Abstract
Devices for continuous glucose monitoring (CGM) are currently a major focus of research in the area of diabetes management. It is envisioned that such devices will have the ability to alert a diabetes patient (or the parent or medical care giver of a diabetes patient) of impending hypoglycemic/hyperglycemic events and thereby enable the patient to avoid extreme hypoglycemic/hyperglycemic excursions as well as minimize deviations outside the normal glucose range, thus preventing both life-threatening events and the debilitating complications associated with diabetes. It is anticipated that CGM devices will utilize constant feedback of analytical information from a glucose sensor to activate an insulin delivery pump, thereby ultimately realizing the concept of an artificial pancreas. Depending on whether the CGM device penetrates/breaks the skin and/or the sample is measured extracorporeally, these devices can be categorized as totally invasive, minimally invasive, and noninvasive. In addition, CGM devices are further classified according to the transduction mechanisms used for glucose sensing (i.e., electrochemical, optical, and piezoelectric). However, at present, most of these technologies are plagued by a variety of issues that affect their accuracy and long-term performance. This article presents a critical comparison of existing CGM technologies, highlighting critical issues of device accuracy, foreign body response, calibration, and miniaturization. An outlook on future developments with an emphasis on long-term reliability and performance is also presented.
Keywords: accuracy, calibration, continuous glucose monitoring, glucose detection, glucose sensors, invasiveness
Introduction
Diabetes Mellitus: A Cause of Concern
Diabetes mellitus is a metabolic disorder in which the blood glucose levels fluctuate outside the normal range as a result of underproduction or underutilization of the hormone insulin.1 Diabetes is classified into two types, namely, type 1 diabetes mellitus (T1DM), which results from underproduction of insulin as result of loss of insulin-producing beta cells in the pancreas, and type 2 diabetes (T2DM), which is due to underutilization of insulin produced in the pancreas.1 There are currently about 230 million diabetes patients around the world, out of which approximately 90% have T2DM.2 Complications arising from diabetes can be both acute and long term and include hypoglycemia, ketoacidosis, coma, renal failure, amputations, neuropathy, and retinal damage.1,3
Continuous Monitoring of Glucose
As suggested by the Diabetes Control and Complications Trial report, complications arising from diabetes can be reduced and even prevented via careful management that includes regular checking of glucose levels.4 It is recommended that a T1DM patient should check his/her glucose levels at least four times per day, while a T2DM patient should check his/her glucose levels at least two times per day.5 For this, at present, most diabetes patients rely on glucose strips along with hand-held glucose meters that record glucose levels in blood drawn via finger pricking [self-monitoring of blood glucose (SMBG)].6 However, the pain associated with finger pricking together with the inability of test strips to reflect the overall trend in the glucose level of individual patients, i.e., the direction (whether the glucose levels are increasing or decreasing at any point in time) and the pattern associated with the patients’ daily habits, renders user-independent continuous glucose monitoring (CGM) a highly desirable proposition. Use of CGM devices will enable the identification of glucose trends, thereby assisting physicians in optimizing treatment plans and facilitating appropriate clinical decisions in cases of emergency. In addition, theoretical modeling has predicted that an additional 5 years of life, 8 years of sight, 6 years free from kidney disease, and 6 years free from amputations can be gained by a diabetes patient who follows tight CGM glucose control versus the standard SMBG.7 Imagine the improvement that could be made with a CGM linked to an insulin delivery pump and fully realizing the concept of artificial pancreas! With considerable research efforts, it could also be possible to eventually achieve glucose control that mirrors that of a normally functioning body. The potential beneficial effects of CGM has spurred a vast amount of research and developmental activities.8
Scope of This Review
This review surveys the current status of devices for continuous monitoring of glucose with an emphasis on the various technologies that are under investigation. After a brief overview of the invasiveness of CGM devices and the core sensing technologies employed, factors that are likely to dictate their success in terms of patient benefit and patient compliance (such as accuracy, reliability, lifetime, and comfort) are discussed. While most of the concepts, benefits, and drawbacks are specific for particular CGM devices and technologies, there is applicability to other CGM approaches employing similar principles. Particular emphasis is placed on technologies that have been tested in vivo. Last but not least, this article concludes with an overview of future opportunities and developments required for the widespread use of CGM devices. Here, the reader is advised that this article focuses only on home CGM approaches and does not explicitly discuss the class of blood-based continuous glucose systems being developed for inpatient use.
Current Sensor Technologies
A CGM device typically consists of (i) a glucose sensor that continuously measures physiological (blood or interstitial fluid [ISF]) glucose levels, (ii) an electronic processing unit that is in communication (wired or wireless) with the glucose sensor, and (iii) a data display unit. These data may then be used to determine whether the patient requires insulin. In futuristic closed-loop CGM systems, in addition to the aforementioned components, an insulin delivery unit and possibly a glucagon delivery unit will be incorporated. Through a patient-specific algorithm, the correct dosing of insulin or glucagon will be provided to the patient via feedback from the electronic processing units.
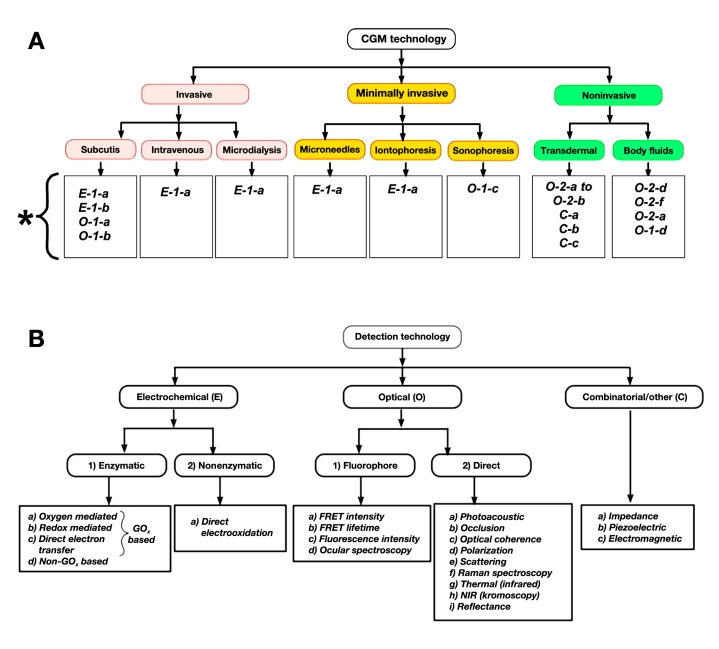
Placement of the glucose sensor (whether it penetrates the skin or not) and its communication with the electronic processing unit defines the invasiveness of a CGM device. On this basis, CGM devices can be classified into three categories: (1) invasive (totally implantable) sensors, (2) minimally invasive sensors, and (3) noninvasive sensors (Figure 1A). Placement of the glucose sensor, in turn, is dictated by the principle of its transduction mechanism, and various glucose sensing mechanisms have been reported (Figure 1B). In this section, a brief explanation of various sensing technologies is presented followed by an overview of the invasiveness of the CGM devices. The reader is advised that this section focuses only on the principle of operation. The advantages and disadvantages of each of these approaches are listed in tables and are analytically discussed later in this article.
Figure 1.
Classification of various CGM technologies according to their (a) invasiveness and (b) transduction mechanism of the sensor. Code definitions in Figure 1A correspond to the respective transduction mechanisms shown in Figure 1B. The asterisk in Figure 1a displays code definitions corresponding to the detection technologies shown in Figure 1B. For example, code E-1-a corresponds to electrochemical (E) detection based on enzymatic (1) reaction that is oxygen mediated (a).
Glucose Detection Methodologies
Glucose Detection Based on Electrochemical Approaches
Glucose detection using electrochemical-based methods can be broadly categorized under enzymatic and non-enzymatic approaches.
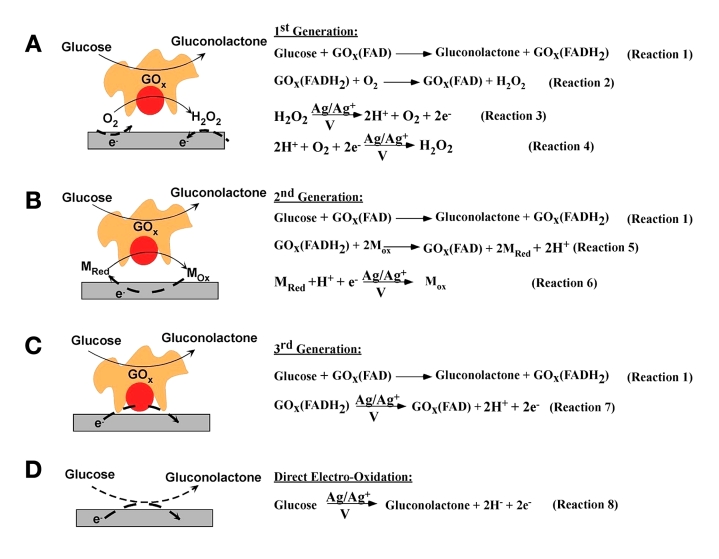
Figure 2 illustrates various enzymatic (Figures 2A–C) and non-enzymatic (Figure 2D) approaches for D-glucose (dextrose monohydrate) detection. Typically, the glucose- specific enzyme [glucose oxidase (GOx)] catalyzes the oxidation of glucose to gluconolactone. In this process, the enzyme (i.e., the enzyme’s redox cofactor, flavin adenine mononucleotide) is converted to its reduced form (flavin adenine dinucleotide) as shown in reaction 1 of Figure 2. The process by which the reduced form of the enzyme is converted back to its oxidized form defines the “generation” of biosensors.
Figure 2.
Various modes of electrochemical detection of glucose: (A) first-generation biosensors based on the use of natural oxygen cofactor, (B) second-generation biosensors based on artificial redox mediators, (C) third-generation biosensors based on direct electron transfer between GOx and the electrode, and (D) direct electro-oxidation of glucose.
In first-generation biosensors, the reduced form of the enzyme is converted back to the oxidized form by ambient oxygen. This leads to the production of H2O2 as shown in reaction 2 of Figure 2A.9,10 The glucose concentration can be correlated to the amperometric signal obtained either via the electrochemical oxidation of the produced H2O2 (reaction 3 of Figure 2A) or via the electrochemical reduction of O2 (reaction 4 of Figure 2A) at the working electrode.
In second-generation biosensors, the oxidation of the reduced form of the enzyme is carried out by redox mediators (reaction 5 of Figure 2B), which compete with the natural cosubstrate oxygen. The glucose concentration can be correlated to the amperometric signal obtained via the electrochemical oxidation of the reduced mediator (reaction 6 of Figure 2B).11–16
In third-generation biosensors, the redox cofactor of the enzyme is covalently or electrochemically linked to the working electrode, thereby facilitating the re-reduction (or reoxidation) of enzymes to be carried out by direct electron transfer from (or to) the working electrode, and the obtained amperometric signal can be correlated to glucose concentration. (Reaction 7 of Figure 2C).17,18
Apart from oxidase-based enzymes, glucose dehydro-genases19–24 and quinoprotein-based glucose dehydro-genases21,25–28 have been utilized for enzymatic glucose sensing. These dehydrogenases utilize biological redox mediators like nicotinamide adenine dinucleotide (NAD+) and quinones for the oxidation of the reduced form of the enzyme.
Nonenzymatic glucose detection involves the direct electro-oxidation of glucose to gluconic acid at nanostructured electrodes [such as platinum nanoforests,29 platinum–leadalloy nanowires,30 gold nanoparticles,31 alloy nano-structures (containing platinum, lead, gold, palladium, and rhodium)]32 that possess high surface area and electrocatalytic activity (reaction 8 of Figure 2D).
Glucose Detection Based on Optical Approaches
Glucose detection based on optical approaches can be broadly classified as (1) fluorophore-based and (2) direct (nonfluorophore)-based techniques.
