Abstract
Background
Glucagon-like peptide-1 (GLP-1) and its analogs are associated with a gamut of physiological processes, including induction of insulin release, support of normoglycemia, β-cell function preservation, improved lipid profiles, and increased insulin sensitivity. Thus, GLP-1 harbors significant therapeutic potential for regulating type 2 diabetes mellitus, where its physiological impact is markedly impaired. To date, GLP-1 analogs are only available as injectable dosage forms, and its oral delivery is expected to provide physiological portal/peripheral concentration ratios while fostering patient compliance and adherence.
Methods
Healthy, fasting, enterically cannulated pigs and beagle canines were administered a single dose of the exenatide-based ORMD-0901 formulation 30 min before oral glucose challenges. Blood samples were collected every 15 min for evaluation of ORMD-0901 safety and efficacy in regulating postchallenge glucose excursions.
Results
Enterically delivered ORMD-0901 was well tolerated by all animals. ORMD-0901 formulations RG3 and AG2 led to reduced glucose excursions in pigs when delivered prior to a 5 g/kg glucose challenge, where area under the curve (AUC)0–120 values were up to 43% lower than in control sessions. All canines challenged with a glucose load with no prior exposure to exenatide, demonstrated higher AUC0–150 values than in their exenatide-treated sessions. Subcutaneous exenatide delivery amounted to a 51% reduction in mean glucose AUC0–150, while formulations AG4 and AG3 prompted 43% and 29% reductions, respectively.
Conclusions
When delivered enterically, GLP-1 (ORMD-0901) is absorbed from the canine and porcine gastrointestinal tracts and retains its biological activity. Further development of this drug class in an oral dosage form is expected to enhance diabetes control and patient compliance.
Keywords: exenatide, glucagon-like peptide-1, oral, ORMD-0901, type 2 diabetes mellitus
Introduction
Glucagon-like peptide-1 (GLP-1) is a 30-amino-acid gut-derived peptide secreted by the intestinal L cells within minutes of food ingestion. Its release is controlled by a combination of neural and endocrine stimuli and by direct nutrient contact with L cells.1 Glucagon-like peptide-1 and its analogs induce pleiotropic physiological effects manifested via stimulation of insulin secretion, glucagon secretion inhibition, reduced appetite and delayed gastric emptying.2,3 In addition, these peptides have been shown to support prolonged normoglycemia, preserve β-cell function, reduce oxidative stress, improve cardiac function, lower blood pressure, improve lipid profiles, reverse fatty liver, and increase insulin sensitivity.3 In this manner, GLP-1 harbors significant therapeutic potential in the management of type 2 diabetes mellitus (T2DM). The effect of GLP-1 on β-cell responsiveness measured upon glucose-induced insulin secretion in T2DM patients and in healthy volunteers has been shown to occur in a dose-dependent manner.4 Furthermore, accruing clinical evidence correlates its plasma concentrations to sustained reductions in hemoglobin A1c and weight loss. Thus, as a novel class of drugs for T2DM, GLP-1-based therapies are attracting increasing attention because of their unique and beneficial mechanisms of action.
However, clinical use of the native form of GLP-1 is limited due to its rapid enzymatic inactivation by the ubiquitous dipeptidyl peptidase-4 (DPP-4) enzymes, resulting in a 2–3 min circulating half-life. To overcome this obstacle, both natural and synthetic, long-acting degradation-resistant peptides, GLP-1 mimetic agents, as well as DPP-4 enzyme inhibitors have been designed and introduced to the clinic. The first GLP-1 mimetic to be approved for clinical use in the United States was the naturally occurring lizard salivary gland exendin-4 peptide (exenatide/Byetta, Amylin Pharmaceuticals), a potent GLP-1 receptor (GLP-1R) agonist with a half-life of 2.4 h. A once-weekly, long-acting exenatide release formulation is currently in advanced clinical trials for T2DM management. Subcutaneous delivery of Liraglutide, a synthetic fatty acylated GLP-1 analog bearing 97% homology to human GLP-1 and a more sustained duration of action (t1/2 ∼13 h), has been recently approved in Europe and the United States for the treatment of T2DM in adults. Oral Sitagliptin (Januvia, Merck and Co., Inc.) and Saxagliptin (OnglyzaTM, AstraZeneca and Bristol-Myers Squibb) represent selective DPP-4-inhibiting agents and have received Food and Drug Administration clearance for the treatment of T2DM.
