Abstract
Objective
The aim of this study was to evaluate the impact of personalized decision support (PDS) on metabolic control in people with diabetes and cardiovascular disease.
Research Design and Methods
The German health insurance fund BKK TAUNUS offers to its insured people with diabetes and cardiovascular disease the possibility to participate in the Diabetiva® program, which includes PDS. Personalized decision support is generated by the expert system KADIS® using self-control data and continuous glucose monitoring (CGM) as its data source. The physician of the participating person receives the PDS once a year, decides about use or nonuse, and reports his/her decision in a questionnaire. Metabolic control of participants treated by use or nonuse of PDS for one year and receiving CGM twice was analyzed in a retrospective observational study. The primary outcome was hemoglobin A1c (HbA1c); secondary outcomes were mean sensor glucose (MSG), glucose variability, and hypoglycemia.
Results
A total of 323 subjects received CGM twice, 289 had complete data sets, 97% (280/289) were type 2 diabetes patients, and 74% (214/289) were treated using PDS, resulting in a decrease in HbA1c [7.10 ± 1.06 to 6.73 ± 0.82%; p < .01; change in HbA1ct0–t12 months -0.37 (95% confidence interval -0.46 to -0.28)] and MSG (7.7 ± 1.6 versus 7.4 ± 1.2 mmol/liter; p = .003) within one year. Glucose variability was also reduced, as indicated by lower high blood glucose index (p = .001), Glycemic Risk Assessment Diabetes Equation (p = .009), and time of hyper-glycemia (p = .003). Low blood glucose index and time spent in hypoglycemia were not affected. In contrast, nonuse of PDS (75/289) resulted in increased HbA1c (p < .001). Diabetiva outcome was strongly related to baseline HbA1c (HbA1ct0; p < .01) and use of PDS (p < .01). Acceptance of PDS was dependent on HbA1ct0 (p = .049).
Conclusions
Personalized decision support has potential to improve metabolic outcome in routine diabetes care.
Keywords: computerized medical decision support, continuous glucose monitoring, expert system, outpatient diabetes care, personalized advisory system
Introduction
Decision support systems are an attractive tool for physicians tasked with improving the outcome of diabetes care in their patients. The Karlsburg Diabetes Management System (KADIS®) is an interactive computerized personalized decision support (PDS) system for type 1 and type 2 diabetes. It allows visualization of the current characteristic daily glucose profile, identification of individual weak points, and interactive simulations procedures to predict outcome of therapeutic strategies and lifestyle changes on glucose profiles.1,2
Since 2000, driven by the political leadership and initiatives of the Federal Ministry of Health, the German Bundestag has passed several laws establishing the legal basis for health insurance funds to enable providers of decision support systems to enter selective integrated care contracts. Under these integrated care contracts, health care can be provided in networks and is financed by the Statutory Health Insurance budget.3
Diabetiva® is the provider network for integrated diabetes care of the German health insurance fund BKK TAUNUS. Insured people with diabetes and cardiovascular diseases can be enrolled in the Diabetiva network and receive PDS from their physicians.
The aim of the observational study was to investigate and evaluate the outcome, usefulness, and acceptance of PDS. We retrospectively analyzed data from Diabetiva after 20 months of running the program.
Research Design and Methods
Diabetiva
BKK TAUNUS runs the Diabetiva program on contract for Integrated Health Care according to Section 140 of the German Social Law Book SGB V. Diabetiva is open to insured people diagnosed with diabetes and cardiovascular disease meeting all the following preconditions:
type 1 or type 2 diabetes,
at least 18 years old,
diagnosed with diabetes >1 year ago,
able to perform continuous glucose monitoring (CGM), and
diagnosed with coronary vascular disease [angina pectoris, history of myocardial infarct, or heart failure (New York Heart Association grade 3-4)].
Exclusion criteria are
inability to give consent,
unwillingness to undertake blood glucose testing,
concurrent severe diseases,
end-stage diabetes-related complications, or
pregnancy.