The fluorophore-based approaches utilize an affinity sensor principle wherein glucose and a fluorophore bind competitively with a receptor that is site specific to both ligands.33,34 For example, concanavalin A (ConA) can be used as the receptor molecule as it has four glucose- binding sites and its competitive binding can be assessed against other binders such as fluorescein-labeled dextran, α-methyl mannoside, and glycated protein.35,36 Various spectroscopic techniques have been utilized to measure glucose concentration. Some of these are listed here:
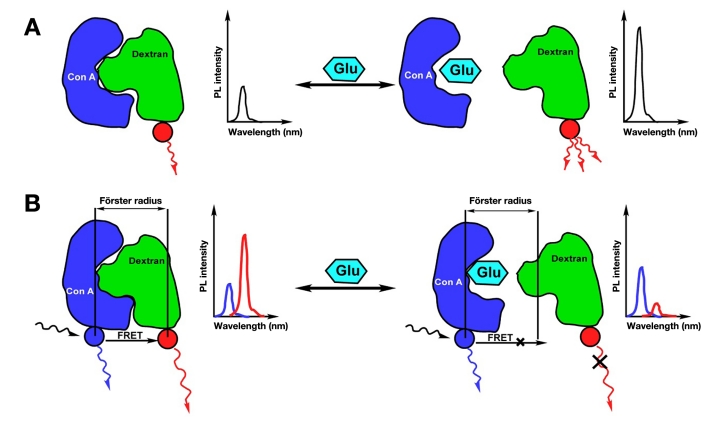
The binding of fluorescein-labeled dextran to ConA results in charge transfer and subsequent quenching in the fluorescence intensity of fluorescein-labeled dextran. Glucose preferentially binds to ConA compared to fluorescein-labeled dextran, thereby the presence of glucose causes an increase in the amount of free (unbound) fluorescein-labeled dextran. This results in an increase in the intensity of the fluorescence emission (Figure 3A). Thus the intensity of the fluorescence emission from the binder molecule (fluorescein-labeled dextran) is used as a measure of glucose concentration. The higher the glucose concentration, the higher the fluorescence intensity of fluorescein-labeled dextran.37
Fluorescence emission that occurs as a result of the Förster resonance energy transfer (FRET) between the allophycocyanin labeled ConA (donor) and fluorescein labeled dextran (acceptor) when they are within atomic distances (Förster radius) of each other.34,35 Any glucose molecule present binds to ConA, thereby increasing the distance between the two (greater than the Förster radius), leading to a decrease in FRET-induced fluorescence emission (Figure 3B). Thus glucose concentration can be evaluated by monitoring changes in the FRET-induced fluorescence emission intensity of fluorescein-labeled dextran.38–41
The occurrence of FRET between the donor and acceptor molecules is also accompanied by a decrease in the lifetime of the donor. Based on this and the fact that the presence of glucose decreases the possibility of FRET (Figure 3B), an increase in the lifetime of the donor can be seen with increasing glucose concentration.34 Thus glucose concentration can be evaluated by monitoring changes in the lifetime of the donor molecule that is in close proximity to a acceptor molecule.42
Fluorescence intensity of human tissue, using glucose itself as the fluorophore. When human tissue is excited with light at 308 nm wavelength, the glucose molecules become excited and the fluorescence emission can be detected at either 340, 380, or 400 nm.43 Thus glucose concentrations can be evaluated by direct tissue excitation at 308 nm and monitoring the emission intensity at 380 nm, the wavelength at which glucose has the strongest emission.
Ocular spectroscopy utilizes synthetic boronic acid derivatives (loaded within a polymer matrix) that specifically, yet reversibly, bind to glucose. In addition, these molecules are coupled to fluorescent moieties that allow their spectroscopic analysis. The boronic acid group, which is in its sp2 hybridized trigonal form, turns into a more electron-rich sp3 hybridized, tetrahedral geometry upon interaction with glucose, thereby leading to changes in the spectra of the fluorescent moiety.44,45
Figure 3.
Optical detection of glucose based on (A) fluorescence intensity decrease as a result of affinity binding and (B) decrease in FRET-induced fluorescence intensity.
Nonfluorophore-based optical detection of glucose utilizes light of variable frequencies to investigate changes in the absorbance, reflection, or refraction (scattering) of tissue containing various concentrations of glucose.33,34 In particular, light with wavelengths in the near-infrared (NIR) range have been found to pass through the human stratum corneum with minimal tissue absorption. Moreover, the fact that the absorption of water creates a window in the NIR (0.8–1.4 µm) region, where tissue components do not absorb, allows light to pass deep into the epidermis and subcutaneous (SC) space, independent of skin pigmentation. This renders NIR light as a possible means to investigate glucose-induced changes in the optical properties of the SC tissue. For example, variations in glucose concentration alter the dielectric strength, polarizability, and permittivity of the SC tissue, thereby registering changes in the absorbance, reflection, and refraction of NIR radiation, respectively. Based on these changes, a number of optical techniques that involve no fluorophores are listed here:
Optical coherence tomography and chromoscopy quantify glucose via assessment of the intensity of the reflected/scattered and transmitted light upon interaction with the SC tissue glucose concentration.46,47
Polarimetric technique utilizes the ability of glucose to rotate linearly polarized light to subsequently quantify glucose concentration, based in the degree of optical rotation.48,49
Thermal infrared spectroscopy utilizes changes in the refractive index of the tissue upon illumination with a time-modulated light that creates change in the temperature of the local tissue. Such local heat changes create microcirculation that induces change in the refractive index of the tissue. These refractive index changes are dependent on the amount of glucose present in the SC tissue.50
Photoacoustic spectroscopy that is based on the principle of adsorption of light by tissue-localized heating and subsequent generation of ultrasonic waves as a result of volumetric expansion. For glucose detection using photoacoustic spectroscopy, the tissue is illuminated with NIR wavelength light and the velocity of the generated ultrasonic wave is recorded. Since the velocity of the generated ultrasonic wave is dependent on the specific heat of the irradiated tissue (which, in turn, is dependent on the glucose concentration), a quantitative assess-ment of glucose concentration can be made.51,52
Raman spectroscopy that is based on inelastic scattering of photons. When monochromatic light interacts with glucose, owing to the Raman effect, there will be a shift in the energy of the photons proportional to the vibrational or rotational energy of glucose.53,54 Since the Raman spectrum is characteristic of a specific intramolecular motion (vibrational or rotational) of the molecular bonds of glucose, it can provide selective information about the concentration of glucose. For example, Raman spectroscopy can be utilized to differentiate between galactose and glucose, two epimers with the same chemical composition but structurally different in the position of one atom.
Photonic crystal-based glucose sensors work on the principle of shift in the wavelength of diffracted light from a hydrogel-based crystalline colloidal array. The sensor consists of a polyacrylamide-polyethylene glycol polymer network with an embedded crystalline colloidal array and a recognition element (such as a boronic acid derivative) that specifically binds to glucose. Interaction of glucose with the recognition element results in the formation of cross links (such as bis-bidentate) that shrinks the hydrogel volume. This volume shrinkage causes a blue shift in the diffraction from the crystalline colloidal array that is proportional to the bound glucose. Such color changes can be perceived visually (without instrumentation) across the visible spectral region, from red to blue over physiologically relevant glucose concentrations.55 Because this scheme involves chemically-induced swelling (i.e., corresponding to mechanical deforma-tion) to the optical properties of the photonic crystal, this technology can also be considered as chemo-mechano-optical transduction.
Other Approaches
Apart from electrochemical and optical approaches, glucose detection based on electric or electromagnetic transduction has also been reported. Some of these include the following:
Impedance spectroscopy. An increase in the local glucose concentration results in a decrease in the sodium levels and an increase in the potassium levels of the plasma, changing the dielectric strength, permittivity, and conductivity of the plasma.56 This forms the principle of glucose detection using impedance spectroscopy, which utilizes the transport of alternating current through the tissue to measure changes in plasma conductivity and subsequently relates this to glucose concentrations.57–61
Electromagnetic spectroscopy utilizes changes in the electromagnetic coupling between two inductors to measure glucose concentration. Because electro-magnetic coupling is dependent on the permittivity of the media, which, in turn, is dependent on the local glucose concentration, quantitative assessment of glucose levels can be achieved.62,63
Continuous Glucose Monitoring Devices: Their Location and Degree of Invasiveness
Invasive Devices
Invasive devices constitute sensors that are completely implanted (either subcutaneously or intravenously) and interface with an external controller via wireless communication. Upon implantation, the sensor continuously measures glucose levels and feeds this information back to an external controller for display and various other actions. While most of implantable sensors are based on enzymatic oxidation of glucose and subsequent assay (electrochemical or optical) of the enzymatic products, communication to the external controller is achieved either via radio frequency or optical signaling.17,64
In a different approach of invasive sensors, microdialysis technology utilizes a catheter housing a dialysis membrane inserted within the SC tissue to continuously pass glucose-free isotonic fluid across the skin. Upon passage through the skin, the isotonic fluid picks up glucose that is assayed externally using optical or electrochemical techniques.65–67 Along these lines, catheter-shaped sensors have also been introduced, wherein the sensing element (mostly electrochemical based) is located at the tip of the catheter, while the transmitter is located at its other end. The user inserts the catheter within the skin in such a way that the sensing element resides within the skin, while the transmitter resides outside the skin, and as such, these devices can be categorized as invasive, transcutaneous CGM devices.68,69 Similarly, a disposable, invasive optical fiber has also been introduced70 that is capable of percutaneous glucose monitoring via spectroscopic measurement.
Minimally Invasive Devices
In an attempt to avoid the continuous presence of a foreign object (sensor) in the body (as in the case of invasive devices), minimally invasive devices are being developed that measure glucose concentrations from fluid (ISF or blood) obtained from the skin tissue. In these devices, both the glucose sensor and the controller are located outside the body and are connected to a fluid-drawing device that is externally located. The feedback loop to the hypoglycemic/hyperglycemic alarms and the insulin pump is similar to invasive devices and hence can be essentially considered the “wired” version of the “wireless” invasive devices. Various methods to draw ISFs include the following:
Iontophoresis employs a low electrical current applied across the skin by two electrodes that are located adjacent to one another. This current causes charged species (and, by induction and other inter-molecular forces, uncharged species) to move across the dermis through the skin pores. Through this action, a minute amount of ISF is withdrawn that contacts an externally located sensor to determine glucose levels. Typically, the glucose concentration in the collected fluid is sensed using a GOx-coated platinum working electrode (E-1-a in Figure 1B).71,72
Sonophoresis uses low-frequency ultrasound to increase skin permeability by causing expansion and contraction of gaseous inclusions within the stratum corneum, which facilitates the collection of the ISF. Similarly, the extracted ISF is assayed externally using an electrochemical or optical glucose sensor.73–75
Skin blister technique employs a small local vacuum on the skin to create a blister at the dermal/epidermal layers of the skin, and ISF can be collected from this blister and assayed externally.34,76
Micropore technology utilizes laser ablation to create an array of microscopic holes in the stratum corneum of the skin and collects ISF (using a small vacuum) to be assayed using an externally located glucose sensor.77
Microneedle technology is based on silicon micro-needles of similar size to that of human hair. A hand-held electronic meter is loaded with disposable sampling devices. Each disposable device consists of a microneedle and a micropouch into which the blood sample is withdrawn. The micro- needle frequently penetrates the skin and draws a very small volume of blood into the miniaturized pouch, where the blood-glucose concentration is measured.8
Since, iontophoresis and sonophoresis do not cause significant and permanent skin damage, these techniques can essentially be categorized as minimally invasive, transcutaneous CGM devices.
Noninvasive Devices
The need to minimize discomfort and the potential risk of infection from fluid-withdrawing probes protruding through the skin along with avoiding the foreign body response that otherwise can compromise analyte permeability has spurred the development of noninvasive sensors that measure glucose concentrations without penetrating the skin. For this, a variety of spectroscopic techniques have been employed to assay various body fluids/gases including saliva, tears, and breath.78 Representative noninvasive devices include the following:
Transdermal sensors that pass NIR light across the stratum corneum to detect glucose concentrations as described earlier under optical approaches (O-2-a and O-2-b in Figure 1B).33,34,79,80
External assays of body-fluids (i.e., saliva, tears, and breath) using various optical and electrochemical detection methodologies shown in Figure 1B (E-1 and O-2 categories).33,34,79,80
Factors That Dictate the Success of a Continuous Glucose Monitoring Device
This section gives an overview of various factors that need to be taken into consideration for widespread adoption of these devices. Each subsection gives an overview of the various issues that effect the different types of CGMs and the strategies currently being used to overcome these. Discussion in this section closely follows Tables 1 and 2, which list the merits and drawbacks of each technology.
Table 1.
Comparison of Various Continuous Glucose Monitoring Technologies Categorized According to Their Invasiveness.
| Modality | Merits | Drawbacks | |
|---|---|---|---|
| Invasive | SC | 1. No open wound | 1. Calibration inaccuracy due to lack of correlation between ISF and blood glucose |
| 2. No subject-to-subject variability | 2. Foreign body response and biofouling-induced sensor degradation | ||
| 3. Comfort and ease of adaptability | 3. Sensor migration and difficulty in extraction | ||
| 4. Ease of implantation | |||
| Intravenous | 1. No open wound | 1. Foreign body response and biofouling-induced sensor degradation in addition to sensor damage due to shearing forces of blood flow | |
| 2. No subject-to-subject variability | 2. Sensor migration and difficulty in extraction as well as tedious implantation procedures | ||
| 3. Comfort and ease of adaptability | |||
| Microdialysis | 1. Sensor is outside the body and so no foreign body response and biofouling-induced degradation | 1. Open wound with significant tissue inflammation | |
| 2. No subject-to-subject variability | 2. Calibration inaccuracy due to lack of correlation between ISF and blood glucose | ||
| 3. Large response times needed for the ISF fluid to reach the sensor | |||
| 4. Discomfort because of presence of protruding microdialysis probes | |||
| Transcutaneous | 1. No subject-to-subject variability | 1. Open wound with significant tissue inflammation | |
| 2. No sensor migration and ease of extraction | 2. Foreign body response and biofouling-induced sensor degradation | ||
| 3. Calibration inaccuracy due to lack of correlation between ISF and blood glucose | |||
| Minimally invasive | Micropores and microneedles | 1. Directly determines blood glucose and so no calibration-induced accuracy | 1. Generation of multiple wounds is prone to infection, irritation, bleeding (important for hemophiliacs) and allows potential for allergic reactions |
| 2. Long duration time required to collect sufficient amount of sample | |||
| Iontophoresis (considered transcutaneous in this manuscript) | 1. No open wound | 1. Long warm-up time needed to get glucose readings | |
| 2. The amount of fluid drawn is low and so no effect of oxygen on the enzymatic glucose sensor | 2. Calibration inaccuracy due to lack of correlation between ISF and blood glucose | ||
| 3. Skin filters most of the large molecules and so less electrode fouling | 3. Cannot be used in conditions of excessive perspiration | ||
| 4. Application of low currents can cause skin erythema | |||
| Sonophoresis | (considered transcutaneous in this manuscript) | 1. No open wound | 1. Subject-to-subject variability in the degree of ISF collection |
| 2. Calibration inaccuracy due to lack of correlation between ISF and blood glucose | |||
| Noninvasive | Transdermal | 1. Pain free | 1. Inaccuracies as a result of skin pigmentation, body water content, and hydration |
| 2. Comfort and patient adaptability | 2. Nonspecificity to glucose | ||
| 3. Strong effect of temperature on glucose response | |||
| Body Fluids | 1. Pain free | 1. Inaccuracies due to lack of correlation between the glucose levels of blood and body fluids | |
| 2. Comfort and patient adaptability | 2. Nonspecificity to glucose |
Table 2.