As T2DM and obesity commonly coexist along with a correlative reduction in insulin sensitivity, drug influence on body weight is an especially important consideration when treating such patients. While DPP-4 inhibitors have been proven potent and are available as oral agents, GLP-1R agonists have been observed to be more effective in their glucose-lowering and anorectic effects. However, to date, GLP-1 analogs are only available in injectable dosage forms. Oral dosage forms of GLP-1 mimetics, in the form of a tablet or capsule, are likely to foster compliance and adherence among patients when compared to parenteral delivery. Such dosage forms would also provide a simpler means of introducing treatment regimens at earlier stages of diabetes and, in turn, improve treatment outcome. Moreover, oral delivery of GLP-1 analogs would preserve portal/peripheral ratios naturally experienced upon its secretion, thereby more closely mimicking its physiological situation. In this study, we sought to examine the efficacy of ORMD-0901, a novel oral exenatide-based formulation designed along the principles of Oramed’s drug delivery system. The study assessed the pharmacodynamic effect of the active ingredient in reducing glucose excursions after a glucose challenge in two animal models. The efficacy of the administered exenatide demonstrated in this study establishes the protective nature of the orally delivered formulation and sets the stage for its further development for the treatment of T2DM.
Methods
The study protocol was approved by the Institutional Animal Care and Use Committee of the Hebrew University and Hadassah Hospital.
Drug
A number of ORMD-0901 formulations were prepared with varying ratios of exenatide [porcine model: Assia Chemical Industries, Ltd., Beer Sheva, Israel; canine model: GL Biochem (Shanghai), Ltd., Shanghai, China] and adjuvants (Table 1). Each formulation was tested on independent test days, with a minimum two-day “washout” period between each.
Table 1.
Composition of Oral GLP-1 Analog Formulations
| Formulation | Carrier (mg) | Adjuvant (mg) | Exenatide (µg) | Dosage volume (ml) | Animal model |
|---|---|---|---|---|---|
| AG2 | 150 | 125 | 50 | 0.8 | porcine |
| AG3 | 150 | 125 | 75 | 0.8 | canine |
| AG4 | 150 | 125 | 100 | 0.8 | canine and porcine |
| AG6 | 150 | 125 | 150 | 0.8 | porcine |
| EG3 | 150 | 75 | 75 | 0.8 | porcine |
| RG3 | 100 | 75 | 75 | 0.8 | porcine |
Study Design
Porcine Model
A single ORMD-0901 dose, containing 50–150 µg exenatide, was administered to healthy, fasting female commercial pigs (29.9 ± 6.3 kg) via an indwelling jejunal cannula. Animals were then challenged with a 3 g/kg (n = 6) or 5 g/kg (n = 3) oral glucose load delivered per os 30 min after ORMD-0901 dosing. Control treatment sessions were performed on the same pigs challenged with an oral glucose load without any prior delivery of ORMD-0901.
Canine Model
A single ORMD-0901 dose, containing 75–100 µg exenatide, was administered to healthy, fasting beagle canines (11.5 ± 1.5 kg) via an indwelling jejunal cannula. Animals (n = 4) were then challenged with an oral glucose load of an 80 ml 50% dextrose solution + 100 g Mugatu dog food (7% protein, 6% fat, 1.2% fiber, 80% moisture) delivered 30 min after ORMD-0901 dosing. Control sessions included glucose challenge in the absence of exenatide or, alternatively, subcutaneous injection of 2.5 µg exenatide (GL Biochem (Shanghai) Ltd.), prepared as per the manufacturer’s instructions and administered to the dogs’ shoulder 30 min before glucose challenge.
All Models
For both models, blood samples were collected every 15 min for up to 2.5 h after dosing to allow for pharmaco-dynamics analyses. Glucose concentration measurements were assessed by glucometer (FreeStyle Lite, Abbott Laboratories, Abbott Park, IL). Tolerance and adverse effects were also monitored.