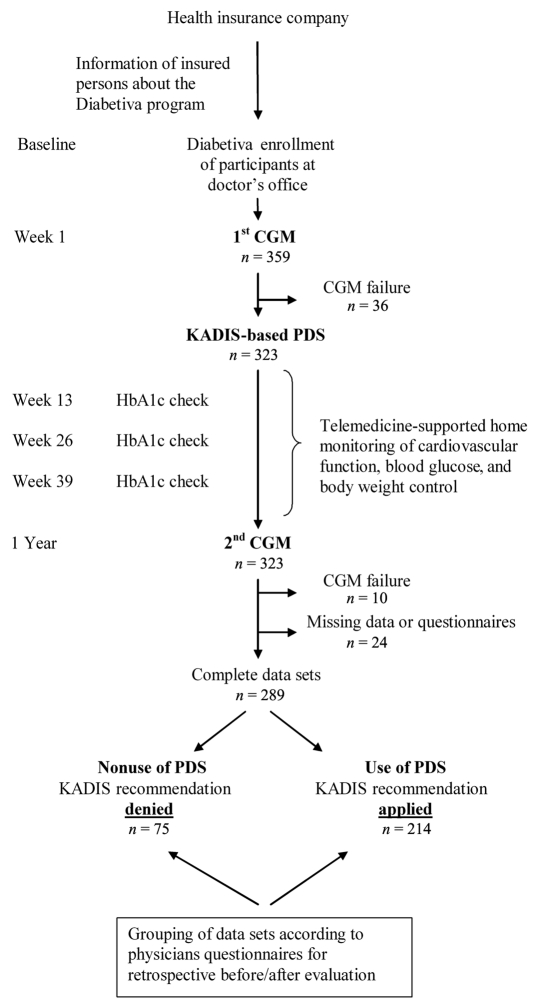
Enrollment into the program is available throughout Germany. BKK TAUNUS informs all insured people eligible for the Diabetiva program. All participants of Diabetiva had the same follow-up (Figure 1). First, the participant initiates the enrollment process and contacts the attending physician. After the enrollment, all participants receive a medical checkup by their general practitioner or diabetes specialist, including venous blood sampling for measurement of hemoglobin A1c (HbA1c). These health checks were repeated every three months. Telemedicine-based observation and analysis of the electrocardiogram was performed by a specialized telemedicine provider (SHL, Düsseldorf, Germany) as described previously.4
Figure 1.
Design of the observational study of the Diabetiva program. To evaluate the impacted of PDS, the metabolic outcome was analyzed and compared retrospectively in patients receiving CGM twice, one at baseline and one after 12 months, and grouped in user or nonuser of PDS.
At enrollment and then once per year, a case manager of the Diabetes Service Center (DCC®) in Karlsburg, Germany, specially trained in glucose monitoring, registers the diabetes history of each participant, records self-control data, performs 72 h CGM, and provides support during CGM.1 Thereafter, the CGM and self-control data are uploaded into the data bank of the DCC Karlsburg. Personalized decision support is generated in the DCC using the model-based expert system KADIS5 for all participants and provided to the attending physicians. The physician decides about use and nonuse of PDS for the next year and documents the decision in a questionnaire without detailing the reasons. To determine the impact of PDS on metabolic control, HbA1c values and CGM at baseline and after one year were compared and analyzed according to the defined outcome parameters; participants were grouped into users and nonusers of PDS as determined by the questionnaire completed by responsible physicians (Figure 1).
KADIS-Based Decision Support
Personalized decision support for type 1 and 2 diabetes5 is generated in four steps using a computerized advisory system.
First, CGM sensor profiles and self-control data (carbohydrate intake, oral hyperglycemic agents, physical activity) are transferred to the KADIS system.
Second, the identification feature of the computer program begins, allowing estimation of personalized model parameters describing the individual insulin/glucose metabolism of a given patient and generating a personalized in silico copy of the patient’s current metabolic status on the computer.
Third, after successful identification, different therapeutic strategies (oral hyperglycemic agents, insulin of different formulas, carbohydrate intake, and exercise) can be tested on a personalized basis by in silico simulation strategies according to the guidelines of the German Diabetes Association in order to predict and to select a therapeutic regimen that may provide the best individual glycemic control.
