Abstract
Background
Despite advances in insulin pen design and functionality, the selection of pens available for children with diabetes is limited. This study assessed the usability, functionality and attitudes towards NovoPen Echo®, a new durable insulin pen designed for pediatric patients that combines a simple memory function with half-increment dosing, versus NovoPen® Junior and HumaPen® Luxura™ HD in pediatric subjects, their parents, and health care professionals (HCPs).
Methods
Pens were evaluated in random order during 1:1 interviews in the three target groups (pediatric subjects, parents, and HCPs) in Germany, France, and Canada. Study participants were asked to prepare each pen, perform injections into foam cushions, and provide feedback via a standardized questionnaire.
Results
In total, 205 participants were included in the study. On a scale of 1–6 (1 = most favorable; 6 = least favorable regarding overall appearance, shape, colors, thickness and length), NovoPen Echo received the most favorable rating for design and overall appearance (mean ± standard deviation = 1.71 ± 0.79) compared with NovoPen Junior (2.02 ± 0.93) and HumaPen Luxura HD (2.36 ± 1.01). Furthermore, 89% of pediatric subjects and 94% of parents rated the memory function of NovoPen Echo as very easy/easy to use. When asked to rate the pens overall, 80% of participants preferred NovoPen Echo to the other pens (p < 0.0001).
Conclusions
The results demonstrate a high overall level of satisfaction with NovoPen Echo among pediatric subjects, parents, and HCPs. The novel design aspects of NovoPen Echo, namely t1468-he simple memory function, half-increment units and, ease of use and design, may contribute towards promoting treatment adherence, which is essential in the pediatric setting.
Keywords: adolescents, children, diabetes mellitus, insulin, insulin pen, medical devices, type 1 diabetes
Introduction
Before insulin pens were introduced, insulin was administered by vials and syringes. However, for many patients with diabetes, the fear of injections, dosing inaccuracy, pain, anxiety, inconvenience, and social acceptability may have presented barriers to this method of administration.1–3 These drawbacks have contributed to the increased popularity of alternative insulin administration systems such as pen delivery devices.4
Benefits of pens include simplicity, improved adherence, freedom and flexibility.5 Pens therefore help to overcome some issues associated with vial/syringe methods of insulin delivery and to improve adherence to treatment regimens in type 1 and 2 diabetes.6,7 Additionally, some pens provide half-increment dosing, which is particularly important in young pediatric patients.8
Adherence issues in children and adolescents with diabetes are higher than in adult patients,5 and evidence shows that some children and young adults develop ketoacidosis due to poor adherence to insulin therapy.9 As long-term poor metabolic control increases the risk of diabetic complications and cardiovascular disease,10 it is necessary to improve self-care in children and adolescents with diabetes. The level and need for self-care also differs with age; children with diabetes who were diagnosed at an older age have more success with self-care.11 Any attempt to improve self-care in children and adolescents must, therefore, be addressed through age-appropriate care and education. An easy-to-use insulin pen may, therefore, be of great importance in pediatric patients.
In a Diabetes Attitudes, Wishes and Needs (DAWN) Youth survey, 6 out of 10 children with diabetes did not manage their diabetes successfully at school, while children with good support at school had a better quality of life and were less burdened with diabetes.11 Children and adolescents, especially those lacking support at school, may therefore benefit from a simple-to-use pen, as it could help to improve their ability to manage diabetes.
Continuous subcutaneous insulin infusion (CSII)/insulin pump therapy also improves adherence; however, bolus insulin omission is common in adolescents.12 Furthermore, CSII is expensive and hence, less accessible to some patients.13 Additionally, insulin sensitivity changes with age and puberty; studies show that prepubertal children require smaller doses of insulin than older children— mainly due to the circadian secretory patterns of growth hormone14,15 that increase substantially in puberty. Sensitivity changes highlight the importance of accurate and convenient insulin delivery devices that can tailor dosing according to the needs of all pediatric age groups.
Especially in Europe, pens have widespread use as a delivery device for children with type 1 diabetes.3,16 Moreover, there is a continued drive to improve pen technology and to enhance the device’s functionality and usability. However, despite recent advances in design and functionality, the selection of pens available for children is limited. To increase adherence and reduce the fear and/or risk of missing a dose or double dosing, Novo Nordisk A/S (Bagsværd, Denmark) has developed NovoPen Echo®, a durable pediatric-specific insulin pen that combines a user-friendly memory function with half-increment dosing.