Comparison of Various Glucose Sensing Technologies, Grouped According to Their Transduction Mechanism.
| Detection Technology | Merits | Drawbacks | ||
|---|---|---|---|---|
| Electrochemical | Enzymatic | First generation | 1. Highly specific to glucose | 1. Interferences from co-substrate (i.e., oxygen) and endogenous species |
| 2. High sensor sensitivity | 2. High operating potential required | |||
| 3. Must use outer membranes, which increase sensor response times | ||||
| Second generation | 1. Highly specific to glucose and free of changes in levels of co-substrate | 1. Mediators used may be toxic | ||
| 2. Low overpotential renders the sensor free of interferences | 2. Competition between mediators and oxygen still exists | |||
| Third generation | 1. Highly specific to glucose and free of changes in the level of co-substrate | 1. Toxicity and biocompatibility of required nanomaterials is untested | ||
| 2. Low overpotential renders the sensor free from interferences | 2. The issue of repeatability is still untested | |||
| Non-GOx based | 1. Does not use oxygen as co-substrate and so no interferences from oxygen | 1. Shown to oxidize other sugars as well as common alcohols | ||
| Nonenzymatic | 1. No enzymes used and so no question of degradation | 1. Not specific to glucose | ||
| 2. Substantial electrode fouling by the products of glucose oxidation | ||||
| Optical | Fluorophore-based | Fluorescence or FRET intensity | 1. Highly specific to glucose because of the use of fluorophore with binding specificity to glucose | 1. Photobleaching of the fluorophore and scattering in tissue |
| 2. Dependent on skin pigmentation, redness, epidermal thickness | ||||
| FRET lifetime | 1. Independent of scattering in tissue | 1. Miniaturization of photodetectors and time resolved spectrometers is not trivial | ||
| 2. Independent of fluorophore concentration and so no issue of photobleaching or fluorophore loss through leaching | 2. Fool-proof demonstration in animals and humans is yet to be demonstrated | |||
| Ocular spectroscopy | 1. Truly noninvasive since it measures glucose concentration in tears | 1. Leaching of boronic acid derivative | ||
| 2. No handheld instruments | 2. Effected by pH and ionic strength | |||
| 3. Glucose levels can be assessed visually | 3. When used in tears, a lag between the blood and tear glucose is observed | |||
| Nonfluorophore based | Optical coherence tomography | 1. Unlike other optical techniques, it is not affected by urea, ionic strength, temperature, heart rate, and hematocrit | 1. Shown to be affected by motion and tissue heterogeneity | |
| Polarimetry | 1. Can utilize visible light, easily available | 1. Effected by scattering in the tissue, pH, and temperature | ||
| 2. All the components can be easily miniaturized | 2. Lack of specificity as molecules such as albumin and ascorbic acid are known to polarize light | |||
| Thermal infrared spectroscopy | 1. Same as polarimetry | 1. Effected by scattering in the tissue, pH, probe position, fever, and temperature | ||
| Photoacoustic spectroscopy | 1. Unlike other optical techniques, it is not affected by ionic strength or albumins | 1. Effected by scattering in the tissue | ||
| 2. Miniaturization of instrument is not trivial | ||||
| Raman spectroscopy | 1. Unlike NIR, it shows sharper peaks and less overlap | 1. Longer stabilization times | ||
| 2. No interference from luminescence and fluorescence | 2. Effected by tissue density, thickness, hematocrit | |||
| Combinatorial | Impedance spectroscopy | 1. Can measure glucose levels in the vascular compartment, so no lag time in sensor response | 1. Temperature and disease state of the body may affect measurements | |
| 2. Changes in blood dielectric properties are not specific to glucose | ||||
| Electromagnetic spectroscopy | 1. Same as impedance spectroscopy | 1. Body temperature, sweating, and motion affect glucose measurements | ||
Accuracy
As with any other analytical medical device, accuracy forms the most important requirement of a CGM device. Accuracy in CGM devices is required not only to transition these from stand-alone continuous sensing devices to “closed-loop” artificial pancreas, but also to increase confidence among patients and physicians.
Standards and Evaluation
As per the International Organization for Standardization guidelines (ISO 15197), a glucose sensor is considered accurate if its error is within 0.83 mM when measuring a hypoglycemic event of less than 4.2 mM glucose. Error values of 20% or lower are acceptable when testing in the range above 4.2 mM.81,82 Even though this standard is exclusive for SMBG, similar standards need to be employed for CGM devices to ensure adequate accuracy. Here the reader is also referred to the POCT05-A document (co-jointly developed by the Clinical and Laboratory Standards Institute and the Diabetes Technology Society), which summarizes some of the performance metrics and approved guidelines for continuous interstitial glucose monitoring.83,84
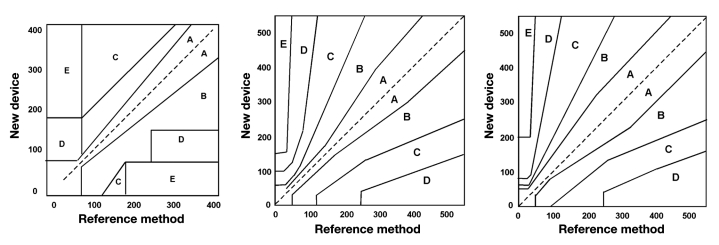
At present, there is no consensus on the criteria to be used for evaluation of CGM device performance.82 Most of the existing methods include comparing values obtained from the CGM device against corresponding reference values using linear regression analysis, error grid analysis, predicted error sum of squares, and mean absolute deviation.34,85,86 In particular, researchers and clinicians tend to use the Clarke error grid analysis, which expresses the relationship of the difference between the CGM-measured glucose concentration and that measured using a clinical analyzer (“true” blood glucose), as a guide for evaluating sensor accuracy.87,88 As shown in Figure 4A, a typical Clarke’s grid is a plot of the “true” blood glucose value on the x-axis and that obtained from the sensor under development on the y-axis. A perfect correlation between the two yields a graph where all the points fall on a 45° line passing through the origin. If this is not the case, the data must be interpreted very carefully. For example, a measurement that falls in zone A correctly reflects the actual glucose concentration, whereas one that falls in zone E reflects a significant error. A clinical decision based on a measurement that falls in zone E, could have a potentially harmful outcome. Zone B indicates benign glucose levels, zone C represents overcorrected values, and zone D represents failure to detect glucose levels.87,88 To quantify the occurrence of data points in zones A and B, two parameters are often used: (i) r value of the correlation between the“true” blood glucose value and the response of the sensor under development and (ii) percentage of experimental data points that fall in zones A and B. Table 3 lists the best accuracies of the various CGM technologies reported so far.
Table 3.
Best Accuracies of Various Continuous Glucose Monitoring Devices Grouped According to Their Invasiveness.a
| Invasiveness | Modality | Accuracy | ||
|---|---|---|---|---|
| R value | Points in zone A and B | Model used | ||
| Noninvasive | Optical coherence tomography | 0.9546 | n/a | Humans |
| Chromoscopy | n/a | n/a | n/a | |
| Polarimetry | >0.9989 | n/a | Ex vivo | |
| Thermal infrared spectroscopy | 0.87 | 100%50 | Humans | |
| Photoacoustic spectroscopy | n/a | n/a | ||
| Raman spectroscopy | 0.8353 | n/a | Humans | |
| Impedance spectroscopy | 0.4961 | 78.4%61 | Humans | |
| Electromagnetic spectroscopy | n/ab | n/a | Ex vivo | |
| Minimally invasive | Iontophoresis | 0.972,90,91 | 98.9%72,90,91 | Humans |
| Sonophoresis | 0.792 | 92%92 | Humans | |
| Skin Blister Technique | n/a | n/a | Humans | |
| Micropores | 0.9473 | 93%73 | Humans | |
| Invasive | SC | 0.8593 | 98.4%94 | Humans |
| Intravenous | 0.9395 | 95.8%95 | Humans | |
| Microdialysis | 0.996 | 95.5%96,97 | Humans | |
This information is available to the general public and to the best of the authors’ knowledge. n/a, not available.
Standard deviation between the actual and recorded glucose levels.98
A major disadvantage with Clarke’s grid is that the zone boundaries are not connected sequentially, which means that a small change in glucose concentration reported by a sensor can move a result from the correct value zone A to the critical zone D, or vice versa. With this in mind, Parkes’ grid was developed (Figures 4b and 4c), wherein boundaries of the zones are sequential, which prevents the possibility of a glucose level falling in the wrong zone by virtue of a small measurement error.99 Moreover, unlike the Clarke’s grid, Parkes’ grid is separate for T1DM and T2DM, allowing increased accuracy and better treatment. However, Parkes’ grid is patient specific and is not universal to all CGMs. While the merits and drawbacks of each of these grids are continuously being evaluated and updated, it is worth mentioning that the percentage of data points in zone A deduced using Clarke’s and Parkes’ grids for the same CGM device do not coincide. For example, a given CGM device rated 98.6% accurate based on Parkes’ grid is shown to be only 95% accurate based on Clarke’s grid.34 Here it is worth mentioning that both Clarke and Parkes error grids have been originally developed for static glucose readings. In order to provide a more accurate analysis for continuous glucose data points (as obtained from a CGM device), Clarke’s grid has been modified.86 The modified Clarke error grid is based on temporal characteristics of continuous CGM devices and the lag time between measuring glucose concentrations in blood (for reference values) versus ISF (for continuous glucose values).86 Analysis based on the updated Clarke’s grid is typically affected by the sampling , and an assumption of 7 min constant lag time is made (something that may change from user to user).34
Figure 4.
Clarke error grid applicable for both T1DM and T2DM patients, (B) Parkes error grid applicable to T1DM patients, and (C) Parkes error grid applicable to T2DM patients.
Here it should be noted that, irrespective of the type of error-grid used, accuracy analysis can be performed either retrospectively or prospectively. In the retrospective mode, the CGM data are collected over a certain period of time and then correlated with values obtained from the reference glucose meter. On the other hand, in the prospective mode, the CGM data are correlated with the reference glucose meter in real time. While retrospective analysis substantially improves accuracy by taking into account the lag time between the blood and ISF glucose event, prospective analysis is more appropriate, as it correctly reflects the real-time performance of the CGM device.
Sources of Inaccuracy
A CGM device contains various components, all of which are potential sources of error. Currently, external glucose measuring devices (i.e., finger-prick test strips) are used to calibrate CGM devices to ensure accuracy. However, this type of calibration may not be ideal, as discussed here.