Fourth, the results of the simulation procedure with the best possible outcome are summarized in a KADIS report, which visualizes and explains the therapeutic strategies for improvement of metabolic control to the treating physician in order to support his/her decision-making process. The physician decides independently whether the PDS is used as suggested, used in a modified version, or not used. Feedback regarding use or nonuse is provided by the physician to the DCC Karlsburg by a questionnaire.
Statistical Analysis
All statistical analyses were carried out using the Statistical Package for the Social Sciences Version 17.0 (SPSS, Chicago, IL). Results are given as mean ± standard deviation for normal distributed parameters or as median and interquartile range for nonnormal distributed parameters. Glycemic variability was described in terms of quality of glycemic control by low blood glucose index (LBGI) and high blood glucose index (HBGI)6 and Glycemic Risk Assessment Diabetes Equation (GRADE).7 Parameters based on CGM were calculated from a continuously recorded glucose profile for each day and were averaged. Hypoglycemia was defined as sensor glucose <3.3 mmol/liter and hyperglycemia as sensor glucose >8.9 mmol/liter. Change in HbA1ct0–t12months (ΔHbA1c) is presented as mean change of the individual values. Group comparisons were made using unpaired Student’s t-test or the Mann–Whitney U test as appropriate. Categorical variables were compared using chi-square test. Within-group changes were tested by paired t-test or Wilcoxon test as appropriate. Multiple regression analysis with stepwise forward selection was performed to reveal which variables were related to ΔHbA1c. Independent variables were HbA1c at study entry, diabetes type, gender, age, body mass index (BMI), type of doctor, therapy, and use or nonuse of decision support. A stepwise logistic regression was used to test the acceptance of PDS. The level of statistical significance was set at p = .05.
Results
Patient Characteristics
A total of 359 participants were enrolled into the Diabetiva program (Figure 1) and followed for more than one year. 323 participants received CGM twice and their attending physician received an offer for PDS, and 34 participants had to be excluded from the final analysis due to CGM failure or missing data or questionnaires. Outcome of Diabetiva was finally retrospectively compared for 289 participants treated with use (214/289) or nonuse (75/289) of KADIS-based PDS as documented in the physician questionnaire. The majority of participants was diagnosed with type 2 diabetes (280; 96.9%) and was greater than 65 years old (Table 1). Participants who were treated according PDS were older compared to participants treated without PDS.
Table 1.
Patient Demographics and Primary and Secondary Outcomesa
| Parameter | PDS | |||
|---|---|---|---|---|
| Use | Nonuse | |||
| Gender (female/male) | 65/149 | 26/49 | ||
| Age (years) | 66.3 ± 8.6 | 63.8 ± 10.0b | ||
| Diabetes duration (years) | 11 (6–19) | 9 (5–17) | ||
| BMI (kg/m2) | 30.6 ± 4.9 | 31.1 ± 5.7 | ||
| Diabetes type (1/2) | 8/206 | 1/74 | ||
