guardian RT CGMS low BG alarm set to 80 mg/dl, together with specific instructions to follow in the event of an alarm, significantly reduces the incidence and duration of hypoglycemic episodes by 44 and 64%, respectively. Although its value in warning users of impending hypoglycemia is somewhat limited, most likely because the CGMSs tested here overestimate BG in the low BG range, the marked effect that its short-term use has on the frequency and duration of hypoglycemia indicates that even wearing a CGMS occasionally for a short period of time may provide an effective tool for the prevention of hypoglycemia.
Acknowledgments
The authors acknowledge financial support of the National Health and Medical Research Council and Juvenile Diabetes Research Foundation Program Grants to Timothy Jones and Paul Fournier and Medtronic who supplied the Guardian units and glucose sensors for this study.
Abbreviations
- (BG)
blood glucose
- (CGMS)
continuous glucose monitoring system
- (RT)
real time
- (T1DM)
type 1 diabetes mellitus
References
- 1.Chase HP, Roberts MD, Wightman C, Klingensmith G, Garg SK, VanWyhe M, Desai S, Harper W, Lopatin M, Bartkowiak M, Tamada J, Eastman RC. Use of the GlucoWatch biographer in children with type 1 diabetes. Pediatrics. 2003;111((4 Pt 1)):790–794. doi: 10.1542/peds.111.4.790. [DOI] [PubMed] [Google Scholar]
- 2.Chico A, Vidal-Rios P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care. 2003;26(4):1153–1157. doi: 10.2337/diacare.26.4.1153. [DOI] [PubMed] [Google Scholar]
- 3.Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 4.Garg SK, Schwartz S, Edelman SV. Improved glucose excursions using an implantable real-time continuous glucose sensor in adults with type 1 diabetes. Diabetes Care. 2004;27(3):734–738. doi: 10.2337/diacare.27.3.734. [DOI] [PubMed] [Google Scholar]
- 5.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O’Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 6.Pitzer KR, Desai S, Dunn T, Edelman S, Jayalakshmi Y, Kennedy J, Tamada JA, Potts RO. Detection of hypoglycemia with the GlucoWatch biographer. Diabetes Care. 2001;24(5):881–885. doi: 10.2337/diacare.24.5.881. [DOI] [PubMed] [Google Scholar]
- 7.Ruxer J, Mozdzan M, Loba J, Barański M, Ruxer M, Markuszewski L. Usefulness of continuous glucose monitoring system in detection of hypoglycaemic episodes in patients with diabetes in course of chronic pancreatitis. Pol Arch Med Wewn. 2005;114(4):953–957. [PubMed] [Google Scholar]
- 8.Schaepelynck-Belicar P, Vague P, Simonin G, Lassmann-Vague V. Improved metabolic control in diabetic adolescents using the continuous glucose monitoring system (CGMS) Diabetes Metab. 2003;29(6):608–612. doi: 10.1016/s1262-3636(07)70076-9. [DOI] [PubMed] [Google Scholar]
- 9.Bode B, Gross K, Rikalo N, Schwartz S, Wahl T, Page C, Gross T, Mastrototaro J. Alarms based on real-time sensor glucose values alert patients to hypo- and hyperglycemia: the guardian continuous monitoring system. Diabetes Technol Ther. 2004;6(2):105–113. doi: 10.1089/152091504773731285. [DOI] [PubMed] [Google Scholar]
- 10.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a trans-cutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29(1):44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 11.Caplin NJ, O’Leary P, Bulsara M, Davis EA, Jones TW. Subcutaneous glucose sensor values closely parallel blood glucose during insulin-induced hypoglycaemia. Diabet Med. 2003;20(3):238–241. doi: 10.1046/j.1464-5491.2003.00837.x. [DOI] [PubMed] [Google Scholar]
- 12.McGowan K, Thomas W, Moran A. Spurious reporting of nocturnal hypoglycemia by CGMS in patients with tightly controlled type 1 diabetes. Diabetes Care. 2002;25(9):1499–1503. doi: 10.2337/diacare.25.9.1499. [DOI] [PubMed] [Google Scholar]
- 13.Monsod TP, Flanagan DE, Rife F, Saenz R, Caprio S, Sherwin RS, Tamborlane WV. Do sensor glucose levels accurately predict plasma glucose concentrations during hypoglycemia and hyperinsulinemia? Diabetes Care. 2002;25(5):889–893. doi: 10.2337/diacare.25.5.889. [DOI] [PubMed] [Google Scholar]
- 14.Djakouré-Platonoff C, Radermercker R, Reach G, Slama G, Selam JI. Accuracy of the continuous glucose monitoring system in inpatient and outpatient conditions. Diabetes Metab. 2003;29((2 Pt 2)):159–162. doi: 10.1016/S1262-3636(07)70023-X. [DOI] [PubMed] [Google Scholar]