Calibration
Continuous assessment of glucose concentration using a CGM device requires a reliable and reproducible method of calibration. The calibration is performed to convert the raw data points (e.g., response current or optical intensity) to useful glucose readings as well as to deduce blood glucose values from the glucose values measured in the ISF (particularly relevant to CGM devices that sample glucose in the ISF). However, user physiology may not permit direct translation of an in vitro calibration routine to patient use. Moreover, given that SC glucose concentrations exhibit a time lag compared to blood glucose concentrations (as a result of diffusional barriers between the blood and the SC fluid), it is important that this delay is incorporated in the calibration routine. In addition, in the case of invasive CGM devices, calibration routines should be performed hours after the sensor implantation to allow equilibration. The timing for the first calibration is variable and is dependent on sensor technology, user physiology, and state (rest or exercise).100 Moreover, as a result of physiological changes, an initial in vivo calibration chart cannot be used continuously as a basis for assessing glucose concentration throughout the lifetime of the CGM device. Physiological changes that can occur include (i) foreign body response that continuously changes glucose permeability toward the sensing element of the CGM device (particularly relevant for invasive devices)101 and (ii) constantly changinguser physiology (exercise, diet, medication, anoxia, or hypoxia) that may result in false sensory responses. Based on these factors and the fact that calibration routine is dependent on the type of the sensing technology employed, all current CGM devices (in particular, invasive and minimally invasive ones) mandate that the patient calibrate his/her CGM device by means of conventional test strips at least once per day, and in some cases more often.100
Two types of calibration procedures are proposed. One-point calibration, which is the most commonly used, requires the user to input only one glucose reading for calibration and is based on one of the following assumptions: (i) the background and interferant’s current are known and remain constant, meaning that the intercept of the sensor response versus concentration can be taken as a second calibration point and (ii) the background and interferant’s current is zero or nonsubstantial, meaning that the response versus concentration curve passes through the origin, which is taken as a second calibration point.102 Despite considerable innovations and careful analysis, one-point calibration could still be affected because of constantly changing membrane permeability and degrading sensory components, which pose a serious problem for all CGM devices irrespective of their invasiveness.102 The development of a two-point calibration method, wherein the user calibrates his/her CGM device using two glucose readings, could show some improvement over one-point calibration methods, even though the timing of these two glucose readings plays a critical role. At present, there seems to be no universal guidelines on the timing of these glucose readings. In addition, as briefly mentioned earlier, a number of aspects can influence the validity of these calibrations, such as the following:
Glucose inequalities between blood and SC tissue glucose in both time and concentration. These inequalities are typically complex and vary considerably according to the physical state of the patient, such as resting, hyperventilation, exercise, anoxia, and hypoxia.103,104 For example, this physical lag time between the occurrence of the glycemic event in the blood and ISF is typically between 12 and 20 min and can be extended in minimally invasive as well as microdialysis-based CGM devices, wherein the ISF must be transferred from the SC tissue to the externally located sensor.104–106
The internal sensor response lag time (typically in the order of seconds to a few minutes) can add to the physical lag time between the blood and ISF glucose to further complicate calibration. This isparticularly important for invasive sensors whoseinternal response lag might vary because of in vivo-induced biofouling and/or scar-tissue encapsulation that can retard glucose permeability to the sensing elements.102,107,108
The glucose strips (typically used for external calibration) can provide their own source of error due to the inherent (approximately 5%) inaccuracies as well as variability as a result of changes in climate or storage life.109
Keeping these issues in mind, various researchers are attempting to correlate blood glucose levels with ISF glucose levels under various physiological states, such as diet, exercise, and medication, to investigate if there is a universal relationship between the two. While the chances of obtaining a linear relationship are remote, more complex calibration functions involving the “rate of changes in glucose levels” as opposed to “individual points” could potentially address this problem. Moreover, inclusion of a secondary glucose monitoring device that periodically checks the primary glucose sensor could alleviate some of the aforementioned issues. A few reports describing the advantages of this multiple sensory approach and the high accuracy afforded have emerged.110–112
Selectivity of Sensor
Selectivity of a CGM device corresponds to its ability to respond only to changes in glucose within the pool of metabolites present in the body. However, there are many molecules with electrochemical and/or optical signature similar to D-glucose (the biologically active form), which renders selectivity of a sensor a major obstacle to CGM devices.
In the case of CGM devices employing enzymatic oxidation of glucose (both in optical and electrochemical), sensor selectivity hinges on the particular enzyme employed. Oxidases, the most commonly used enzymes for glucose sensing, render high selectivity to glucose. However, this selectivity is often negated by the need for high oxygen concentrations as a co-substrate to produce H2O2 in a linear fashion.113 In order to decrease oxygen dependence of these biosensors, outer membranes that significantly decrease the analyte flux while keeping the oxygen flux (due to its smaller size) more or less altered are typically utilized.114 This approach is in accordance with the need to equip implantable devices with outer membranes that are also necessary to prevent unwanted in vivo biofouling and foreign body response, which is discussed in detail later in this review.
In the case of oxidase-based electrochemical sensors (E-1-a configuration shown in Figure 1B), the high potential required for sensor operation renders them susceptible to interferences from other endogenous species in the body (such as ascorbic acid, uric acid, dopamine, and nitric oxide). Interferences from endogenous species have been minimized through the use of perm-selective membranes (i.e., Nafion, polyester sulfonic acid, and cellulose acetate) on top of the working electrode. However, the use of these perm-selective membranes reduces sensor sensitivity and increases response time. In addition, these membranes may degrade upon implantation as a result of biofouling or calcification, and if due attention is not exerted, the CGM device may record large deviations from actual physiological glucose response. A logical approach is to use a blank secondary working electrode along with the primary working electrode for detection of background current. This background current is subsequently subtracted from the response of the primary working electrode.115–117 An alternate approach is to apply two different potentials on the same working electrode, one of which corresponds to background current.118
Sensor selectivity may not be a critical issue in second generation electrochemical biosensors (E-1-b configuration shown in Figure 1B), which use redox mediators. These sensors do not require high potential because the redox mediators, which replace H2O2, operate at low potential (i.e., 0.2–0.3 V versus silver/silver chloride reference electrode, reaction 6 in Figure 2B). Redox mediators based on osmium and ferrocene complexes,119 carbon nanotubes,120 conducting polymers,14,121 as well as combinations thereof16 have been reported. However, it should be noted that only the osmium complexes are used in the current Food and Drug Administration- approved CGM device. Similarly, third-generation electro- chemical glucose sensors (E-1-c configuration shown in Figure 1B) that utilize nanomaterials are highly selective even though the biocompatibility of these nanostructured materials needs to be considered.
Biosensors utilizing dehydrogenases and quinoprotein- based dehydrogenases (E-1-d configuration shown in Figure 1B) are not susceptible to interferences from oxygen and other endogenous species. However, their specificity toward other redox mediators is not clearly addressed. Interference from other sugars (i.e., lactose, galactose, maltose, cellobiose, and xylose) and common alcohols has been reported.122,123 Biosensors utilizing direct electro-oxidation of glucose (E-2-a configuration shown in Figure 1B) also lack specificity and are susceptible to interferences from other sugars.
Sensor selectivity is a more critical issue in optical methods of glucose detection (O-2-a to O-2-i configurations shown in Figure 1B) because of the vast number of metabolites present that have a similar optical signature to glucose. Moreover, optical interferences arising from instrumental or physiological variations are significantly higher than the magnitude of the change in optical signal as a result of changes in glucose concentration.124 This inherently renders their selectivity a major issue. Some of the sources of low sensor selectivity in CGM devices employing optical-based glucose detection (O-2-a to O-2-i configurations shown in Figure 1B) arise from the following:
Similarity of the NIR spectrum of glucose to that of other sugars, especially fructose, which often is prescribed to diabetes patients as an alternative to glucose.124
Overlap of the NIR overtone of glucose with several other overtones (e.g., water, fat, or hemoglobin). These overtones significantly complicate glucose detection via absorption, reflectance, and scattering methods, rendering it, at best, nonspecific.124
Sensitivity of the intensity of the tissue water absorption band to the solute concentration (i.e., glucose and other metabolites) and temperature. Typically, as solute concentration increases, water absorption decreases. This effect, often referred to as “water displacement,” is not analyte specific and can be a potential source of nonspecificity in absorption-based glucose detection. Changes in the optical signal could be a result of changes arising from other metabolites rather than glucose.124
The refractive index and extinction coefficient of the tissue are also affected by the water displacement effect. As a result of this, glucose detection based on glucose-induced changes in tissue refractive index may not be specific. For example, it was shown that changes in the sensor optical signal could be a result of changes in other metabolites such as creatine or albumin.33,34
Extreme susceptibility of NIR spectra to various physiological factors such as pigmentation, hydration, blood flow, temperature, pH, as well as physical parameters (i.e., probe placement and orientation).124
Some of these problems can be solved by developing more sensitive and stable instrumentation. Raman spectroscopy is being investigated as an alternative to absorption-based detection methods. This method is highly specific to glucose since detection is based on changes in fundamental molecular vibrations. Optical detection methods based on fluorescent molecules attached to glucose-specific recognizer moieties (i.e., enzymes and binder; O-1-a to O-1-d configurations shown in Figure 1B) are also highly specific to glucose. However, as mentioned earlier, these are susceptible to changes in oxygen concentration. In addition, interference as a result of enzyme denaturation and photobleaching of fluorophores affects the long-term performance of these sensors.35,125
Operational Lifetime of a Continuous Glucose Monitoring Device
The prospect of home-based CGM devices can be achieved only if they are affordable and acceptable to patients; this hinges on increasing their lifetime in a cost-effective manner. This is particularly true for minimally invasive and invasive sensors, where the patient is unlikely to tolerate frequent implantation/extraction procedures due to pain, inconvenience, and high costs.
Table 4 lists the best reported lifetime of various invasive CGM devices. In general, failure modes originate from enzyme degradation, electrode degradation, biofouling, membrane delamination, battery discharge, component failure of telemetry packs,126,127 and electronic package failures.128 Advances in the semiconductor industry have made a significant contribution to addressing electronics- related failures. Despite considerable research efforts, sensor in vivo stability (biofouling, foreign body response, enzyme, and fluorophore degradation) still constitutes a major problem. The following subsections are structured to provide an overview of the in vivo failure modes of minimally invasive and invasive CGM devices.
Table 4.
Best Reported Lifetimes of Invasive Continuous Glucose Monitoring Devices Grouped According to Their Modality.
| Modality of invasive sensors | Reported lifetime | Model used | |
|---|---|---|---|
| SC | 120 daysa64 | 7 daysb | Humans |
| Intravenous | 259 daysa,64 | Humans | |
| Microdialysis | 3–7 days | Humans |
Reported life time.
U.S. Food and Drug Administration approved.
Inflammation, Foreign Body Response, and Biofouling
Biofouling inhibits analyte transport to the sensing element. The foreign body response can cause fluctuations in glucose readings due to edema and the influx of scavenger cells (acute inflammatory phase) and can deprive the sensor of adequate levels of analyte (glucose and co-factors such as O2 and NAD+) due to fibrotic encapsulation (chronic inflammatory phase).18,129–133
The inflammatory processes that occur in the local tissue following implantation can be categorized into three phases: (1) acute inflammatory phase, which sets in immediately following implantation and invokes migration of inflammatory cells and plasma proteins toward the implant; (2) intermediate phase, which involves adsorption of phagocytes onto the surface of the implant in an attempt to destroy it, the release of oxygen species and secretion of proteolytic enzymes intended to degrade the foreign body, as well as deposition of fibrinogen in the wound area and neovascularization; and (3) chronic inflammatory phase, consisting of the formation of multinucleate giant cells and the deposition of fibrinogen, resulting in the formation of a fibrotic capsule around the implant (i.e., scarring), lasting from days to years.101,134
Initial approaches to overcome the foreign body reaction have included the use of biocompatible materials to coat the sensor. Typical biocompatible coatings are based on materials that are resistant to immunogenicity, such as Nafion,135–138 hyaluronic acid,139 and humic acids.107,140 However, even the use of biocompatible materials that have no toxic effects on the surrounding tissue are found to evoke a host of immune responses associated with the action of implantation.101 Based on this and the fact that the foreign body response is largely dictated by implant specifics (such as shape, size, chemical and physical properties, and extent of injury134,141), researchers have started to develop advanced sensor coatings. These can be classified as (i) biofouling-resistant coatings and (ii) drug-releasing biocompatible coatings that actively combat tissue inflammation. The primary purpose of both these approaches is to promote integration of the sensor with the surrounding tissue. The biofouling resistant coatings reduce protein adsorption via creation of a hydrophilic interface between the sensor surface and the tissue fluids. The drug-releasing biocompatible coatings minimize inflammation caused by tissue injury and implantation-induced hemorrhage, along with suppressing ischemia by enhancing vascularity so that a constant flux of analytes is maintained over long periods of time.142,143
Biofouling-resistant coatings reported include hydrophilic polymers such as phosphorylcholine,144 2-methacryloyloxy-ethyl phosphorylcholine,145 polyurethanes with phospho-lipid polar groups,146 and water-rich hydrogels.147 These are solely intended to reduce protein adsorption on sensor surfaces and thereby mitigate the foreign body reaction. On the other hand, reduction in local tissue inflammation and enhancement in vascularity have been achieved through drug delivery approaches that utilize biocompatible sensor coatings capable of delivering tissue response modifiers (TRMs) at the implant site. For example, anti- inflammatory drugs (dexamethasone142,148,149), neovasculari-zation-inducing growth factors (vascular endothelial growth factor and platelet-derived growth factor143,150), vasodilators (nitric oxide151–153), and anti-coagulants (heparin154) have been incorporated into sensor coatings. However, while these TRMs are able to inhibit the foreign body response, their beneficial action can only be maintained so long as their release is sustained.142 Although this is a promising approach, a question remains on whether there are any systemic effects from the local delivery of TRMs. However, because the dosage of the TRMs is both minimal and highly localized, systemic effects are likely to be negligible. In addition, it has been shown in animal studies that TRM blood levels associated with this local therapy are minimal.155
Nanotextured surfaces have been shown to enhance vascularity around implants.156–159 These nanostructured surfaces include nanoporous titania,160,161 nanoporous silicon,162–165 and nanoporous carbon.166 These nanomaterial-based surfaces possess high aspect ratio features that are believed to alter cell phenotype, proliferation, and differentiation.162,167 However, the applicability of these nanostructured surfaces hinges on their immunogenicity. For example, there are a number of reports debating the biocompatibility and possible toxicity of nanostructured materials.151–153 Clearly, the need to obtain a better picture of the long-term effects of nanostructured materials is mandated in order to move forward with their applicability in implantable biosensor platforms.113
Enzyme Degradation
Enzymes form an important functional component of many CGM devices (operating in either the optical or electrochemical mode) in view of their ability to render the device specific to glucose, as shown in Figures 2 and 3. The long-term stability and activity of the immobilized enzymes is, however, a cause of concern for the long-term operation of CGM devices. Two common enzyme immobilization techniques involve (i) enzyme/bovine serum albumin cross linked with glutaraldehyde168 and (ii) electrochemical growth of a conductive matrix that incorporates the enzyme as counter ions.169 While sensor degradation due to loss of enzyme activity is rare,170 reports have indicated that inhibition of enzyme catalysis can be caused by transition metal ions, such as Zn2+ and Fe2+,171 low molecular weight serum components,129 and hydrogen peroxide. The latter in particular could be more problematic, considering that hydrogen peroxide is also the signaling molecule in first generation electro-chemical glucose sensors (reaction 4 in Figure 2). This mandates significant optimization in terms of enzyme loading with respect to the glucose permeability of outer sensor coatings.9 Other studies have shown that, while glucose sensors loaded with excess GOx could extend sensor lifetime,172 any excess free (un-cross linked) GOx originally entrapped within the glutaraldehyde cross-linked enzyme layer can slowly leach out and contribute to a decline in sensitivity over time.168 These factors should be taken into consideration to extend long-term in vivo sensor life. Enzyme denaturation is another concern for long-term enzymatic decay. Reports have begun to emerge on nanotechnology-based venues to reduce natural enzyme denaturation.173–176 For example, the catalytic activity of an enzyme has been shown to be preserved when immobilized on nanostructured supports such as zeolites and phosphates. These are promising strategies that could be applied to prolong the lifetime of CGM devices.