| Therapy (diet/OHA/OHA+insulin/insulin) | 25/60/49/80 | 4/29/18/24 | ||
| Carbohydrate intake (bread exchange unit) | 11.5 ± 3.2 | 12.0 ± 3.9 | ||
| Diabetes specialist/general practitioner | 108/106 | 34/41 | ||
| Before | After | Before | After | |
| HbA1c (%) | 7.10 ± 1.06 | 6.73 ± 0.82c | 6.83 ± 0.81b | 7.28 ± 0.83a,a |
| HbA1c <6.5 (%) | 32.7 | 46.7c | 34.7 | 16.0c,4 |
| MSG (mmol/liter) | 7.68 ± 1.64 | 7.39 ± 1.26e | 8.07 ± 1.62 | 8.13 ± 1.92d |
| Glucose variability: | ||||
| range (mmol/liter) | 7.65 ± 2.88 | 7.21 ± 2.95e | 8.22 ± 3.11 | 8.22 ± 2.72d |
| Hyperglycemia (h/day) | 4.2 (1.6–9.3) | 3.6 (1.1–7.1)e | 5.7 (3.3–9.3)b | 6.1 (2.9–10.8)d |
| Hypoglycemia (min/day) | 0 (0–20) | 0 (0–15) | 0 (0–11) | 0 (0–20) |
| HBGI | 1.3 (0.6–2.7) | 1.1 (0.5–2.2)c | 1.8 (1.1–3.0)b | 1.8 (0.9–3.5)d |
| LBGI | 0.2 (0.04–0.7) | 0.3 (0.04–0.6) | 0.1 (0.03–0.5) | 0.3 (0.04–0.6) |
| GRADE | 5.1 (3.1–7.7) | 4.7 (3.1–6.9)e | 5.9 (4.5–7.7)b | 5.8 (3.7–8.6)d |
| Carbohydrate intake (bread exchange unit) | 11.5 ± 3.2 | 11.7 ± 3.1 | 12.0 ± 3.9 | 12.5 ± 3.7 |
| Insulin (IU) | 53 (34–77) | 55 (32–80) | 46 (35–76) | 48 (26–79) |
| BMI (kg/m2) | 30.6 ± 4.9 | 30.5 ± 4.8 | 31.1 ± 5.7 | 31.4 ± 5.3 |
Data are given as mean ± standard deviation or median (interquartile range). Euglycemic range for blood glucose is 3.9–8.9 mmol/liter. OHA; oral hyperglycemic agent; IU, insulin units.
p < .05 PDS use versus nonuse.
p < .01 versus before.
p < .01 PDS use versus nonuse.
p < .05.
Outcome of Diabetiva after Use or Nonuse of Personalized Decision Support
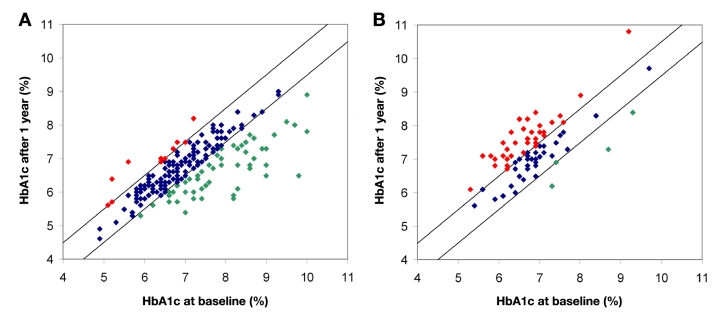
Use of PDS resulted in improved metabolic control (Table 1; Figure 2) as demonstrated by a significant decrease in HbA1c [7.10 ± 1.06% to 6.73 ± 0.82%; p < .01; ΔHbA1c -0.37 (95% confidence interval (CI) -0.46 to -0.28)], which was associated with improved mean sensor glucose (MSG) (7.7 ± 1.6 versus 7.4 ± 1.3 mmol/liter; p = .011). In contrast, nonuse of decision support was associated with increased HbA1c [p < .01; ΔHbA1c 0.44 (95% CI 0.30 to 0.59)].
Figure 2.
Comparison of HbA1ct0 and 12 months later in patients (A) treated with PDS and (B) treated conventional without use of PDS. For each patient, HbA1ct0 (x axis) is plotted versus HbA1c after one year (y axis) treated by use or nonuse of KADIS-based PDS. The diagonal area represents HbA1ct0 ± 0.5%. Green dots are patients in whom the HbA1c decreased over 0.5%. Red dots represent patients were the HbA1c increased >0.5%.
The outcome of Diabetiva was dependent on baseline HbA1c (HbA1ct0). If the HbA1ct0 was <6.5%, the HbA1c in PDS-treated patients was stable. The HbA1c decreased if the HbA1ct0 was above 6.5% [HbA1ct0 6.5–7.0: 6.78→6.56% (n = 70); HbA1ct0 >7.0–7.5: 7.28→6.88% (n = 45); HbA1ct0 >7.5–8.0: 7.78→7.38% (n = 38); HbA1ct0 >8.0: 8.87→7.58% (n = 26); Figure 3]. Usage of PDS did not increase BMI.
Figure 3.
Outcome of the KADIS-based PDS in dependence of HbA1ct0. Comparison is given for patients with use (left) or nonuse (right) of PDS. HbA1ct0 is grouped into <6.5, 6.5–7.0, 7.0–7.5, 7.5–8.0, and >8.0. The asterisks represent p < .05 versus HbA1ct0.