In this proof of concept study in animal models, we did not measure hormonal changes or GLP-1 levels. It has been, however, demonstrated by Beglinger and colleagues5 that GLP-1 can be absorbed when given orally, induces a potent effect on insulin release, and suppresses ghrelin levels.
Data Analysis
Parameters measured included safety and monitoring of glucose behavior, including Cmax and Tmax and area under the curve (AUC) of the 0–120 min or 0–150 min postdosing period for the porcine and canine models, respectively. Mean plasma glucose response curves were plotted as a function of time. Comparisons were made between formulations and control sessions, where animals were challenged with a glucose load without any prior treatment with ORMD-0901. Glucose AUCs were calculated using the trapezoidal rule. Paired t tests were used to compare the significance of changes in glucose levels seen in control versus ORMD-0901 treatment session. Due to small sample sizes, p-value directionality was employed to determine rejectability of the null hypothesis and to indicate any treatment-related trends.
Results
Enterically delivered ORMD-0901 was well tolerated by all animals, and no adverse reactions were noted throughout the study.
Porcine Response to ORMD-0901
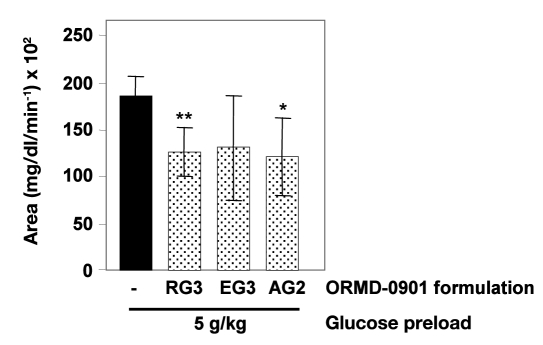
Exenatide delivered in conjunction with Oramed’s proprietary oral delivery formulations significantly reduced glucose excursions in pigs receiving formulations RG3 (75 µg exenatide) or AG2 (50 µg exenatide) prior to a 5 g/kg glucose challenge (Figure 1; p = 0.004 and .041, respectively). Mean AUC values of pigs pretreated with ORMD-0901 were up to 43% lower than control sessions, where animals were treated with glucose alone (Table 2, Figure 1). Individual animal responses demonstrated up to 100% lower glucose Cmax values, relative to baseline, after 5 g/kg glucose loads, when pretreated with the various GLP-1 analog formulations (Table 2). Animals pretreated with ORMD-0901 formulations AG4 and AG6 prior to a 3 g/kg glucose challenge exhibited a 20–82% drop in Cmax values, relative to baseline, when compared to nontreated animals (data not shown). However, overall AUC calculations of AG4 and AG6 sessions did not show any significant decrease in ORMD-0901-dosed pigs when compared to their respective control sessions.
Figure 1.
Pharmacodynamic evaluation of ORMD-0901 formulations in glucose-challenged pigs: mean AUC0–120 of glucose responses of healthy, cannulated pigs to ORMD-0901. Pigs were treated with various formulations of ORMD-0901 containing 50 µg (formulation AG2, n = 4) or 75 µg (formulations RG3 and EG3, n = 6 and n = 3, respectively) exenatide 30 min prior to a glucose challenge of 5 g/kg. Blood was drawn every 15 min from start of experiment, and blood glucose levels were determined using a glucometer. The single asterisk represents p = .041, and the double asterisk represents p = .004.
Table 2.
Pharmacodynamic Effect of ORMD-0901 Formulations in Porcines Challenged with 5 g/kg Glucose
| Treatment | ORMD-0901 formulation | Exenatide dose (µg) | Pig ID | Cmax (mg/dl) | % drop from control Cmax | Tmax (min) | AUC0–120 (mg/dl*min-1) | % drop from control AUC |
|---|---|---|---|---|---|---|---|---|
| 5 g/kg glucose | 2011a | 182 | 75 | 17550 | ||||
| 2099a | 207 | 45 | 19752 | |||||
| 1952 | 117 | 30 | 16778 | |||||
| ORMD-0901 + 5 g/kg glucose | RG3 | 75 | 2011b | 122 | 33 | 50 | 12238 | 30 |
| 2099a | 137 | 34 | 68 | 14654 | 26 | |||
| 1952 | 94 | 20 | 75 | 9630 | 43 | |||
| EG3 | 75 | 2011a | 164 | 10 | 45 | 15588 | 11 | |
| 1952 | — | 100 | — | 8078 | 52 | |||
| AG2 | 50 | 2099 | 136 | 34 | 120 | 11183 | 43 | |
| 2011a | 160 | 12 | 53 | 15494 | 12 | |||
| 1952 | — | 100 | — | 6893 | 59 |
Value represents average of two sessions with identical treatment.