Degradation of Fluorophore
In the case of CGM devices that employ fluorophore-based optical detection of glucose (Figure 3), degradation of the fluorophore itself is of concern. This is because the magnitude of the emitted signal from the sensor is dependent on the fluorophore concentration, which, in turn, is dependent on the fluorophore stability.35,125 However, most of the fluorophores reported to date are susceptible to photobleaching within a short period of time, which drastically decreases CGM device lifetime. The higher the rate of photobleaching, the shorter the sensor lifetime. For example, a glucose sensor employing an IR-Fye-78-CA with a photobleaching rate of 250 h-1 has a nominal sensor lifetime of 3.2 s, which is 3000 times lower than a sensor employing a quantum dot-based fluorophore that has a photobleaching rate of 0.082 h-1.125Even though quantum dots that possess lower photo-bleaching rates could be a viable option, concerns about their toxicity remain to be addressed, especially for implantable sensor configurations. The same is the case with fluorophores based on carbon nanotubes,40,41,125 which have been utilized for optical detection of glucose and have been shown to be resistant to photobleaching.
Continuous Glucose Monitoring Device Size and Miniaturization
The size of a CGM device has been argued to be a critical factor in device development. Among equally performing devices (such as cell phones, laptops, and media players), the smallest device in a given device class typically presents the greatest appeal to consumers. This general rule is also expected to be applied to CGM devices. As mentioned earlier, the size of the implant also dictates the extent of trauma inflicted via device implantation, which, in turn, affects the extent of the foreign body reaction. With respect to CGM device size, two kinds of CGM device configurations need to be addressed: (i) semi-implantable devices that are based on either microdialysis-based or transcutaneous-based implants and (ii) completely implantable architectures housing all powering, sensing, and wireless communication within a small footprint to minimize device-induced inflammation. In the case of microdialysis-based systems, the fluid-withdrawing tube is located inside the body and the sensing element is attached to the surface of the body with a portable powering/wireless device. On the other hand, transcutaneous-based systems have the sensory element located inside the body with the electrode leads connecting to the powering and wireless electronics located on the surface of the skin. A major difference between the implantable and semi-implantable sensors is the presence of an open wound in the latter, which could act as a site of infection and thereby inadvertently negate the benefits of a minimized, catheter-sized sensor footprint. In addition, semi-implantable devices have a shorter in vivo functionality and are less convenient and less acceptable to the patient.
With respect to miniaturization of electrochemical-based implanted CGM devices, the emerging field of micro- and nanofabrication is expected to provide a significant impetus in the coming years. Similar trends can be expected with optically based, implantable CGM devices, although alignment of optical components might present a greater challenge. As nanotechnology concepts continue to advance, manufacturing in semiconductor and optical communication industries are also expected to experience significant advances that could help streamline miniaturization of driving electronics, power management, optical components, and wireless trans-mission. At present, a major challenge for CGM device miniaturization is the powering of the sensor and wireless telecommunication circuits. The emerging field of miniaturized batteries along with wireless power transmission, biofuel cells and piezoelectric powering systems (to harvest energy from motion) provide viable opportunities to address the long-term power requirements of implantable CGM devices.177–179
For invasive CGM devices, the sensing component should be small enough to allow ready implantation and explantation (for example, needle assisted) without the need for surgery. The implantable device should be extremely small, which calls for unprecedented miniaturization of the various functional components such as electrodes, power sources, signal processing units, and sensory elements. Moreover, as mentioned earlier, miniaturized biosensors implanted through ultrafine needles (similar to the ones used in acupuncture) cause less tissue damage and therefore less inflammation and foreign body response.134 The sensing elements for most of the reported invasive devices are based on either immobilization of enzymes onto ultrathin platinum wires (diameters less than 25 µm) and carbon nanofibers180–184 or packing the fluorophore within a miniaturized polymeric capsule.125 The emerging field of top down micro-/nanofabrication involving traditional semiconductor processes such as photolithography and micromachining is proving to be a facile avenue for further sensor miniaturization. For example, Errachid and colleagues185 and Johnson and associates186 have reported a silicon micromachined needle-shaped structure for glucose monitoring. These needle-shaped biosensors, along with channels for fluid flow, are created by wet and dry etching processes, while the titanium/platinum working and silver/silver chloride reference electrodes located at the tip of the needle-shaped biosensors are patterned by photolithography. Even though these reports are focused on electrochemical glucose detection, they can be easily extended to optical glucose detection by replacing the electrode channels with fluorophore filled channels.
Summary and Outlook
The well-documented beneficial aspects of CGM for diabetes management has stimulated the substantial growth in the development of CGM devices. With more than four decades of sustained research and development efforts, there is considerable evolution in various CGM technologies. In this review, an effort has been made to provide a comprehensive comparison of various CGM technologies with an emphasis on their detection mechanism, invasiveness, and factors that dictate their widespread acceptance.
In conclusion, critical evaluation of various CGM technologies must not only focus on a given advantage afforded by a certain technology but rather holistically evaluate various physicochemical and physiological aspects that are closely linked to device performance and the lifestyle of the patient. For example, unlike invasive CGMs, noninvasive devices do not pose problems of implantation/extraction and foreign body response but have issues with sensor selectivity and accuracy. Although, minimally invasive devices could be a middle ground between the invasive and noninvasive approaches, the patient’s discomfort, the presence of an open wound, and device longevity must be considered. On the other hand, invasive devices could provide greater comfort and freedom to the patient with only periodic (1–3 times per year) outpatient visits for sensor implantation/explantation. Another alternative is the reported biodegradable, tattoo-like technologies.187,188 Unfortunately, this technology is not highly selective and requires frequent calibration via finger pricking.
Among the sensing technologies, the electrochemical mode of glucose detection and the fluorophore-based optical detection appear most promising in view of their selectivity and sensitivity. Sensing technologies based on nonfluorophore optical detection of glucose are more challenging due to the greater environmental variability with respect to parameters such as temperature, pH, tissue scattering, skin pigmentation, and level of hydration. Irrespective of sensing technology or invasiveness, a major problem with all CGM devices has been the calibration-induced inaccuracies and the complexity of correlating ISF glucose levels to actual blood glucose levels. There are a plethora of sources for such correlation inaccuracies that are difficult to narrow down. While future developments are focused on complex algorithms to correlate ISF to blood glucose levels, more research is needed to delineate the interrelationship among different metabolites that could one day help us attain a universal correlation. To this end, devices capable of simultaneously monitoring more than one metabolite can play a crucial role in building sensor robustness and accuracy, and this is expected to increase confidence levels in glucose readings.189
With respect to invasive devices, a pressing issue is the foreign body response. At present, there seems to be no comprehensive study that relates the extent of the foreign body response with sensor size and in vivo location. The realization that utilization of simple immunogenic-resistant materials for invasive sensor fabrication has resulted in only a marginal reduction in the foreign body response and has spurred the research community to adopt complex approaches such as drug-delivery systems, stimuli-responsive materials, and biomimetics. These approaches, however, have been solely tested as viable methodologies to suppress the foreign body response and are yet to be tested as drug–device combinations. In addition, future studies should aim to correlate implant size and sensor coatings to various interrelated factors such as immunogenicity, cyto- toxicity, genotoxicity, tissue sensitization and irritation, intracutaneous reactivity, hemocompatibility, chronic toxicity, and biodegradation. In order to tackle all the aforementioned factors, advanced CGM device architectures together with multiple strategies from multidisciplinary research involving chemists, material scientists, engineers, pharmacists, and physicians are needed. Given enough time and resources, the confidence level is high that an ideal CGM device is within reach.
Glossary
Abbreviations
- (CGM)
continuous glucose monitoring
- (ConA)
concanavalin A
- (FRET)
Förster resonance energy transfer
- (GOx)
glucose oxidase
- (ISF)
interstitial fluid
- (NAD)
nicotinamide adenine dinucleotide
- (NIR)
near infrared
- (SC)
subcutaneous
- (SMBG)
self-monitoring of blood glucose
- (T1DM)
type 1 diabetes mellitus
- (T2DM)
type 2 diabetes mellitus
- (TRM)
tissue response modifier
References
- 1.American Diabetes Association Diabetes statistics. http://www.diabetes.org/diabetes-basics/diabetes-statistics/. Accessed September 28, 2010.
- 2.International Diabetes Federation IDF diabetes atlas. Data. http://www.diabetesatlas.org/content/regional-data. Accessed September 28, 2010.
- 3.World Health Organization Diabetes fact sheet. http://www.who.int/mediacentre/factsheets/fs312/en/index.html.