We assessed usability, functionality and attitudes towards NovoPen Echo versus NovoPen® Junior (Novo Nordisk) and HumaPen® Luxura™ HD (Eli Lilly and Company, Indianapolis, IN) in pediatric subjects, their parents and health care professionals (HCPs).
Methods
Participants
Participants from six study sites in Germany, France and Canada were included. Pediatric subjects included children and adolescents (aged 7–18 years) with type 1 diabetes who had been receiving insulin for ≤6 months and were self-injecting insulin (they may also have been using CSII). Subgroup data were also collected on children (aged 7–12 years) and adolescents (13–18 years).
Male or female parents of the pediatric subjects participated. One family, with twins who had diabetes, had more than one child in the study. Health care professionals (working as diabetes nurse educators or physicians/pediatricians when the study was conducted) had ≤2 years’ experience [mean ± standard deviation (SD): 8.1 ± 5.6 years] in treating and consulting patients using pens.
Protocol-defined exclusion criteria for all participants included involvement in diabetes-related usability tests 6 months before the study; mental or physical incapacity, unwillingness to take part in the study or language barriers precluding adequate understanding or cooperation (for pediatric subjects or parents) and any disease or condition that might have interfered with the test.
Insulin Pens and Study Design
NovoPen Echo is a new insulin pen with a memory function and half-increment dosing; it will also be available in red or blue with a choice of “skins” (i.e., decorative/protective covers typically used for mobile phones and MP3 players). NovoPen Echo was compared with NovoPen Junior and HumaPen Luxura HD, both durable devices specifically designed for the pediatric market.
Logos and labels were removed from the pens, but because design features were being tested, this study was unblinded. Pictures of different skins showing available options for NovoPen Echo customization were provided. The three pens were evaluated in a computer-generated random order during 1:1 interviews in three groups: pediatric subjects, their parents, and HCPs. Interviews lasted either 45 minutes (pediatric subjects and parents) or 90 minutes (HCPs). Pediatric subjects and their parents were interviewed simultaneously; however, only one person answered the questions. When asked to handle the pen’s injector as they normally would, if pediatric subjects picked up the pen, then they would answer the questions and vice versa.
Before performing the assigned tasks, HCPs were asked to state which pens they were familiar with or currently using. If they could not remember the names, they pointed to a picture of the pen from a pictured list provided: NovoPen® 3 (Novo Nordisk); NovoPen® 4 (Novo Nordisk); SoloSTAR® (sanofi-aventis U.S., Bridgewater, NJ); HumaPen Luxura HD; FlexPen® (Novo Nordisk); OptiPen® Pro (sanofi-aventis); NovoPen Junior; HumaPen Memoir™ (Eli Lilly); BerliPen® (Berlin-Chemie, Berlin, Germany) and Autopen® Junior (Owen Mumford, Oxford, UK). Health care professionals could also answer “other” or “don’t know”. From the same pictured list, pediatric subjects or their parents were asked which pen they or their children were currently using.
Session Structure
The study was divided into two parts: a usability assessment and a preference assessment. Participants were asked to complete the following tasks: (1a) set up the pen, adjust, and inject a dose of insulin into a foam cushion; (1b) operate the memory function (a task was considered successfully completed when all subtasks were easily completed by participants); and (2) subjectively assess the pens. At the end of each session, each pen was ranked separately by the participants, using rating scales, including scales for young children. Feedback was provided via a standardized nonvalidated online questionnaire. Answers were recorded by the interviewer.
Data Analysis
A standard χ2 test with Yates correction for continuity, using 2 × 2 contingencies, for independent samples wasused to test the equality of two proportions. A paired t-test was used to compare independent samples. The significance level was set at p < .05; instances where p > .05 have not been reported.
Results
Baseline Demographics
A total of 205 participants (79 children, 78 parents and 48 HCPs) were included in the study. Baseline demo-graphics of the pediatric subjects are shown in Table 1. Of the 10 pens included in the prestudy survey, HCPs were most familiar/experienced with NovoPen 4 (83%), NovoPen 3 (77%) and HumaPen Luxura HD (69%). Most pediatric subjects used NovoPen Junior (46%), SoloSTAR (19%) and HumaPen Luxura HD (13%).