- 15.Sachedina N, Pickup JC. Performance assessment of the Medtronic-MiniMed Continuous Glucose Monitoring System and its use for measurement of glycaemic control in Type 1 diabetic subjects. Diabet Med. 2003;20(12):1012–105. doi: 10.1046/j.1464-5491.2003.01037.x. [DOI] [PubMed] [Google Scholar]
- 16.Rebrin K, Steil GM, van Antwerp WP, Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am J Physiol. 1999;277((3 Pt 1)):E561–E571. doi: 10.1152/ajpendo.1999.277.3.E561. [DOI] [PubMed] [Google Scholar]
- 17.Thennadil SN, Rennert JL, Wenzel BJ, Hazen KH, Ruchti TL, Block MB. Comparison of glucose concentration in interstitial fluid, and capillary and venous blood during rapid changes in blood glucose levels. Diabetes Technol Ther. 2001;3(3):357–365. doi: 10.1089/15209150152607132. [DOI] [PubMed] [Google Scholar]
- 18.Wentholt IM, Vollebregt MA, Hart AA, Hoekstra JB, DeVries JH. Comparison of a needle-type and a microdialysis continuous glucose monitor in type 1 diabetic patients. Diabetes Care. 2005;28(12):2871–2876. doi: 10.2337/diacare.28.12.2871. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm B, Forst S, Weber MM, Larbig M, Pfützner A, Forst T. Evaluation of CGMS during rapid blood glucose changes in patients with type 1 diabetes. Diabetes Technol Ther. 2006;8(2):146–155. doi: 10.1089/dia.2006.8.146. [DOI] [PubMed] [Google Scholar]
- 20.Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003;52(11):2790–2794. doi: 10.2337/diabetes.52.11.2790. [DOI] [PubMed] [Google Scholar]
- 21.Steil GM, Rebrin K, Mastrototaro J, Bernaba B, Saad MF. Determination of plasma glucose during rapid glucose excursions with a subcutaneous glucose sensor. Diabetes Technol Ther. 2003;5(1):27–31. doi: 10.1089/152091503763816436. [DOI] [PubMed] [Google Scholar]
- 22.Steil GM, Rebrin K, Hariri F, Jinagonda S, Tadros S, Darwin C, Saad MF. Interstitial fluid glucose dynamics during insulin-induced hypoglycaemia. Diabetologia. 2005;48(9):1833–1840. doi: 10.1007/s00125-005-1852-x. [DOI] [PubMed] [Google Scholar]
- 23.Sternberg F, Meyerhoff C, Mennel FJ, Mayer H, Bischof F, Pfeiffer EF. Does fall in tissue glucose precede fall in blood glucose? Diabetologia. 1996;39(5):609–612. doi: 10.1007/BF00403309. [DOI] [PubMed] [Google Scholar]
- 24.Thome-Duret V, Reach G, Gangnerau MN, Lemonnier F, Klein JC, Zhang Y, Hu Y, Wilson GS. Use of a subcutaneous glucose sensor to detect decreases in glucose concentration prior to observation in blood. Anal Chem. 1996;68(21):3822–3826. doi: 10.1021/ac960069i. [DOI] [PubMed] [Google Scholar]
- 25.Jansson PA, Fowelin J, Smith U, Lönnroth P. Characterization by microdialysis of intracellular glucose level in subcutaneous tissue in humans. Am J Physiol. 1988;255((2 Pt 1)):E218–E220. doi: 10.1152/ajpendo.1988.255.2.E218. [DOI] [PubMed] [Google Scholar]
- 26.Diabetes Research in Children Network (Direcnet) Study Group The accuracy of the CGMS in children with type 1 diabetes: results of the diabetes research in children network (DirecNet) accuracy study. Diabetes Technol Ther. 2003;5(5):781–789. doi: 10.1089/152091503322526987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg SK, Smith J, Beatson C, Lopez-Baca B, Voelmle M, Gottlieb PA. Comparison of accuracy and safety of the SEVEN and the Navigator continuous glucose monitoring systems. Diabetes Technol Ther. 2009;11(2):65–72. doi: 10.1089/dia.2008.0109. [DOI] [PubMed] [Google Scholar]
- 28.Guerci B, Floriot M, Bohme P, Durain D, Benichou M, Jellimann S, Drouin P. Clinical performance of CGMS in type 1 diabetic patients treated by continuous subcutaneous insulin infusion using insulin analogs. Diabetes Care. 2003;26(3):582–589. doi: 10.2337/diacare.26.3.582. [DOI] [PubMed] [Google Scholar]
- 29.Kovatchev BP, Gonder-Frederick LA, Cox DJ, Clarke WL. Evaluating the accuracy of continuous glucose-monitoring sensors: continuous glucose-error grid analysis illustrated by TheraSense Freestyle Navigator data. Diabetes Care. 2004;27(8):1922–1928. doi: 10.2337/diacare.27.8.1922. [DOI] [PubMed] [Google Scholar]
- 30.Maia FF, Araujo LR. Accuracy, utility and complications of continuous glucose monitoring system (CGMS) in pediatric patients with type 1 diabetes. J Pediatr (Rio J) 2005;81(4):293–297. [PubMed] [Google Scholar]