Value represents average of three sessions with identical treatment.
Canine Response to ORMD-0901
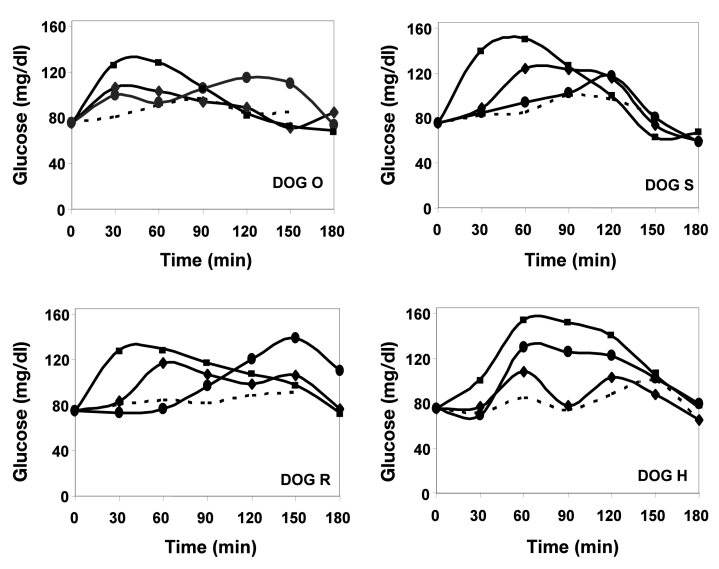
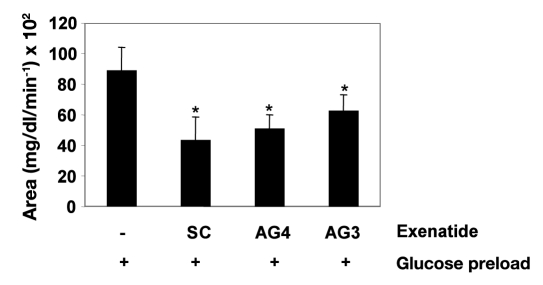
In efforts to assess ORMD-0901 efficacy in an additional animal model, beagle canines were treated with varying formulations of the exenatide-based drug. Of the 20 ORMD-0901 treatment sessions, 18 exhibited lower glucose Cmax values in comparison to their counterpart nontreated glucose test days (Figure 2). Although AG3-dosed dog R demonstrated Cmax values above those recorded for its glucose-only sessions (Figure 2), mean AUC0–150 calculations were still 18% lower under ORMD-0901 influence. ORMD-0901 formulation AG4 (100 µg exenatide) led to a mean 19 ± 8.2% reduction in glucose Cmax values, relative to baseline, which peaked within 30 min of glucose intake. As reference, subcutaneous injection of 2.5 µg exenatide led to a mean attenuation of Cmax values by 32 ± 5.3 % when compared to exenatide-free glucose challenges. All four canines challenged with a glucose load with no prior exposure to ORMD-0901 formulations, or to subcutaneously injected exenatide, demonstrated higher AUC0–150 values than their exenatide-treated sessions (Figure 3). Subcutaneous exenatide delivery amounted to a 51% reduction in mean glucose AUC0–150, while formulations AG4 (100 µg exenatide) and AG3 (75 µg exenatide) prompted 43% and 29% reductions, respectively (p = 0.068, the lowest p value possible for a four-sample group size).
Figure 2.