Table 1.
Baseline Demographics of the Pediatric Subjects (n = 79)
| Characteristics | Subjects (%) |
|---|---|
| Age (years)a | |
| 7–12 | 44 |
| 13–18 | 56 |
| Sex | |
| Male | 48 |
| Female | 52 |
| Self-injecting insulinb (number of years) | |
| <1 | 10 |
| ≥1 – <2 | 22 |
| ≥2 – <4 | 37 |
| ≥4 – <6 | 16 |
| ≥6 – <10 | 9 |
| ≥10 | 6 |
Mean ± SD = 12.9 ± 2.8 years
Mean ± SD = 4.0 ± 3.5 years
Task 1a: Setting Up, Adjusting, and Injecting
Setting up, adjusting, and injecting with NovoPen Echo was problem-free for most participants. Overall, 84% of participants successfully set up NovoPen Echo, compared with 88% for NovoPen Junior and 91% for HumaPen Luxura HD. This task was considered incomplete if participants did not pull out the dose button. The ease of dialing the dose was highly rated for all pens [using a scale of 1–6 (1 = “very easy to dial”; 6 = “very difficult to dial”)]. Overall, 77% of study participants rated NovoPen Echo as “easy”/”very easy” compared with 75% for NovoPen Junior and 87% (p = .007) for HumaPen Luxura HD.
It was easier for study participants to adjust and inject when using NovoPen Echo and HumaPen Luxura HD compared to NovoPen Junior [95%, 97% and 60% completion, respectively (p < .0001 versus NovoPen Echo)]. The pressure required to inject a dose into a foam cushion was considered ideal by ∼70% of study participants using NovoPen Echo and HumaPen Luxura HD, compared with 58% (p = .005) using NovoPen Junior.
Subgroup data were collected on pediatric subjects [children (7–12 years) and adolescents (13–18 years)] (Table 2). Fewer adolescents dialed a dose successfully with NovoPen Echo compared with adolescents using NovoPen Junior and HumaPen Luxura HD; but the difference was only statistically significant when compared with HumaPen Luxura HD (p < .05 versus NovoPen Echo). Also, more children completed the dialing task successfully with NovoPen Echo and NovoPen Junior compared with children using HumaPen Luxura HD. More children found it “easy”/”very easy” to dial with NovoPen Junior compared with NovoPen Echo; but the difference was only statistically significant when compared with HumaPen Luxura HD (p = .009 versus NovoPen Echo).
Table 2.
Rating of Completion of Subtasks and Ease of Use (Pediatric Subjects)
| Age 7–12 years (n = 35)(%) | Age 13–18 years (n = 44) (%) | |||||
|---|---|---|---|---|---|---|
| NovoPen Echo | NovoPen Junior | HumaPen Luxura HD | NovoPen Echo | NovoPen Junior | HumaPen Luxura HD | |
| Completion of subtasks | ||||||
| Dialing dose | 86 | 86 | 77 | 86 | 93 | 98a,h |
| Adjusting/injecting dose | 91 | 54b,h | 91 | 95 | 70c,h | 98 |
| Ease of use (tasks rates 1–2)d | ||||||
| Dialing dose | 66 | 86 | 91e,h | 75 | 68 | 86 |
| Reading dose | 86 | 91 | 89 | 84 | 73 | 86 |
| Adjusting dose | 83 | 43f,h | 95 | 84 | 46g,h | 95 |
p < .05
p = .0005
p = .002
1 = very easy; 6 = very difficult
p = .009
p = .0005
p = .0002
p values versus NovoPen Echo
Task 1b: Memory Function
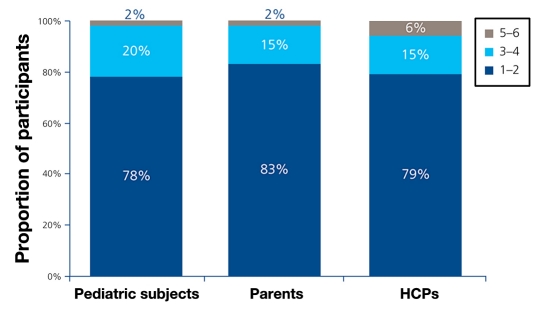
Participants evaluated the memory function of NovoPen Echo. (NovoPen Junior and HumaPen Luxura HD do not have a memory function.) Overall, 89% of pediatric subjects and 94% of parents rated the user-friendliness as “very easy”/”easy” to use [using a scale of 1–6 (1 = “very easy to use”; 6 = “very difficult to use”)]. In total, 87% of pediatric subjects and 81% of parents successfully activated the memory function and read the amount and time of the last dose. The proportion of pediatric subjects, grouped by age, rating the user-friendliness of the memory function and reading the time and amount of the last dose is shown in Table 3. When asked: “To what extent does the memory function meet your needs/your child’s/your patient’s needs?”, most pediatric subjects, parents, and HCPs considered the memory function of NovoPen Echo as completely meeting their needs (Figure 1).