In contrast, patients without PDS treatment had an increase in HbA1c with the exception of the group where HbA1ct0 was above 8%. Multiple regression analysis demonstrated that achieved reduction of HbA1c (ΔHbA1c) was strongly related to HbA1ct0 (b = -0.386, standard error = 0.032, p < .01) and use or nonuse of PDS (β = -0.719,standard error = 0.074, p < .01, R2 = 48.9%). Age, gender, diabetes duration, type of diabetes, therapy, type of doctor, bread exchange unit (1 bread exchange unit equals 12–15 g carbohydrate), and BMI had no influence on outcome.
Evaluation of questionnaires revealed that PDS was used by 74% (214/289) of participating physicians (Table 1). The frequency of usage was greater in patients with higher HbA1ct0. Logistic regression analysis demonstrated that use of PDS was significantly dependent on HbA1ct0 (β = 0.290 ± 0.148; p = .049). There was no relation to gender, type of doctor, type of diabetes, BMI, or diabetes duration.
Effect of Use or Nonuse of Decision Support on Glucose Variability
After one year of running KADIS-based PDS, variability of the CGM-profiles was significantly reduced if PDS was used (Table 1). Use of PDS resulted in reduced HBGI (1.3 to 1.1; p = .006), range (7.6 to 7.2 mmol/liter; p = .039), GRADE (5.1 to 4.7; p = .027), and time in hyperglycemia (4.2 to 3.6 h/day; p = .004). In contrast, there was no improvement in glucose variability within 12 months in the nonuser group in terms of HBGI [1.8 to 1.8; not significant (ns)], range (8.2 to 8.2 mmol/liter; ns), GRADE (5.9 to 5.8; ns), or time in hyperglycemia (5.7 to 6.1 h/day; ns). Differences in glucose variability were significant between the user and nonuser groups. Time in hypoglycemia and LBGI were not affected in both groups.
Discussion
Current diabetes care systems do not sufficiently meet the personal needs and requirements necessary to significantly improve outcomes in diabetes patients. This has particularly notable consequences for patients with both diabetes and cardiovascular disease, as these patients are at a much higher risk for severe cardio-vascular events, including sudden death.8 Additionally, they frequently suffer from poor metabolic control, high glucose variability, and more frequent hypoglycemic events. Innovative approaches are therefore needed to improve care.9 The chronic care model10 suggests the benefit of integrating decision support and clinical information systems in the management of chronic diseases. We introduced a PDS system into routine diabetes care using a model-based computerized expert system. KADIS generates a virtual copy of the glucose/insulin metabolism of a given patient and allows performance of N of 1 trials11 in silico to identify the most appropriate individual therapeutic strategies rapidly and safely.
The Diabetiva program offers KADIS-based PDS on a broad scale for use in routine diabetes care of patients with concomitant cardiovascular disease. If this PDS is applied, insured persons apparently benefit with improved metabolic control after one year of treatment. Use of PDS reduced HbA1c and MSG without increased time below the target glucose value of 3.3 mmol/liter. At present, there is no similar system available that can be used readily in routine diabetes care.
The term “decision support“ is not clearly defined,12–17 making comparison of our results with other studies applying decision support difficult. For example, decision support is in some cases defined as integration of guidelines into the care process combined with computerized feedback systems. Computerized advisory systems have been used to illustrate care pathways and to implement expert suggestions in finding appropriate therapeutic strategies in diabetes patients.1,18 In general, there is great variety in the characteristics and features of computerized decision support systems and its expected impact on metabolic outcome.13,16,17 For example, DiasNet, a diabetes advisory system for communication and education via the Internet, achieved a HbA1c reduction of 1.2% in poorly controlled diabetes patients.12 In this application, a “general“ patient is used for in silico simulations, and the patients included had HbA1ct0 values above 8–9%.