- 4.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association Clinical practice recommendations 1997. Diabetes Care. 1997;20(Suppl 1):S1–S70. [PubMed] [Google Scholar]
- 6.Reach G, Wilson GS. Can continuous glucose monitoring be used for the treatment of diabetes. Anal Chem. 1992;64(6):381A–386A. doi: 10.1021/ac00030a001. [DOI] [PubMed] [Google Scholar]
- 7.The Diabetes Control and Complications Trial Research Group Lifetime benefits and costs of intensive therapy as practiced in the diabetes control and complications trial. JAMA. 1996;276(17):1409–1415. [PubMed] [Google Scholar]
- 8.Newman JD, Turner AP. Home blood glucose biosensors: a commercial perspective. Biosens Bioelectron. 2005;20(12):2435–2453. doi: 10.1016/j.bios.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Vaddiraju S, Burgess DJ, Jain FC, Papadimitrakopoulos F. The role of H2O2 outer diffusion on the performance of implantable glucose sensors. Biosens Bioelectron. 2009;24(6):1557–1562. doi: 10.1016/j.bios.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaddiraju S, Singh H, Burgess DJ, Jain FC, Papadimitrakopoulos F. Enhanced glucose sensor linearity using poly(vinyl alcohol) hydrogels. J Diabetes Sci Technol. 2009;3(4):863–874. doi: 10.1177/193229680900300434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degani Y, Heller A. Direct electrical communication between chemically modified enzymes and metal electrodes. I. Electron transfer from glucose oxidase to metal electrodes via electron relays, bound covalently to the enzyme. J Phys Chem. 1987;91(6):1285–1289. [Google Scholar]
- 12.Pishko MV, Katakis I, Lindquist SE, Ye L, Gregg BA, Heller A. Direct electrical communication between graphite electrodes and surface adsorbed glucose oxidase/redox polymer complexes. Angewandte Chemie Internat Ed. 1990;29(1):82–84. [Google Scholar]
- 13.Dong S, Wang B, Liu B. Amperometric glucose sensor with ferrocene as an electron transfer mediator. Biosens Bioelectron. 1992;7(3):215–222. [Google Scholar]
- 14.Palys B, Bokun A, Rogalski J. Poly-o-phenylenediamine as redox mediator for laccase’. Electrochim Acta. 2007;52(24):7075–7082. [Google Scholar]
- 15.Yu X, Sotzing GA, Papadimitrakopoulos F, Rusling JF. Wiring of enzymes to electrodes by ultrathin conductive polyion underlayers: enhanced catalytic response to hydrogen peroxide. Anal Chem. 2003;75(17):4565–4571. doi: 10.1021/ac034188r. [DOI] [PubMed] [Google Scholar]
- 16.Joshi PP, Merchant SA, Wang Y, Schmidtke DW. Amperometric biosensors based on redox polymer-carbon nanotube-enzyme composites. Anal Chem. 2005;77(10):3183–3188. doi: 10.1021/ac0484169. [DOI] [PubMed] [Google Scholar]
- 17.Vaddiraju S, Tomazos I, Burgess DJ, Jain FC, Papadimitrakopoulos F. Emerging synergy between nanotechnology and implantable biosensors: a review. Biosens Bioelectron. 2010;25(7):1553–1565. doi: 10.1016/j.bios.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J. Glucose biosensors: 40 years of advances and challenges. Electroanalysis. 2001;13:983–988. [Google Scholar]
- 19.Malinauskas A, Kuzmarskytė J, Meškys R, Ramanavičius A. Bioelectrochemical sensor based on PQQ-dependent glucose dehydrogenase’. Sens Actuators B. 2004;100:387–394. [Google Scholar]
- 20.Blaedel WJ, Jenkins RA. Study of a reagentless lactate electrode. Anal Chem. 1976;48(8):1240–1247. doi: 10.1021/ac50002a045. [DOI] [PubMed] [Google Scholar]
- 21.Newman JD, Turner AP. Home blood glucose biosensors: a commercial perspective. Biosens Bioelectron. 2005;20(12):2435–2453. doi: 10.1016/j.bios.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Skoog M, Johansson G. Internal supply of coenzyme to an amperometric glucose biosensor based on a chemically modified electrode. Biosens Bioelectron. 1991;6(5):407–412. doi: 10.1016/0956-5663(91)87005-v. [DOI] [PubMed] [Google Scholar]
- 23.Gorton L, Bremle G, Csöregi E, Jönsson-Pettersson G, Persson B. Amperometric glucose sensors based on immobilized glucose-oxidizing enzymes and chemically modified electrodes. Anal Chim Acta. 1991;249(1):43–54. [Google Scholar]
- 24.Mizutani F, Yabuki S, Katsura T. Amperometric enzyme electrode with the use of dehydrogenase and NAD(P)H oxidase. Sens Actuator B. 1993;13-14:574–575. [Google Scholar]
- 25.Laurinavičius V, Kurtinaitienė B, Liauksminas V, Ramanavicius A, Meškys R, Rudomanskis R. Oxygen insensitive glucose biosensor based on PQQ-dependent glucose dehydrogenase. Anal Lett. 1999;32(2):299–316. [Google Scholar]
- 26.Habermuller K, Ramanavičius A, Laurinavičius V, Schuhmann W. An oxygen-insensitive reagentless glucose biosensor based on Osmium-complex modified polypyrrole. Electroanal. 2000;12(17):1383–1389. [Google Scholar]
- 27.Loew N, Scheller FW, Wollenberger U. Characterization of self-assembling of glucose dehydrogenase in mono- and multilayers on gold electrodes. Electroanal. 2004;16((13-14)):1149–1154. [Google Scholar]
- 28.Yamazaki T, Kojima K, Sode K. Extended-range glucose sensor employing engineered glucose dehydrogenases. Anal Chem. 2000;72(19):4689–4693. doi: 10.1021/ac000151k. [DOI] [PubMed] [Google Scholar]
- 29.Yuan JH, Wang K, Xia XH. Highly ordered platinum-nanotubule arrays for amperometric glucose sensing. Adv Funct Mater. 2005;15(5):803–809. [Google Scholar]
- 30.Wang J, Thomas DF, Chen A. Nonenzymatic electrochemical glucose sensor based on nanoporous PtPb networks. Anal Chem. 2008;80(4):997–1004. doi: 10.1021/ac701790z. Feb 15. [DOI] [PubMed] [Google Scholar]
- 31.Zhou YG, Yang S, Qian QY, Xia XH. Gold nanoparticles integrated in a nanotube array for electrochemical detection of glucose. Electrochem Commun. 2009;11(1):216–219. [Google Scholar]
- 32.Myung Y, Jang DM, Cho YJ, Kim HS, Park J. Nonenzymatic amperometric glucose sensing of platinum, copper sulfide, and tin oxide nanoparticle-carbon nanotube hybrid nanostructures. J Phys Chem C. 2009;113(4):1251–1259. [Google Scholar]
- 33.Tura A, Maran A, Pacini G. Non-invasive glucose monitoring: assessment of technologies and devices according to quantitative criteria. Diabetes Res Clin Pract. 2007;77(1):16–40. doi: 10.1016/j.diabres.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 34.Oliver NS, Toumazou C, Cass AE, Johnston DG. Glucose sensors: a review of current and emerging technology. Diabet Med. 2009;26(3):197–210. doi: 10.1111/j.1464-5491.2008.02642.x. [DOI] [PubMed] [Google Scholar]
- 35.Pickup JC, Hussain F, Evans ND, Rolinski OJ, Birch DJ. Fluorescence-based glucose sensors. Biosens Bioelectron. 2005;20(12):2555–2565. doi: 10.1016/j.bios.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Moschou EA, Sharma BV, Deo SK, Daunert S. Fluorescence glucose detection: advances toward the ideal in vivo biosensor. J Fluoresc. 2004;14(5):535–547. doi: 10.1023/b:jofl.0000039341.64999.83. [DOI] [PubMed] [Google Scholar]
- 37.Mansouri S, Schultz JS. A miniature optical glucose sensor based on affinity binding. Biotechnol. 1984;2:885–890. [Google Scholar]
- 38.Meadows D, Schultz JS. Fiber-optic biosensors based on fluorescence energy transfer. Talanta. 1988;35(2):145–150. doi: 10.1016/0039-9140(88)80053-5. [DOI] [PubMed] [Google Scholar]
- 39.Russell RJ, Pishko MV, Gefrides CC, McShane MJ, Coté GL. A fluorescence-based glucose biosensor using concanavalin A and dextran encapsulated in a poly(ethylene glycol) hydrogel. Anal Chem. 1999;71(15):3126–3132. doi: 10.1021/ac990060r. [DOI] [PubMed] [Google Scholar]
- 40.Barone PW, Strano MS. Reversible control of carbon nanotube aggregation for a glucose affinity sensor. Angew Chem Int Ed Engl. 2006;45(48):8138–8141. doi: 10.1002/anie.200603138. [DOI] [PubMed] [Google Scholar]
- 41.Barone PW, Parker RS, Strano MS. In vivo fluorescence detection of glucose using a single-walled carbon nanotube optical sensor: design, fluorophore properties, advantages, and disadvantages. Anal Chem. 2005;77(23):7556–7562. doi: 10.1021/ac0511997. [DOI] [PubMed] [Google Scholar]
- 42.Liang F, Pan T, Sevick-Muraca EM. Measurements of fluorescence resonance energy transfer in the concanavalin A-dextran affinity system using frequency-domain lifetime spectroscopy. Proc SPIE. 2005;5702:7–14. [Google Scholar]
- 43.Khalil OS. Non-invasive glucose measurement technologies: an update from 1999 to the dawn of the new millennium. Diabetes Technol Ther. 2004;6(5):660–697. doi: 10.1089/dia.2004.6.660. [DOI] [PubMed] [Google Scholar]
- 44.Domschke A, March WF, Kabilan S, Lowe C. Initial clinical testing of a holographic non-invasive contact lens glucose sensor. Diabetes Technol Ther. 2006;8(1):89–93. doi: 10.1089/dia.2006.8.89. [DOI] [PubMed] [Google Scholar]
- 45.Badugu R, Lakowicz JR, Geddes CD. A glucose-sensing contact lens: from bench top to patient. Curr Opin Biotechnol. 2005;16(1):100–107. doi: 10.1016/j.copbio.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larin KV, Eledrisi MS, Motamedi M, Esenaliev RO. Noninvasive blood glucose monitoring with optical coherence tomography: a pilot study in human subjects. Diabetes Care. 2002;25(12):2263–2267. doi: 10.2337/diacare.25.12.2263. [DOI] [PubMed] [Google Scholar]
- 47.Helwig AM, Arnold MA, Small GW. Evaluation of Kromoscopy: resolution of glucose and urea. Appl Opt. 2000;39(25):4715–4720. doi: 10.1364/ao.39.004715. [DOI] [PubMed] [Google Scholar]
- 48.Rabinovitch B, March WF, Adams RL. Noninvasive glucose monitoring of the aqueous humor of the eye: Part I. Measurement of very small optical rotations. Diabetes Care. 1982;5(3):254–258. doi: 10.2337/diacare.5.3.254. [DOI] [PubMed] [Google Scholar]
- 49.Rawer R, Stork W, Müller-Glaser KD. Polarimetric methods for measurement of intra ocular glucose concentration. Biomed Tech (Berl) 2002;47(Suppl 1)(Pt 1):186–188. doi: 10.1515/bmte.2002.47.s1a.186. [DOI] [PubMed] [Google Scholar]
- 50.Malchoff CD, Shoukri K, Landau JI, Buchert JM. A novel noninvasive blood glucose monitor. Diabetes Care. 2002;25(12):2268–2275. doi: 10.2337/diacare.25.12.2268. [DOI] [PubMed] [Google Scholar]
- 51.Weiss R, Yegorchikov Y, Shusterman A, Raz I. Noninvasive continuous glucose monitoring using photoacoustic technology-results from the first 62 subjects. Diabetes Technol Ther. 2007;9(1):68–74. doi: 10.1089/dia.2006.0059. [DOI] [PubMed] [Google Scholar]
- 52.MacKenzie HA, Ashton HS, Spiers S, Shen Y, Freeborn SS, Hannigan J, Lindberg J, Rae P. Advances in photoacoustic noninvasive glucose testing. Clin Chem. 1999;45(9):1587–1595. [PubMed] [Google Scholar]
- 53.Enejder AM, Scecina TG, Oh J, Hunter M, Shih WC, Sasic S, Horowitz GL, Feld MS. Raman spectroscopy for noninvasive glucose measurements. J Biomed Opt. 2005;10(3):031114. doi: 10.1117/1.1920212. [DOI] [PubMed] [Google Scholar]
- 54.Lyandres O, Yuen JM, Shah NC, VanDuyne RP, Walsh JT, Glucksberg MR. Progress toward an in vivo surface-enhanced Raman spectroscopy glucose sensor. Diabetes Technol Ther. 2008;10(4):257–265. doi: 10.1089/dia.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alexeev VL, Das S, Finegold DN, Asher SA. Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin Chem. 2004;50(12):2353–2360. doi: 10.1373/clinchem.2004.039701. [DOI] [PubMed] [Google Scholar]
- 56.Hillier TA, Abbott RD, Barrett EJ. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med. 1999;106(4):399–403. doi: 10.1016/s0002-9343(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 57.Amaral CE, Wolf B. Effects of glucose in blood and skin impedance spectroscopy. Presented at: IEEE AFRICON 2007; September 25–28, 2007; Windhoek/Namibia. pp. 1–7. [Google Scholar]
- 58.Huber D, Talary M, Dewarrat F, Caduff A. The compensation of perturbing temperature fluctuation in glucose monitoring technologies based on impedance spectroscopy. Med Biol Eng Comput. 2007;45(9):863–876. doi: 10.1007/s11517-007-0229-3. [DOI] [PubMed] [Google Scholar]
- 59.Caduff A, Dewarrat F, Talary M, Stalder G, Heinemann L, Feldman Y. Non-invasive glucose monitoring in patients with diabetes: a novel system based on impedance spectroscopy. Biosens Bioelectron. 2006;22(5):598–604. doi: 10.1016/j.bios.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 60.Pfützner A, Caduff A, Larbig M, Schrepfer T, Forst T. Impact of posture and fixation technique on impedance spectroscopy used for continuous and noninvasive glucose monitoring. Diabetes Technol Ther. 2004;6(4):435–441. doi: 10.1089/1520915041705839. [DOI] [PubMed] [Google Scholar]