Table 3.
Rating Ease of Use of the Memory Function of NovoPen Echo and Reading the Time and Amount of the Last Dose
| Rating of memory function | Age | |
|---|---|---|
| 7–12 years (n = 35) (%) | 13–18 years (n = 44) (%) | |
| 1 = very easy | 71 | 73 |
| 2 | 9 | 23 |
| 3 | 17 | 4 |
| 4 | 3 | 0 |
| 5 | 0 | 0 |
| 6 = very difficult | 0 | 0 |
Figure 1.
Participants’ rating of the memory function of NovoPen Echo. Participants were asked: “To what extent does the memory function meet your needs/your child’s needs/your patient’s needs?” Scale: 1 = meets needs completely; 6 = does not meet needs.
Task 2: Subjective Assessment of Pens and Participants’ Preference
Participants rated the size, weight, and design of all three pens; 70% of participants rated NovoPen Echo as ideal in size compared with 54% (p = .005) rating NovoPen Junior and 20% (p < .0001) rating HumaPen Luxura HD as ideal in size. Overall, 53% of participants rated the weight of NovoPen Echo as ideal compared with NovoPen Junior [42% (p = .02)] and HumaPen Luxura HD [36% (p = .0005)].
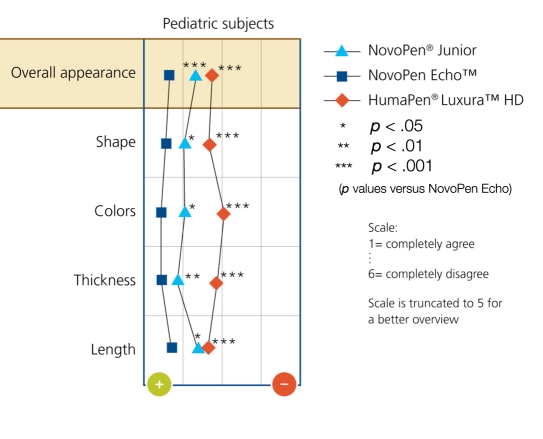
The pen designs were rated using a scale of 1–6 (1 = most favorable; 6 = least favorable for overall appearance, shape, colors, thickness, and length). NovoPen Echo was rated highest (1–2) for design and overall appearance (87%), compared with NovoPen Junior (77%, p = .03) and HumaPen Luxura HD (46%, p < .0001). Pediatric subject ratings are shown in Figure 2. The design of NovoPen Junior was also rated highly by the participants. Notably, the color of NovoPen Echo was preferred over the other pens.
Figure 2.
Rating of design aspects of insulin pens. Pediatric subjects were asked to rate the pen in terms of design by giving their opinion on the following statements: “I like the overall appearance/shape/colors/thickness/length of the pen.”
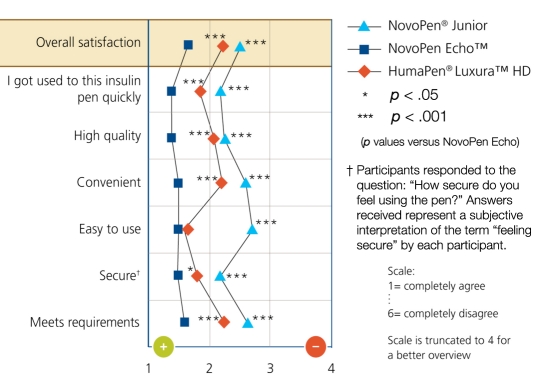
After using all three pens, 89% of participants favored NovoPen Echo (rated it as 1–2) for satisfaction using a scale of 1–6 (1 = most favorable; 6 = least favorable). “Getting used to the pen quickly”, quality, convenience, ease of use, security (feeling secure that they had completely injected the dose) and meeting requirements all contributed to the higher overall satisfaction compared with the other pens (Figure 3).