Glucose responses in glucose-challenged dogs pretreated with ORMD-0901. Mean glucose responses of healthy, cannulated dogs were determined after treatment with glucose alone (squares, n = 4 for each dog) and were graphed as a function of time. Glucose values of dogs treated with subcutaneously delivered exenatide (dashed line, n = 3 for all dogs aside from dog H, where n = 1) or enterically administered ORMD-0901 formulations AG3 (circles, 75 µg exenatide, n = 2 for each dog) or AG4 (diamonds, 100 µg exenatide, n = 3 for each dog) 30 min before glucose challenge were also measured. Blood was drawn every 15 min from start of experiment, and blood glucose levels were determined using a glucometer.
Figure 3.
Pharmacodynamic evaluation of ORMD-0901 formulations in glucose-challenged dogs: mean AUC0–150 of glucose responses of healthy, cannulated dogs to ORMD-0901. Dogs were treated with ORMD-0901 formulation AG3 (n = 8) or AG4 (n = 12), containing 75 or 100 µg exenatide, respectively, 30 min prior to an oral glucose challenge of 80 ml of a 50% dextrose solution + 100 g commercial dog food (7% protein, 6% fat, 1.2% fiber, 80% moisture). For controls, glucose challenge in the absence of exenatide (n = 16) or glucose challenge after subcutaneous injection of 2.5 µg exenatide (n = 10) was performed. Blood was drawn every 15 min from start of experiment, and blood glucose levels were determined using a glucometer. A single asterisk represents p = .068. SC, subcutaneous.
Discussion
While the oral route of drug administration is considered safest, most practical, and associated with higher degrees of patient compliance, polypeptide drugs such as GLP-1 and its analogs are degraded to their constituent amino acids by enzymes located throughout the gastrointestinal tract. These microcomponents are eventually absorbed by the gastrointestinal tract but lack the biological activity of the original molecule. Despite the challenges involved in formulation of oral polypeptide and protein drug preparations, the past few decades have witnessed a great deal of research focusing on attempts to develop noninvasive methods of delivering such drugs, with the oral route clearly being the most convenient and desirable.6
Oramed Pharmaceuticals has developed a drug delivery platform enabling the conversion of parenteral polypeptide drugs into oral dosage forms. The technology employs protectants in combination with an absorption enhancer, all of which are approved pharmacopoeial adjuvants with extensive safety track records. In this manner, passage of active ingredients through the gastrointestinal tract proceeds with minimal degradation. Oramed has demonstrated the efficacy of its technology with insulin-based formulations applied in preclinical as well as in clinical trials in healthy volunteers and in patients with type 1 diabetes mellitus and T2DM.7–9
The current study focused on the performance of the exenatide-based ORMD-0901 and its pharmacodynamic effect on postprandial glucose excursions in two animal species. Formulations RG3 or AG2 effectively blunted glucose surges in a porcine model when compared to controls. Similarly, formulations AG4 and AG3 significantly controlled canine responses to glucose challenges. These data demonstrate that the unique protective mixture allowed for retention of the active ingredient’s biological activity.
Aside from convenience and practicality, oral drug administration may provide for tangible physiological advantages. Such a delivery route recreates hormonal gradients natural to GLP-1 secretion and absorption as determined by the hepatic-portal system, triggering glucose disposal independent of both pancreatic activity and portal vein GLP-1Rs.10,11 Upon release by L cells in response to food ingestion, GLP-1 is rapidly degraded by DPP-4 in the intestinal capillaries and liver. Less than 25% of the secreted GLP-1 leaves the gut in an intact, active form and traverses the intestinal capillaries draining into the portal vein. Of the GLP-1 that reaches the liver, approximately 40–50% is degraded, leaving only approximately 10–15% of the original secreted GLP-1 to reach the systemic circulation.12 Thus a portal/peripheral gradient of 3–4/1 is established for GLP-1, a gradient that is slightly higher than that of insulin (2–3/1). Several lines of evidence alluded to in the following paragaphs suggest that the postprandial portal concentration of the hormone may have physiological and consequently salutary clinical implications.