- 61.Wentholt IM, Hoekstra JB, Zwart A, DeVries JH. Pendra goes Dutch: lessons for the CE mark in Europe. Diabetologia. 2005;48(6):1055–1058. doi: 10.1007/s00125-005-1754-y. [DOI] [PubMed] [Google Scholar]
- 62.Gourzi M, Rouane A, Guelaz R, Nadi M, Jaspard F. Study of a new electromagnetic sensor for glycaemia measurement: in vitro results on blood pig. J Med Eng Technol. 2003;27(6):276–281. doi: 10.1080/0309190031000098845. Nov-Dec. [DOI] [PubMed] [Google Scholar]
- 63.Gourzi M, Rouane A, Guelaz R, Alavi MS, McHugh MB, Nadi M, Roth P. Non-invasive glycaemia blood measurements by electromagnetic sensor: study in static and dynamic blood circulation. J Med Eng Technol. 2005;29(1):22–26. doi: 10.1080/03091900410001720247. [DOI] [PubMed] [Google Scholar]
- 64.Renard E. Implantable continuous glucose sensors. Curr Diabetes Rev. 2008;4(3):169–174. doi: 10.2174/157339908785294406. Aug. [DOI] [PubMed] [Google Scholar]
- 65.Lutgers HL, Hullegie LM, Hoogenberg K, Sluiter WJ, Dullaart RP, Wientjes KJ, Schoonen AJ. Microdialysis measurement of glucose in subcutaneous adipose tissue up to three weeks in type 1 diabetic patients. Neth J Med. 2000;57(1):7–12. doi: 10.1016/s0300-2977(00)00022-x. [DOI] [PubMed] [Google Scholar]
- 66.Klaus S, Heringlake M, Bahlmann L. Bench-to-bedside review: Microdialysis in intensive care medicine. Crit Care. 2004;8(5):363–368. doi: 10.1186/cc2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klonoff DC. Microdialysis of interstitial fluid for continuous glucose measurement. Diabetes Technol Ther. 2003;5(4):539–543. doi: 10.1089/152091503322250569. [DOI] [PubMed] [Google Scholar]
- 68.Mastrototaro JJ, Johnson KW, Morff RJ, Lipson D, Andrew CC, Allen DJ. An electroenzymatic glucose sensor fabricated on a flexible substrate. Sens Actuators B Chem. 1991;5(1):139–144. [Google Scholar]
- 69.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29(1):44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 70.Liao KC, Hogen-Esch T, Richmond FJ, Marcu L, Clifton W, Loeb GE. Percutaneous fiber-optic sensor for chronic glucose monitoring in vivo. Biosens Bioelectron. 2008;23(10):1458–1465. doi: 10.1016/j.bios.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 71.Rao G, Glikfeld P, Guy RH. Reverse iontophoresis: development of a noninvasive approach for glucose monitoring. Pharm Res. 1993;10(12):1751–1755. doi: 10.1023/a:1018926215306. [DOI] [PubMed] [Google Scholar]
- 72.Rhee SY, Chon S, Koh G, Paeng JR, Oh S, Woo JT, Kim SW, Kim JW, Kim YS. Clinical experience of an iontophoresis based glucose measuring system. J Korean Med Sci. 2007;22(1):70–73. doi: 10.3346/jkms.2007.22.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gebhart S, Faupel M, Fowler R, Kapsner C, Lincoln D, McGee V, Pasqua J, Steed L, Wangsness M, Xu F, Vanstory M. Glucose sensing in transdermal body fluid collected under continuous vacuum pressure via micropores in the stratum corneum. Diabetes Technol Ther. 2003;5(2):159–166. doi: 10.1089/152091503321827812. [DOI] [PubMed] [Google Scholar]
- 74.Mitragotri S, Coleman M, Kost J, Langer R. Analysis of ultra-sonically extracted interstitial fluid as a predictor of blood glucose levels. J Appl Physiol. 2000;89(3):961–966. doi: 10.1152/jappl.2000.89.3.961. [DOI] [PubMed] [Google Scholar]
- 75.Park EJ, Werner J, Beebe J, Chan S, Smith NB. Noninvasive ultrasonic glucose sensing with large pigs (approximately 200 pounds) using a lightweight cymbal transducer array and biosensors. J Diabetes Sci Technol. 2009;3(3):517–523. doi: 10.1177/193229680900300316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kiistala U. Suction blister device for separation of viable epidermis from dermis. J Invest Dermatol. 1968;50(2):129–137. doi: 10.1038/jid.1968.15. [DOI] [PubMed] [Google Scholar]
- 77.Robert JJ. Continuous monitoring of blood glucose. Horm Res. 2002;57(Suppl 1):81–84. doi: 10.1159/000053321. [DOI] [PubMed] [Google Scholar]
- 78.Khalil OS. Spectroscopic and clinical aspects of noninvasive glucose measurements. Clin Chem. 1999;45(2):165–177. [PubMed] [Google Scholar]
- 79.De Block C, Vertommen J, Manuel-y-Keenoy B, Van Gaal L. Minimally-invasive and non-invasive continuous glucose monitoring systems: indications, advantages, limitations and clinical aspects. Curr Diabetes Rev. 2008;4(3):159–168. doi: 10.2174/157339908785294415. Aug. [DOI] [PubMed] [Google Scholar]
- 80.Ferrante do Amaral CE, Wolf B. Current development in non-invasive glucose monitoring. Med Eng Phys. 2008;30(5):541–549. doi: 10.1016/j.medengphy.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 81.International Organization for Standardization In vitro diagnostic test systems: requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. 2003. ISO 15197:
- 82.Clarke WL, Kovatchev B. Continuous glucose sensors: continuing questions about clinical accuracy. J Diabetes Sci Technol. 2007;1(5):669–675. doi: 10.1177/193229680700100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klonoff D, Bernhardt P, Ginsberg BH, Joseph J, Mastrototaro J, Parker DR, Vesper H, Vigersky R, Clinical and Laboratory Standards Institute Performance metrics for continuous interstitial glucose monitoring; approved guideline. 2008. CLSI document POCT05-A.
- 84.D’Archangelo MJ. New guideline supports the development and evaluation of continuous interstitial glucose monitoring devices. J Diabetes Sci Technol. 2008;2(2):332–334. doi: 10.1177/193229680800200228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 86.Kovatchev BP, Gonder-Frederick LA, Cox DJ, Clarke WL. Evaluating the accuracy of continuous glucose-monitoring sensors: continuous glucose-error grid analysis illustrated by TheraSense Freestyle Navigator data. Diabetes Care. 2004;27(8):1922–1928. doi: 10.2337/diacare.27.8.1922. [DOI] [PubMed] [Google Scholar]
- 87.Cox DJ, Clarke WL, Gonder-Frederick L, Pohl S, Hoover C, Snyder A, Zimbelman L, Carter WR, Bobbitt S, Pennebaker J. Accuracy of perceiving blood glucose in IDDM. Diabetes Care. 1985;8(6):529–536. doi: 10.2337/diacare.8.6.529. [DOI] [PubMed] [Google Scholar]
- 88.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 89.Cameron BD, Anumula H. Development of a real-time corneal birefringence compensated glucose sensing polarimeter. Diabetes Technol Ther. 2006;8(2):156–164. doi: 10.1089/dia.2006.8.156. [DOI] [PubMed] [Google Scholar]
- 90.Tierney MJ, Tamada JA, Potts RO, Jovanovic L, Garg S, Cygnus Research Team Clinical evaluation of the GlucoWatch biographer: a continual, non-invasive glucose monitor for patients with diabetes. Biosens Bioelectron. 2001;16((9-12)):621–629. doi: 10.1016/s0956-5663(01)00189-0. [DOI] [PubMed] [Google Scholar]
- 91.Diabetes Research in Children Network (DirecNet) Study Group Accuracy of the GlucoWatch G2 Biographer and the continuous glucose monitoring system during hypoglycemia: experience of the Diabetes Research in Children Network. Diabetes Care. 2004;27(3):722–726. doi: 10.2337/diacare.27.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kost J, Mitragotri S, Gabbay RA, Pishko M, Langer R. Transdermal monitoring of glucose and other analytes using ultrasound. Nat Med. 2000;6(3):347–350. doi: 10.1038/73213. [DOI] [PubMed] [Google Scholar]
- 93.Sachedina N, Pickup JC. Performance assessment of the Medtronic-MiniMed Continuous Glucose Monitoring System and its use for measurement of glycaemic control in type 1 diabetic subjects. Diabet Med. 2003;20(12):1012–105. doi: 10.1046/j.1464-5491.2003.01037.x. Dec. [DOI] [PubMed] [Google Scholar]
- 94.Weinstein RL, Schwartz SL, Brazg RL, Bugler JR, Peyser TA, McGarraugh GV. Accuracy of the 5-Day FreeStyle Navigator Continuous Glucose Monitoring System: comparison with frequent laboratory reference measurements. Diabetes Care. 2007;30(5):1125–1130. doi: 10.2337/dc06-1602. [DOI] [PubMed] [Google Scholar]
- 95.Renard E, Shah R, Miller M, Kolopp M, Costalat G, Bringer J. Sustained safety and accuracy of central IV glucose sensors connected to implanted insulin pumps and short-term closed-loop trials in diabetic patients. Diabetologia. 2003;46:A47. [Google Scholar]
- 96.Rossetti P, Porcellati F, Fanelli CG, Bolli GB. Evaluation of the accuracy of a microdialysis-based glucose sensor during insulin-induced hypoglycemia, its recovery, and post-hypoglycemic hyperglycemia in humans. Diabetes Technol Ther. 2006;8(3):326–337. doi: 10.1089/dia.2006.8.326. Jun. [DOI] [PubMed] [Google Scholar]
- 97.Nielsen JK, Freckmann G, Kapitza C, Ocvirk G, Koelker KH, Kamecke U, Gillen R, Amann-Zalan I, Jendrike N, Christiansen JS, Koschinsky T, Heinemann L. Glucose monitoring by microdialysis: performance in a multicentre study. Diabet Med. 2009;26(7):714–721. doi: 10.1111/j.1464-5491.2009.02750.x. [DOI] [PubMed] [Google Scholar]
- 98.Gourzi M, Rouane A, Guelaz R, Alavi MS, McHugh MB, Nadi M, Roth P. Non-invasive glycaemia blood measurements by electromagnetic sensor: study in static and dynamic blood circulation. J Med Eng Technol. 2005;29(1):22–26. doi: 10.1080/03091900410001720247. [DOI] [PubMed] [Google Scholar]
- 99.Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143–1148. doi: 10.2337/diacare.23.8.1143. [DOI] [PubMed] [Google Scholar]
- 100.Lodwig V, Heinemann L, Glucose Monitoring Study Group Continuous glucose monitoring with glucose sensors: calibration and assessment criteria. Diabetes Technol Ther. 2003;5(4):572–586. doi: 10.1089/152091503322250596. [DOI] [PubMed] [Google Scholar]
- 101.Onuki Y, Bhardwaj U, Papadimitrakopoulos F, Burgess DJ. A review of the biocompatibity of implantable devices: current challenges to overcome foreign body response. J Diabetes Sci Technol. 2008;2(6):1003–1015. doi: 10.1177/193229680800200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heller A. Implanted electrochemical glucose sensors for the management of diabetes. Annu Rev Biomed Eng. 1999;1:153–175. doi: 10.1146/annurev.bioeng.1.1.153. [DOI] [PubMed] [Google Scholar]
- 103.Wentholt IM, Hart AA, Hoekstra JB, Devries JH. Relationship between interstitial and blood glucose in type 1 diabetes patients: delay and the push-pull phenomenon revisited. Diabetes Technol Ther. 2007;9(2):169–175. doi: 10.1089/dia.2006.0007. [DOI] [PubMed] [Google Scholar]
- 104.Hovorka R, Shojaee-Moradie F, Carroll PV, Chassin LJ, Gowrie IJ, Jackson NC, Tudor RS, Umpleby AM, Jones RH. Partitioning glucose distribution/transport, disposal, and endogenous production during IVGTT. Am J Physiol Endocrinol Metab. 2002;282(5):E992–E1007. doi: 10.1152/ajpendo.00304.2001. [DOI] [PubMed] [Google Scholar]
- 105.Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003;52(11):2790–2794. doi: 10.2337/diabetes.52.11.2790. [DOI] [PubMed] [Google Scholar]
- 106.Kulcu E, Tamada JA, Reach G, Potts RO, Lesho MJ. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 2003;26(8):2405–2409. doi: 10.2337/diacare.26.8.2405. [DOI] [PubMed] [Google Scholar]
- 107.Tipnis R, Vaddiraju S, Jain F, Burgess DJ, Papadimitrakopoulos F. Layer-by-layer assembled semipermeable membrane for ampero-metric glucose sensors. J Diabetes Sci Technol. 2007;1(2):193–200. doi: 10.1177/193229680700100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson GS, Gifford R. Biosensors for real-time in vivo measure-ments. Biosens Bioelectron. 2005;20(12):2388–2403. doi: 10.1016/j.bios.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 109.Cembrowski GC, Smith B, O’Malley EM. Increases in whole blood glucose measurements using optically based self-monitoring of blood glucose analyzers due to extreme canadian winters. J Diabetes Sci Technol. 2009;3(4):661–667. doi: 10.1177/193229680900300407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schoemaker M, Andreis E, Röper J, Kotulla R, Lodwig V, Obermaier K, Stephan P, Reuschling W, Rutschmann M, Schwaninger R, Wittmann U, Rinne H, Kontschieder H, Strohmeier W. The SCGM1 System: subcutaneous continuous glucose monitoring based on microdialysis technique. Diabetes Technol Ther. 2003;5(4):599–608. doi: 10.1089/152091503322250613. [DOI] [PubMed] [Google Scholar]