In agreement with earlier studies, our KADIS-based PDS was effective especially for type 2 diabetes patients.5 Due to the small number of type 1 diabetes patients enrolled, the effect of PDS cannot be determined in this subgroup appropriately. The impact of PDS in type 2 diabetes was strongly dependent on HbA1ct0. Similar to results from other studies, the greatest impact was observed in patients with high HbA1ct0;1,5 in our study, the greatest effect (-1.3%) was seen within 1 year in patients with HbA1ct0 >8%. Similar results were reported by McMahon and colleagues.19 They observed a reduction of HbA1c by 1.9% in poorly controlled diabetes patients with HbA1ct0 ≥9% using a Web-based care management on glucose and blood pressure control over 12 months. In type 2 diabetes, glucose monitoring combined with recommendations provided by short message service using cell phones or Internet communication technology20 resulted in a reduction of HbA1c by 1.1% at HbA1ct0 of 8.0% after 6 months.21
In the case of nonuse of PDS by the participating physician, we observed a tendency of HbA1c reduction only in patients with HbA1ct0 >8%. In fact, there was a significant increase of HbA1c observed in patients with HbA1ct0 between 6% and 8%. The lack of positive outcome in the case of nonuse of PDS suggests that improved data management and analysis of clinical data alone do not necessarily result in improved metabolic control. Similar results were published by Meigs and associates22 who performed a randomized trial designed to assess the impact of a Web-based electronic medical record. In this study, the authors found modest improvement in metabolic control. The physicians ordered more HbA1c tests; the impact of increased test frequency was minor, translating into a HbA1c reduction of 0.2%.21 Interestingly, a meta-analysis of teleconsultation studies did not show a reduction in HbA1c,23 and the authors suggested the need for interactive systems that integrate monitoring and personalized feedback functions in order to achieve better outcomes. Jackson and coworkers24 postulated that information technology may assist physicians and patients in improving outcomes in diabetes care.
Importantly, our findings are in full agreement with the results reported in the COMPETE II randomized trial,18 which applied individualized decision support and reminders as a Web-based diabetes tracker, including the provision of electronic medical record and an automated telephone reminder. In this trial, significant improvement in glycemic control was achieved as demonstrated by HbA1c reduction of -0.28% in the intervention group, with a HbA1ct0 of 7%. These results are similar to our findings. Like Holbrook and colleagues,18 we evaluated a complex approach in our retrospective observational study and achieved, with use of PDS, a reduction of HbA1c by -0.4%. However, the two studies are comparable only to a certain degree due to different decision support approach. In contrast to the online applications, the core of our approach is the generation of PDS by in silico simulations of therapeutic options to find an individually appropriate therapeutic approach, which is provided to the responsible physician. As stated by Campbell and associates,25 the evaluation of complex interventions to improve health care is difficult and requires use of qualitative and quantitative evidence. Future studies are necessary to develop evaluation methods for studies using computerized decision support.
The present observational study provides a retrospective analysis of the first roll out of KADIS-based PDS to a large group of users. The findings imply a great potential for PDS to improve diabetes care. However, there are limitations that need to be addressed in future randomized studies. The enrollment procedure might account for a selection bias. Insured persons decide by themselves whether they participate in the program, which could result in the selection of motivated participants. The logistic regression analysis indicates that the use of PDS by the attending physician is affected by the HbA1ct0. Apparently, higher HbA1ct0 levels caused higher acceptance of PDS. As use and nonuse of PDS are the criteria for the retrospective grouping, the HbA1ct0 values might be affected by this decision behavior of the participating physicians. The questionnaires did not ask for the reasons leading to the decision of the participating physicians.
Importantly, the improved metabolic control after application of KADIS-based PDS was not associated with increased risk of hypoglycemia. By analyzing the sensor profiles, we found no effect of PDS on LBGI and the area under the curve in the below-target range. This is especially important as the Diabetiva program enrolls diabetes patients with concomitant cardiovascular disease. In these patients, hypoglycemia is considered a main cause for increased mortality due to cardiac dysrhythmia.26 The impact of our results could have tremendous impact for patients with diabetes and cardiovascular disease, as the use of PDS resulted in decreased HbA1c and glucose variability, both of which are major risk factors for elevated sudden death due to cardiovascular events.8
The impact of our KADIS-based PDS approach might be even stronger if specially trained case managers and diabetes patients could use the personalized simulation procedure for its own educational and self-management purposes, for example, to test the effect of exercise or diet on individual glucose patterns. An additional option might be to use PDS to optimize quality of home care by generating patient-focused advice that can be seamlessly integrated into the practice workflow.15
Conclusion
We demonstrated in this retrospective observational study that people with diabetes can benefit from PDS without being at higher risk for hypoglycemia. Based on the high prevalence of diabetes, PDS has great potential for improving diabetes care. Patients and physicians as well as the entire diabetes health care system can benefit from application of PDS by improving the quality of health care and reducing the economic burden.