- 31.Wilson DM, Beck RW, Tamborlane WV, Dontchev MJ, Kollman C, Chase P, Fox LA, Ruedy KJ, Tsalikian E, Weinzimer SA. The accuracy of the FreeStyle Navigator continuous glucose monitoring system in children with type 1 diabetes. Diabetes Care. 2007;30(1):59–64. doi: 10.2337/dc06-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reach G. Continuous glucose monitoring and diabetes health outcomes: a critical appraisal. Diabetes Technol Ther. 2008;10(2):69–80. doi: 10.1089/dia.2007.0261. [DOI] [PubMed] [Google Scholar]
- 33.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25(2):275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 34.Cryer PE. Hierarchy of physiological responses to hypoglycemia: relevance to clinical hypoglycemia in type I (insulin dependent) diabetes mellitus. Horm Metab Res. 1997;29(3):92–96. doi: 10.1055/s-2007-978997. [DOI] [PubMed] [Google Scholar]
- 35.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 36.Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143–1148. doi: 10.2337/diacare.23.8.1143. [DOI] [PubMed] [Google Scholar]
- 37.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561–577. [PubMed] [Google Scholar]
- 38.Garg S, Jovanovic L. Relationship of fasting and hourly blood glucose levels to HbA1c values: safety, accuracy, and improvements in glucose profiles obtained using a 7-day continuous glucose sensor. Diabetes Care. 2006;29(12):2644–2649. doi: 10.2337/dc06-1361. [DOI] [PubMed] [Google Scholar]
- 39.Tanenberg R, Bode B, Lane W, Levetan C, Mestman J, Harmel AP, Tobian J, Gross T, Mastrototaro J. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526. doi: 10.4065/79.12.1521. [DOI] [PubMed] [Google Scholar]
- 40.McGarraugh G, Bergenstal R. Detection of hypoglycemia with continuous interstitial and traditional blood glucose monitoring using the FreeStyle Navigator Continuous Glucose Monitoring System. Diabetes Technol Ther. 2009;11(3):145–150. doi: 10.1089/dia.2008.0047. [DOI] [PubMed] [Google Scholar]
- 41.Buckingham B, Wilson DM, Lecher T, Hanas R, Kaiserman K, Cameron F. Duration of nocturnal hypoglycemia before seizures. Diabetes Care. 2008;31(11):2110–2112. doi: 10.2337/dc08-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Di Vincenzo A, Modarelli F, Ciofetta M, Lepore M, Annibale B, Torlone E, Perriello G, De Feo P, Santeusanio F, Brunetti P, Bolli GB. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycaemia, following institution of rational, intensive insulin therapy in IDDM. Diabetologia. 1994;37(12):1265–1276. doi: 10.1007/BF00399801. [DOI] [PubMed] [Google Scholar]
- 43.Amin R, Ross K, Acerini CL, Edge JA, Warner J, Dunger DB. Hypoglycemia prevalence in prepubertal children with type 1 diabetes on standard insulin regimen: use of continuous glucose monitoring system. Diabetes Care. 2003;26(3):662–667. doi: 10.2337/diacare.26.3.662. [DOI] [PubMed] [Google Scholar]
- 44.Bode B, Silver M, Weiss R, Martin K. Evaluation of a continuous glucose monitoring system for home-use conditions. Manag Care. 2008;17(8):40–45. [PubMed] [Google Scholar]
- 45.Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24(11):1858–1862. doi: 10.2337/diacare.24.11.1858. [DOI] [PubMed] [Google Scholar]
- 46.Chase HP, Kim LM, Owen SL, MacKenzie TA, Klingensmith GJ, Murtfeldt R, Garg SK. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics. 2001;107(2):222–226. doi: 10.1542/peds.107.2.222. [DOI] [PubMed] [Google Scholar]
- 47.Guillod L, Comte-Perret S, Monbaron D, Gaillard RC, Ruiz J. Nocturnal hypoglycaemias in type 1 diabetic patients: what can we learn with continuous glucose monitoring? Diabetes Metab. 2007;33(5):360–365. doi: 10.1016/j.diabet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Schiaffini R, Ciampalini P, Fierabracci A, Spera S, Borrelli P, Bottazzo GF, Crinò A. The Continuous Glucose Monitoring System (CGMS) in type 1 diabetic children is the way to reduce hypoglycemic risk. Diabetes Metab Res Rev. 2002;18(4):324–329. doi: 10.1002/dmrr.309. [DOI] [PubMed] [Google Scholar]
- 49.Buckingham B, Block J, Burdick J, Kalajian A, Kollman C, Choy M, Wilson DM, Chase P. Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther. 2005;7(3):440–447. doi: 10.1089/dia.2005.7.440. [DOI] [PMC free article] [PubMed] [Google Scholar]