Figure 3.
Rating of pens after usage: subjective assessment of pens. Participants were asked how the following statements apply to the pen: “I am very satisfied with this insulin pen;” “I got used to this insulin pen quickly;” “This insulin pen is of high quality;” “This insulin pen is convenient for everyday use;” “This insulin pen is easy to use” and “This insulin pen meets my requirements.”
Overall, 92% of participants rated the user-friendliness of NovoPen Echo as “very easy”/”easy” [using a scale of 1–6 (1 = “very easy to use”; 6 = “very difficult to use”)]. Health care professionals rated NovoPen Echo as the easiest pen device to explain to pediatric subjects.
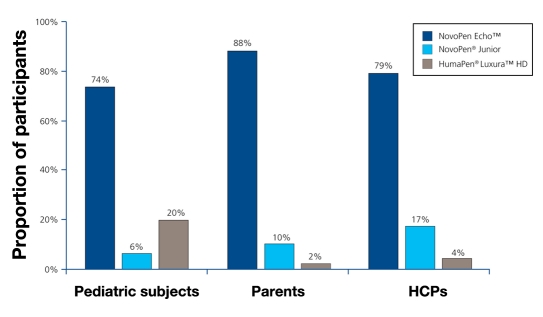
When asked to rate the pens overall, 80% of participants preferred NovoPen Echo to the other pens, while 7% (p < .0001) and 12% (p < .0001) preferred NovoPen Junior and HumaPen Luxura HD, respectively. NovoPen Echo was favored by most pediatric subjects, parents, and HCPs (Figure 4).
Figure 4.
Preference results for overall favorite insulin pen. p < .0001 for NovoPen Echo compared with the other two pens.
Discussion
Available data on insulin pen use in children are sparse. Furthermore, a limited selection of pens is available for children. This study therefore explored the usability, functionality and preference for different insulin pens in pediatric subjects, their parents, and HCPs. NovoPen Echo was preferred over NovoPen Junior and HumaPen Luxura HD by 80% of participants, demonstrating a high overall satisfaction with NovoPen Echo in most parts of the usability study and questionnaire.
Setting up, adjusting, and injecting with NovoPen Echo was problem-free for most participants, indicating that this pen is a good alternative to the currently available pens. The overall completion rate for set-up was 84% with NovoPen Echo; some participants forgot to check if the air had been removed, although the pressure of the test situation may have contributed to this. In the subgroup analyses, we expected to find higher completion rates for certain tasks with adolescents (aged 13–18 years) compared with children (aged 7–12 years), as older children may find it easier to perform particular tasks compared with younger children. Although dialing the dose with NovoPen Echo was rated significantly more difficult compared with HumaPen Luxura HD for children, more children completed the dialing task with NovoPen Echo compared with HumaPen Luxura HD. The proportion of children completing the dialing task with NovoPen Echo was equal to that of adolescents using NovoPen Echo; therefore all pediatric subjects were able to use NovoPen Echo successfully. Problems with understanding that the dose button needed to be pulled out before use were minor, and with the correct training from HCPs, subjects were able to learn the correct process quickly. NovoPen Echo was user-friendly—setting up, adjusting and injecting seemed to be intuitive, therefore decreasing the risk of error. Additionally, this pen was easier to operate compared with its predecessor Novo Pen Junior. As similar completion rates for the “dialing a dose” subtask were observed in children and adolescents using NovoPen Echo, it appeared that younger age does not necessarily hinder pediatric subjects from correctly dialing a dose.
The memory function met the needs of participants and achieved a high ease-of-use rating among pediatric subjects (89%), a characteristic that is crucial to improving acceptance and adherence. NovoPen Echo combines half-increment dosing with a memory function, which can be used to retrieve information about the time and amount of the last dose, potentially reducing the fear of double dosing or missing a dose. The memory function may be particularly important in younger patients in whom insulin is administered by multiple caregivers at home, day care and school settings, and it may help reduce the risk of double injections. HumaPen Memoir is the only other pen available with a memory function; it records the last 16 doses. However, a pen designed for pediatric patients needs to be simple because children may not necessarily require information on multiple previous doses. Furthermore, the HumaPen Memoir device does not allow half-increment dosing, reducing its applicability in a pediatric setting.