A number of studies have described a vagus nerve- mediated GLP-1-sensing pathway within the portal vein, partly responsible for regulating GLP-1 action.13–17 Another work has confirmed expression of a GLP-1R in vagal afferent neurons of the hepatoportal region, where its selective inhibition in the portal circulatory network led to significant impairment of glucose tolerance.18,19 Moreover, emerging evidence supports the hypothesis claiming that the effect of GLP-1 on food intake is at least partially mediated via peripheral GLP-1Rs.20 A study in this context has questioned whether gradients of GLP-1 across the different vascular compartments are differentially affected by DPP-4 inhibitors. By direct sampling of the portal vein following a GLP-1 secretory stimulus combined with intravenous administration of a DPP-4 inhibitor (vildagliptin), a 2–3 times higher GLP-1 level was measured in the portal vein when compared to that of the peripheral circulation.21 The study suggests that the divergent effects of the DPP-4 inhibitors may be due to high portal GLP-1 concentration when compared to that in the periphery.
In further support of possible advantages derived from oral GLP-1 delivery, a pharmacokinetic study of oral GLP-1 formulations administered to fasting healthy subjects at escalating doses demonstrated an extended (hours), robust insulinogenic response despite short (minutes) plasma GLP-1 spikes.5 Prolonged reductions in both plasma glucose and ghrelin levels were observed, indicating a multifaceted means of controlling food intake. These long-lasting effects stimulated by transient GLP-1 presence are suggestive of stimulation of portal neuronal signaling pathways. Thus replication of physiological GLP-1 portal/systemic gradients can be achieved by direct portal administration via its oral administration. In contrast, systemic administration of the concentrations required to reach physiological portal vein concentration is considered impractical and has been associated with nausea and vomiting.22
It is well established that the incretin effect is markedly reduced or lost in T2DM patients and likely contributes to glucose intolerance.23 Supraphysiological levels of GLP-1 have been shown to normalize glucose responsiveness, stimulate an insulinotropic effect in advanced T2DM patients, and exhibit dramatic influence on glycemic control, weight loss, and β-cell function.23,24 An oral GLP-1-based drug that replicates the natural physiologic portal–hepatic route of absorption may be beneficial to achieve glycemic control without the risk of hypoglycemia. Furthermore, an oral dosage form is also likely to foster compliance and adherence among patients and allow for intervention early in the course of T2DM with a GLP-1 analog at a stage where resistance to parenteral treatment is prevalent.
Conclusions
In both the porcine and canine animal models, enteric formulations of exenatide based on Oramed’s drug delivery platform have been shown to be absorbed while retaining their biological activity. These formulations pave the way for the development of oral formulations as the main impediments for polypeptide and protein absorption are the degradation in the gastrointestinal tract and transport across the epithelial lining of this tract. Both impediments have been overcome with Oramed’s technology. An oral form of a GLP-1 analog is likely to be an important added tool to the antidiabetes armamentarium. The oral route of administration is the most practical and the one that fosters most compliance and adherence and thus is predicted to have a significant therapeutic impact both on short-term glycemic control and sustainability of this effect.
Acknowledgments
The authors thank Dr. Yehudit Posen for her assistance in preparation of this manuscript.
Abbreviations
- (AUC)
area under the curve
- (DPP-4)
dipeptidyl peptidase-4
- (GLP-1)
glucagon-like peptide-1
- (GLP-1R)
glucagon-like peptide-1 receptor
- (T2DM)
type 2 diabetes mellitus
References
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 2.Vilsbøll T, Holst JJ, Knop FK. The spectrum of antidiabetic actions of GLP-1 in patients with diabetes. Best Pract Res Clin Endocrinol Metab. 2009;23(4):453–462. doi: 10.1016/j.beem.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60(4):470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52(2):380–386. doi: 10.2337/diabetes.52.2.380. [DOI] [PubMed] [Google Scholar]