Glossary
Abbreviations
- (BMI)
body mass index
- (CI)
confidence interval
- (CGM)
continuous glucose monitoring
- (GRADE)
Glycemic Risk Assessment Diabetes Equation
- (HbA1c)
hemoglobin A1c
- (HbA1ct0)
baseline HbA1c
- (ΔHbA1c)
change in hemoglobin A1ct0–t12months
- (HBGI)
high blood glucose index
- (LBGI)
low blood glucose index
- (MSG)
mean sensor glucose
- (ns)
not significant
- (PDS)
personalized decision support
References
- 1.Augstein P, Vogt L, Kohnert KD, Freyse EJ, Heinke P, Salzsieder E. Outpatient assessment of Karlsburg Diabetes Management System-based decision support. Diabetes Care. 2007;30(7):1704–1708. doi: 10.2337/dc06-2167. [DOI] [PubMed] [Google Scholar]
- 2.Rutscher A, Salzsieder E, Thierbach U, Fischer U, Albrecht G. KADIS—a computer-aided decision support system for improving the management of type-I diabetes. Exp Clin Endocrinol. 1990;95(1):137–147. doi: 10.1055/s-0029-1210946. [DOI] [PubMed] [Google Scholar]
- 3.Schlette S, Lisac M, Blum K. Integrated primary care in Germany: the road ahead. Int J Integr Care. 2009;9:e14. doi: 10.5334/ijic.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birati EY, Malov N, Kogan Y, Yanay Y, Tamari M, Elizur M, Steinberg DM, Golovner M, Roth A. Vigilance, awareness and a phone line: 20 years of expediting CPR for enhancing survival after out-of-hospital cardiac arrest The ‘SHL’-Telemedicine experience in Israel. Resuscitation. 2008;79(3):438–443. doi: 10.1016/j.resuscitation.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Salzsieder E, Augstein P, Vogt L, Kohnert KD, Heinke P, Freyse EJ, Azim Ahmed A, Metwali Z, Salman I, Attef O. Telemedicine-based KADIS combined with CGMS has high potential for improving outpatient diabetes care. J Diabetes Sci Technol. 2007;1(4):511–521. doi: 10.1177/193229680700100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21(11):1870–1875. doi: 10.2337/diacare.21.11.1870. [DOI] [PubMed] [Google Scholar]
- 7.Hill NR, Hindmarsh PC, Stevens RJ, Stratton IM, Levy JC, Matthews DR. A method for assessing quality of control from glucose profiles. Diabet Med. 2007;24(7):753–758. doi: 10.1111/j.1464-5491.2007.02119.x. [DOI] [PubMed] [Google Scholar]
- 8.Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394–403. doi: 10.7326/0003-4819-151-6-200909150-00137. [DOI] [PubMed] [Google Scholar]
- 9.Davidson MB. How our current medical care system fails people with diabetes: lack of timely, appropriate clinical decisions. Diabetes Care. 2009;32(2):370–372. doi: 10.2337/dc08-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warm EJ. Diabetes and the chronic care model: a review. Curr Diabetes Rev. 2007;3(4):219–225. doi: 10.2174/1573399076. [DOI] [PubMed] [Google Scholar]
- 11.Tsapas A, Matthews DR. N of 1 trials in diabetes: making individual therapeutic decisions. Diabetologia. 2008;51(6):921–925. doi: 10.1007/s00125-008-0983-2. [DOI] [PubMed] [Google Scholar]
- 12.Plougmann S, Hejlesen OK, Cavan DA. DiasNet—a diabetes advisory system for communication and education via the internet. Int J Med Inform. 2001;64((2-3)):319–330. doi: 10.1016/s1386-5056(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 13.Hetlevik I, Holmen J, Kruger O, Kristensen P, Iversen H, Furuseth K. Implementing clinical guidelines in the treatment of diabetes mellitus in general practice. Evaluation of effort, process, and patient outcome related to implementation of a computer-based decision support system. Int J Technol Assess Health Care. 2000;16(1):210–227. doi: 10.1017/s0266462300161185. [DOI] [PubMed] [Google Scholar]