Insulin pens with a memory function may provide added security for parents, who need reassurance that their child has received the correct insulin dose. They may also help parents allow other persons, i.e., school staff, personal day care and grandparents, to be responsible for their child’s care. Parents worry about their child having low blood glucose at school so they might administer insufficient insulin in the morning in an attempt to prevent hypoglycemia at school.17 Consequently, the memory function of NovoPen Echo may benefit parents and caregivers; they would be able to check if and when their child took or was given a dose of insulin at school. Due to the lack of adequate support and care in certain schools,11,17 the memory function could provide parents and caregivers with a simple means of testing a child’s adherence to treatment.
Parents can also use the memory function as a form of age-appropriate education to teach their child about the importance of insulin injections, dosing and timing, and to remind their children to take insulin, thus reinforcing the importance of taking medication. Although tight blood glucose control may be difficult to achieve in children and young adults with diabetes, studies in adults show that pens that are easy and efficient to operate correctly with minimal discomfort increase acceptance of, and adherence to, treatment regimens.18 Adult patients using pens have also been shown to be more adherent than patients using vials and syringes.7
The need for precise dosing with half-increments may be the main driver for the choice of pen. The possibility for half-increment dosing is most likely to be of highest importance for bolus administration of rapid-acting insulin; however, highly insulin-sensitive young children may also benefit from half-increment dosing with respect to administration of basal insulin.8
Study participants preferred the color of NovoPen Echo to the other pens and also favored the option of customizing the pen using the range of skins provided. These attributes might aid adherence to treatment. Personalization of pens might remove some of the barriers to taking medication, making children less reluctant to use their insulin pens at school and perform injections in public. Furthermore, adolescents may not want their insulin pens to resemble a medical device; therefore, a choice of skins is likely to be beneficial. None of the other pens used in the study have customizable skin options. NovoPen Echo will be available in two different colors, which is advantageous for children using multiple kinds of insulin. It will allow them and parents/caregivers to distinguish or differentiate easily between the different kinds of insulin (e.g., basal and bolus) and thus prevent injection of the wrong insulin. To date, this kind of differentiation has been possible only when using different brands or models of pen. However, with NovoPen Echo, the ability to distinguish between different types of insulin while using the same kind of pen is facilitated by the availability of different colors and the ability to add skins.8 The appealing design and possibility for customization may increase the willingness of children to perform injections in public and therefore improve adherence.
Although results from the study may not reflect daily practice, as interview sessions only lasted for 45 minutes, clear benefits of NovoPen Echo were still demonstrated. Future studies are required to evaluate the actual performance of NovoPen Echo and its features in a real-life setting.
There are currently no pens with a memory function and half-increment unit dosing dedicated to the pediatric population. Therefore NovoPen Echo is particularly applicable to this patient group.
Conclusions
In conclusion, the novel features of NovoPen Echo, namely the simple memory function (which may provide additional benefits such as confidence and security); half-increment units; ease of use (especially for small hands); and option to customize with fashionable skins, may contribute to the success of this pen in the pediatric setting in terms of usability, functionality and preference.
Acknowledgments
The authors thank SirValUse Consulting GmbH, Hamburg, Germany, for conducting the usability test and providing the statistical analyses. Writing and editorial assistance was provided by Ruth Bond, Ph.D., ScopeMedical Ltd, and funded by Novo Nordisk A/S.