- 5.Beglinger C, Poller B, Arbit E, Ganzoni C, Gass S, Gomez-Orellana I, Drewe J. Pharmacokinetics and pharmacodynamic effects of oral GLP-1 and PYY3-36: a proof-of-concept study in healthy subjects. Clin Pharmacol Ther. 2008;84(4):468–474. doi: 10.1038/clpt.2008.35. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2(4):289–295. doi: 10.1038/nrd1067. [DOI] [PubMed] [Google Scholar]
- 7.Kidron M, Raz I, Kessler K, Wolfensberger M, Schwob H, Schruefer C. Open label study to assess the safety, pharmacokinetics (PK) and pharmacodynamics (PD) of five oral insulin formulations in healthy volunteers. Presented at: European Association for the Study Of Diabetes (EASD); Rome. 2008. http://www.oramed.com/ufiles/EASD008%20poster%20Final%20handout%20version%20n.pdf. [Google Scholar]
- 8.Kidron M, Raz IM, Wolfensberger M, Schwob H, Schruefer C. Pharmacokinetics (PK) and pharmacodynamics (PD) of oral insulin in healthy subjects. Presented at: American Diabetes Association 68th Annual Meeting; San Francisco, CA. 2008. [Google Scholar]
- 9.Eldor R, Kidron M, Arbit E. Open-label study to assess the safety and pharmacodynamics of five oral insulin formulations in healthy subjects. Diabetes Obes and Metab. 2010;12(3):219–223. doi: 10.1111/j.1463-1326.2009.01153.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson KM, Edgerton DS, Rodewald T, Scott M, Farmer B, Neal D, Cherrington AD. Intraportal GLP-1 infusion increases nonhepatic glucose utilization without changing pancreatic hormone levels. Am J Physiol Endocrinol Metab. 2007;293(4):E1085–E1091. doi: 10.1152/ajpendo.00275.2007. [DOI] [PubMed] [Google Scholar]
- 11.Dardevet D, Moore MC, DiCostanzo CA, Farmer B, Neal DW, Snead W, Lautz M, Cherrington AD. Insulin secretion-independent effects of GLP-1 on canine liver glucose metabolism do not involve portal vein GLP-1 receptors. Am J Physiol Gastrointest Liver Physiol. 2005;289(5):G806–G814. doi: 10.1152/ajpgi.00121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 13.Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol. 1996;271((5 Pt 1)):E808–E813. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- 14.Nishizawa M, Nakabayashi H, Kawai K, Ito T, Kawakami S, Nakagawa A, Niijima A, Uchida K. The hepatic vagal reception of intraportal GLP-1 is via receptor different from the pancreatic GLP-1 receptor. J Auton Nerv Syst. 2000;80((1-2)):14–21. doi: 10.1016/s0165-1838(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 15.Kakei M, Yada T, Nakagawa A, Nakabayashi H. Glucagon-like peptide-1 evokes action potentials and increases cytosolic Ca2+ in rat nodose ganglion neurons. Auton Neurosci. 2002;102((1-2)):39–44. doi: 10.1016/s1566-0702(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 16.D’Alessio D, Vahl T, Prigeon R. Effects of glucagon-like peptide 1 on the hepatic glucose metabolism. Horm Metab Res. 2004;36((11-12)):837–841. doi: 10.1055/s-2004-826172. [DOI] [PubMed] [Google Scholar]
- 17.Balkan B, Li X. Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1449–R1454. doi: 10.1152/ajpregu.2000.279.4.R1449. [DOI] [PubMed] [Google Scholar]
- 18.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D’Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148(10):4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- 19.Balkan B. Effects of glucagon-like peptide-1 (GLP-1) on glucose homeostasis and food intake. Appetite. 2000;35(3):269–270. doi: 10.1006/appe.2000.0354. [DOI] [PubMed] [Google Scholar]
- 20.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150(4):1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hjøllund KR, Hughes TE, Deacon CF, Holst JJ. The dipeptidyl peptidase-4 inhibitor vildagliptin increases portal concentrations of active GLP-1 to a greater extent then the peripheral concentrations. Presented at: The American Diabetes Association Annual Meeting.2008. [Google Scholar]
- 22.Nauck MA, Wollschläger D, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Willms B. Effects of subcutaneous glucagon-like peptide 1 (GLP-1 [7-36 amide]) in patients with NIDDM. Diabetologia. 1996;39:1546–1553. doi: 10.1007/s001250050613. [DOI] [PubMed] [Google Scholar]
- 23.Knop FK, Vilsbøll T, Højberg PV, Larsen S, Madsbad S, Vølund A, Holst JJ, Krarup T. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes. 2007;56(8):1951–1959. doi: 10.2337/db07-0100. [DOI] [PubMed] [Google Scholar]
- 24.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in Type 2 diabetes: a parallel-group study. Lancet. 2002;359(9309):824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]