- 14.Holbrook A, Xu S, Banting J. What factors determine the success of clinical decision support systems? AMIA Annu Symp Proc. 2003:862. [PMC free article] [PubMed] [Google Scholar]
- 15.Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, Sam J, Haynes RB. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 16.Klonoff DC, True MW. The missing element of telemedicine for diabetes: decision support software. J Diabetes Sci Technol. 2009;3(5):996–1001. doi: 10.1177/193229680900300501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mollon B, Chong J, Jr, Holbrook AM, Sung M, Thabane L, Foster G. Features predicting the success of computerized decision support for prescribing: a systematic review of randomized controlled trials. BMC Med Inform Decis Mak. 2009;9:11. doi: 10.1186/1472-6947-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holbrook A, Thabane L, Keshavjee K, Dolovich L, Bernstein B, Chan D, Troyan S, Foster G, Gerstein H, COMPETE II Investigators Individualized electronic decision support and reminders to improve diabetes care in the community: COMPETE II randomized trial. CMAJ. 2009;181(1-2):37–44. doi: 10.1503/cmaj.081272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahon GT, Gomes Hohne S, Hickson HS, Hu TM, Levine BA, Conlin PR. Web-based care management in patients with poorly controlled diabetes. Diabetes Care. 2005;28(7):1624–1629. doi: 10.2337/diacare.28.7.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HS, Jeong HS. A nurse short message service by cellular phone in type-2 diabetic patients for six months. J Clin Nurs. 2007;16(6):1082–1087. doi: 10.1111/j.1365-2702.2007.01698.x. [DOI] [PubMed] [Google Scholar]
- 21.Cho JH, Chang SA, Kwon HS, Choi YH, Ko SH, Moon SD, Yoo SJ, Song KH, Son HS, Kim HS, Lee WC, Cha BY, Son HY, Yoon KH. Long-term effect of the Internet-based glucose monitoring system on HbA1c reduction and glucose stability: a 30-month follow-up study for diabetes management with a ubiquitous medical care system. Diabetes Care. 2006;29(12):2625–2631. doi: 10.2337/dc05-2371. [DOI] [PubMed] [Google Scholar]
- 22.Meigs JB, Cagliero E, Dubey A, Murphy-Sheehy P, Gildesgame C, Chueh H, Barry MJ, Singer DE, Nathan DM. A controlled trial of web-based diabetes disease management: the MGH diabetes primary care improvement project. Diabetes Care. 2003;26(3):750–757. doi: 10.2337/diacare.26.3.750. [DOI] [PubMed] [Google Scholar]
- 23.Verhoeven F, van Gemert-Pijnen L, Dijkstra K, Nijland N, Seydel E, Steehouder M. The contribution of teleconsultation and video-conferencing to diabetes care: a systematic literature review. J Med Internet Res. 2007;9(5):e37. doi: 10.2196/jmir.9.5.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson CL, Bolen S, Brancati FL, Batts-Turner ML, Gary TL. A systematic review of interactive computer-assisted technology in diabetes care. Interactive information technology in diabetes care. J Gen Intern Med. 2006;21(2):105–110. doi: 10.1111/j.1525-1497.2005.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, Tyrer P. Framework for design and evaluation of complex interventions to improve health. BMJ. 2000;321(7262):694–696. doi: 10.1136/bmj.321.7262.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill GV, Woodward A, Casson IF, Weston PJ. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes—the ‘dead in bed’ syndrome revisited. Diabetologia. 2009;52(1):42–45. doi: 10.1007/s00125-008-1177-7. [DOI] [PubMed] [Google Scholar]