Abbreviations
- (CSII)
continuous subcutaneous insulin infusion
- (HCP)
health care professional
- (SD)
standard deviation
References
- 1.Graff MR, McClanahan MA. Assessment by patients with diabetes mellitus of two insulin pen delivery systems versus a vial and syringe. Clin Ther. 1998;20(3):486–496. doi: 10.1016/s0149-2918(98)80058-1. [DOI] [PubMed] [Google Scholar]
- 2.Mollema ED, Snoek FJ, Heine RJ, van der Ploeg HM. Phobia of self-injecting and self-testing in insulin-treated diabetes patients: opportunities for screening. Diabet Med. 2001;18(8):671–674. doi: 10.1046/j.1464-5491.2001.00547.x. [DOI] [PubMed] [Google Scholar]
- 3.Pfutzner A, Asakura T, Sommavilla B, Lee W. Insulin delivery with FlexPen: dose accuracy, patient preference and adherence. Expert Opin Drug Deliv. 2008;5(8):915–925. doi: 10.1517/17425247.5.8.915. [DOI] [PubMed] [Google Scholar]
- 4.Stockl K, Ory C, Vanderplas A, Nicklasson L, Lyness W, Cobden D, Chang E. An evaluation of patient preference for an alternative insulin delivery system compared to standard vial and syringe. Curr Med Res Opin. 2007;23(1):133–146. doi: 10.1185/030079906X159524. [DOI] [PubMed] [Google Scholar]
- 5.Lombardo F, Salzano G, Messina MF, De Luca F. Compliance and administration methods in management of type 1 diabetes. Acta Biomed. 2005;76(Suppl 3):66–69. [PubMed] [Google Scholar]
- 6.Gnanalingham MG, Newland P, Smith CP. Accuracy and reproduci-bility of low dose insulin administration using pen-injectors and syringes. Arch Dis Child. 1998;79(1):59–62. doi: 10.1136/adc.79.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther. 2006;28(10):1712–1725. doi: 10.1016/j.clinthera.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Hanas R. Type 1 diabetes in children, adolescents and young adults. 2nd ed. London: Class Publishing; 2004. [Google Scholar]
- 9.Morris AD, Boyle DI, McMahon AD, Greene SA, MacDonald TM, Newton RW. Adherence to insulin treatment, glycaemic control, and ketoacidosis in insulin-dependent diabetes mellitus. The DARTS/MEMO Collaboration. Diabetes Audit and Research in Tayside Scotland. Medicines Monitoring Unit 4251. Lancet. 1997;350(9090):1505–1510. doi: 10.1016/s0140-6736(97)06234-x. [DOI] [PubMed] [Google Scholar]
- 10.Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. J Pediatr. 1994;125(2):177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 11.Peyrot M, Aanstoot H-J. Parent-reported social, psychological, and health care factors associated with youth self-care success in the Multi-national DAWN Youth Survey. Pediatr Diabetes. 2008;9(Suppl 10):28. [Google Scholar]
- 12.Olinder AL, Kernell A, Smide B. Missed bolus doses: devastating for metabolic control in CSII-treated adolescents with type 1 diabetes. Pediatr Diabetes. 2009;10(2):142–148. doi: 10.1111/j.1399-5448.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 13.Medical management of type 1 diabetes. 5th ed. Alexandria, VA: American Diabetes Association; 2008. [Google Scholar]
- 14.Danne T, Battelino T, Kordonouri O, Hanas R, Klinkert C, Ludvigsson J, Barrio R, Aebi C, Gschwend S, Mullis PE, Schumacher U, Zumsteg U, Morandi A, Rabbone I, Cherubini V, Toni S, de Beaufort C, Hindmarsh P, Sumner A, van Waarde WM, van den Berg N, Phillip M. A cross-sectional international survey of continuous subcutaneous insulin infusion in 377 children and adolescents with type 1 diabetes mellitus from 10 countries. Pediatr Diabetes. 2005;6(4):193–198. doi: 10.1111/j.1399-543X.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- 15.Klinkert C, Bachran R, Heidtmann B, Grabert M, Holl RW. Age-specific characteristics of the basal insulin-rate for pediatric patients on CSII. Exp Clin Endocrinol Diabetes. 2008;116(2):118–122. doi: 10.1055/s-2007-990296. [DOI] [PubMed] [Google Scholar]
- 16.Hanel H, Weise A, Sun W, Pfutzner JW, Thome N, Pfutzner A. Differences in the dose accuracy of insulin pens. J Diabetes Sci Technol. 2008;2(3):478–481. doi: 10.1177/193229680800200318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacombs J. Every child matters? Children with type 1 diabetes are being let down by lack of support in school. A report by the UK Children With Diabetes Advocacy Group investigating the range of contemporary educational experiences of children with diabetes at school in the UK. 2007. Available from: www.childrenwithdiabetes.com/uk/Final6207EveryChildMattersCombined.pdf.
- 18.Korytkowski M, Bell D, Jacobsen C, Suwannasari R. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25(11):2836–2848. doi: 10.1016/s0149-2918(03)80337-5. [DOI] [PubMed] [Google Scholar]