- 111.Harman-Boehm I, Gal A, Raykhman AM, Naidis E, Mayzel Y. Noninvasive glucose monitoring: increasing accuracy by combination of multi-technology and multi-sensors. J Diabetes Sci Technol. 2010;4(3):583–595. doi: 10.1177/193229681000400312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Castle JR, Ward WK. Amperometric glucose sensors: sources of error and potential benefit of redundancy. J Diabetes Sci Technol. 2010;4(1):221–225. doi: 10.1177/193229681000400127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vaddiraju S, Tomazos I, Burgess DJ, Jain FC, Papadimitrakopoulos F. Emerging synergy between nanotechnology and implantable biosensors: a review. Biosens Bioelectron. 2010;25(7):1553–1565. doi: 10.1016/j.bios.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang J. In vivo glucose monitoring: towards ‘Sense and Act’ feedback-loop individualized medical systems. Talanta. 2008;75(3):636–641. doi: 10.1016/j.talanta.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 115.Burmeister JJ, Gerhardt GA. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Anal Chem. 2001;73(5):1037–1042. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- 116.Jeong RA, Hwang JY, Joo S, Chung TD, Park S, Kang SK, Lee WY, Kim HC. In vivo calibration of the subcutaneous amperometric glucose sensors using a non-enzyme electrode. Biosens Bioelectron. 2003;19(4):313–319. doi: 10.1016/s0956-5663(03)00219-7. [DOI] [PubMed] [Google Scholar]
- 117.O’Brien KB, Killoran SJ, O’Neill RD, Lowry JP. Development and characterization in vitro of a catalase-based biosensor for hydrogen peroxide monitoring. Biosens Bioelectron. 2007;22(12):2994–3000. doi: 10.1016/j.bios.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 118.O’Neill RD. Microvoltammetric techniques and sensors for monitoring neurochemical dynamics in vivo: a review. Analyst. 1994;119(5):767–779. doi: 10.1039/an9941900767. [DOI] [PubMed] [Google Scholar]
- 119.Dong S, Wang B, Liu B. Amperometric glucose sensor with ferrocene as an electron transfer mediator. Biosens Bioelectron. 1992;7(3):215–222. [Google Scholar]
- 120.Kim SN, Rusling JF, Papadimitrakopoulos F. Carbon nanotubes for electronic and electrochemical detection of biomolecules. Adv Mater. 2007;19(20):3214–3228. doi: 10.1002/adma.200700665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu X, Sotzing GA, Papadimitrakopoulos F, Rusling JF. Wiring of enzymes to electrodes by ultrathin conductive polyion underlayers: enhanced catalytic response to hydrogen peroxide. Anal Chem. 2003;75(17):4565–4571. doi: 10.1021/ac034188r. [DOI] [PubMed] [Google Scholar]
- 122.Yuhashi N, Tomiyama M, Okuda J, Igarashi S, Ikebukuro K, Sode K. Development of a novel glucose enzyme fuel cell system employing protein engineered PQQ glucose dehydrogenase. Biosens Bioelectron. 2005;20(10):2145–2150. doi: 10.1016/j.bios.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 123.Kay CW, Mennenga B, Görisch H, Bittl R. Structure of the pyrroloquinoline quinone radical in quinoprotein ethanol dehydrogenase. J Biol Chem. 2006;281(3):1470–1476. doi: 10.1074/jbc.M511132200. [DOI] [PubMed] [Google Scholar]
- 124.McNichols RJ, Coté GL. Optical glucose sensing in biological fluids: an overview. J Biomed Opt. 2000;5(1):5–16. doi: 10.1117/1.429962. [DOI] [PubMed] [Google Scholar]
- 125.Barone PW, Strano MS. Single walled carbon nanotubes as reporters for the optical detection of glucose. J Diabetes Sci Technol. 2009;3(2):242–252. doi: 10.1177/193229680900300204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Armour JC, Lucisano JY, McKean BD, Gough DA. Application of chronic intravascular blood glucose sensor in dogs. Diabetes. 1990;39(12):1519–1526. doi: 10.2337/diab.39.12.1519. [DOI] [PubMed] [Google Scholar]
- 127.Updike SJ, Shults MC, Rhodes RK, Gilligan BJ, Luebow JO, von Heimburg D. Enzymatic glucose sensors. Improved long-term performance in vitro and in vivo. ASAIO J. 1994;40(2):157–163. [PubMed] [Google Scholar]
- 128.Gilligan BJ, Shults MC, Rhodes RK, Updike SJ. Evaluation of a subcutaneous glucose sensor out to 3 months in a dog model. Diabetes Care. 1994;17(8):882–887. doi: 10.2337/diacare.17.8.882. [DOI] [PubMed] [Google Scholar]
- 129.Gerritsen M, Jansen JA, Lutterman JA. Performance of subcutaneously implanted glucose sensors for continuous monitoring. Neth J Med. 1999;54(4):167–179. doi: 10.1016/s0300-2977(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 130.Kerner W. Implantable glucose sensors: present status and future developments. Exp Clin Endocrinol DiabetesM. 2001;109(Suppl 2):S341–S346. doi: 10.1055/s-2001-18593. [DOI] [PubMed] [Google Scholar]
- 131.Koschinsky T, Heinemann L. Sensors for glucose monitoring: technical and clinical aspects. Diabetes Metab Res Rev. 2001;17(2):113–123. doi: 10.1002/dmrr.188. [DOI] [PubMed] [Google Scholar]
- 132.Shichiri M, Kawamori R, Yamasaki Y. [Use of a wearable artificial pancreas] Nippon Rinsho. 1985;43(12):2629–2634. [PubMed] [Google Scholar]
- 133.Shichiri M, Kawamori R, Yamasaki Y, Hakui N, Abe H. Wearable artificial endocrine pancreas with needle-type glucose sensor. Lancet. 1982;2(8308):1129–1131. doi: 10.1016/s0140-6736(82)92788-x. [DOI] [PubMed] [Google Scholar]
- 134.Kvist PH, Iburg T, Aalbaek B, Gerstenberg M, Schoier C, Kaastrup P, Buch-Rasmussen T, Hasselager E, Jensen HE. Biocompatibility of an enzyme-based, electrochemical glucose sensor for short-term implantation in the subcutis. Diabetes Technol Ther. 2006;8(5):546–559. doi: 10.1089/dia.2006.8.546. [DOI] [PubMed] [Google Scholar]
- 135.Harrison DJ, Turner RF, Baltes HP. Characterization of perfluoro- sulfonic acid polymer coated enzyme electrodes and a miniaturized integrated potentiostat for glucose analysis in whole blood. Anal Chem. 1988;60(19):2002–2007. doi: 10.1021/ac00170a003. [DOI] [PubMed] [Google Scholar]
- 136.Moussy F, Harrison DJ, O’Brien DW, Rajotte RV. Performance of subcutaneously implanted needle-type glucose sensors employing a novel trilayer coating. Anal Chem. 1993;65(15):2072–2077. doi: 10.1021/ac00063a023. [DOI] [PubMed] [Google Scholar]
- 137.Galeska I, Chattopadhyay D, Moussy F, Papadimitrakopoulos F. Calcification-resistant Nafion/Fe3+ assemblies for implantable biosensors. Biomacromolecules. 2000;1(2):202–207. doi: 10.1021/bm0002813. [DOI] [PubMed] [Google Scholar]
- 138.Galeska I, Chattopadhyay D, Papadimitrakopoulos F. Application of polyanion/Fe3 multilayered membranes in prevention of biosensor mineralization. J Macromol Sci Pure Appl Chem. 2002;39(10):1207–1222. [Google Scholar]
- 139.Praveen SS, Hanumantha R, Belovich JM, Davis BL. Novel hyaluronic acid coating for potential use in glucose sensor design. Diabetes Technol Ther. 2003;5(3):393–399. doi: 10.1089/152091503765691893. [DOI] [PubMed] [Google Scholar]
- 140.Galeska I, Hickey T, Moussy F, Kreutzer D, Papadimitrakopoulos F. Characterization and biocompatibility studies of novel humic acids based films as membrane material for an implantable glucose sensor. Biomacromolecules. 2001;2(4):1249–1255. doi: 10.1021/bm010112y. [DOI] [PubMed] [Google Scholar]
- 141.Ward WK, Slobodzian EP, Tiekotter KL, Wood MD. The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants. Biomaterials. 2002;23(21):4185–4192. doi: 10.1016/s0142-9612(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 142.Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. Controlling acute inflammation with fast releasing dexamethasone-PLGA microsphere/PVA hydrogel composites for implantable devices. J Diabetes Sci Technol. 2007;1(1):8–17. doi: 10.1177/193229680700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117(1):68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 144.Yang Y, Zhang SF, Kingston MA, Jones G, Wright G, Spencer SA. Glucose sensor with improved haemocompatibilty. Biosens Bioelectron. 2000;15((5-6)):221–227. doi: 10.1016/s0956-5663(00)00082-8. [DOI] [PubMed] [Google Scholar]
- 145.Ishihara K, Nakabayashi N, Nishida K, Sakakida M, Shichiri M. New biocompatible polymer: application for implantable glucose sensor. Diagnost Biosens Polymer. 1994;556:194–210. [Google Scholar]
- 146.Ishihara K, Tanaka S, Furukawa N, Kurita K, Nakabayashi N. Improved blood compatibility of segmented polyurethanes by polymeric additives having phospholipid polar groups. I. Molecular design of polymeric additives and their functions. J Biomed Mater Res. 1996;32(3):391–399. doi: 10.1002/(SICI)1097-4636(199611)32:3<391::AID-JBM12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 147.Suri JT, Cordes DB, Cappuccio FE, Wessling RA, Singaram B. Continuous glucose sensing with a fluorescent thin-film hydrogel. Angew Chem Int Ed Engl. 2003;42(47):5857–5859. doi: 10.1002/anie.200352405. [DOI] [PubMed] [Google Scholar]
- 148.Hickey T, Kreutzer D, Burgess DJ, Moussy F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. J Biomed Mater Res. 2002;61(2):180–187. doi: 10.1002/jbm.10016. [DOI] [PubMed] [Google Scholar]
- 149.Hickey T, Kreutzer D, Burgess DJ, Moussy F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23(7):1649–1656. doi: 10.1016/s0142-9612(01)00291-5. [DOI] [PubMed] [Google Scholar]
- 150.Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial growth factor and dexa-methasone release from nonfouling sensor coatings affect the foreign body response. J Biomed Mater Res A. 2007;81(4):858–869. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Frost M, Meyerhoff ME. In vivo chemical sensors: tackling biocompatibility. Anal Chem. 2006;78(21):7370–7377. doi: 10.1021/ac069475k. [DOI] [PubMed] [Google Scholar]
- 152.Frost MC, Batchelor MM, Lee Y, Zhang H, Kang Y, Oh BK, Wilson GS, Gifford R, Rudich SM, Meyerhoff ME. Preparation and characterization of implantable sensors with nitric oxide release coatings. Microchem J. 2003;74:277–288. [Google Scholar]
- 153.Frost MC, Meyerhoff ME. Fabrication and in vivo evaluation of nitric oxide-releasing electrochemical oxygen-sensing catheters. Methods Enzymol. 2004;381:704–715. doi: 10.1016/S0076-6879(04)81045-0. [DOI] [PubMed] [Google Scholar]
- 154.Sung WJ, Na K, Bae YH’. Biocompatibility and interference eliminating property of pullulan acetate/polyethylene glycol/heparin membrane for the outer layer of an amperometric glucose sensor. Sens Actuators B Chem. 2004;99((2-3)):393–398. [Google Scholar]
- 155.Kim TK, Burgess DJ. Pharmacokinetic characterization of 14C-vascular endothelial growth factor controlled release micro-spheres using a rat model. J Pharm Pharmacol. 2002;54(7):897–905. doi: 10.1211/002235702760089009. [DOI] [PubMed] [Google Scholar]
- 156.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29(12):1517–1524. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 157.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. J Biomed Mater Res. 1997;37(3):401–412. doi: 10.1002/(sici)1097-4636(19971205)37:3<401::aid-jbm11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 158.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. III. Effective tissue response times. J Biomed Mater Res. 1998;40(4):598–605. doi: 10.1002/(sici)1097-4636(19980615)40:4<598::aid-jbm11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 159.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. II. Plasma-tissue exchange properties. J Biomed Mater Res. 1998;40(4):586–597. doi: 10.1002/(sici)1097-4636(19980615)40:4<586::aid-jbm10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 160.Popat KC, Eltgroth M, Latempa TJ, Grimes CA, Desai TA. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials. 2007;28(32):4880–4888. doi: 10.1016/j.biomaterials.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 161.Sahlin H, Contreras R, Gaskill DF, Bjursten LM, Frangos JA. Anti-inflammatory properties of micropatterned titanium coatings. J Biomed Mater Res A. 2006;77(1):43–49. doi: 10.1002/jbm.a.30642. [DOI] [PubMed] [Google Scholar]
- 162.Lopez CA, Fleischman AJ, Roy S, Desai TA. Evaluation of silicon nanoporous membranes and ECM-based microenvironments on neurosecretory cells. Biomaterials. 2006;27(16):3075–3083. doi: 10.1016/j.biomaterials.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 163.Striemer CC, Gaborski TR, McGrath JL, Fauchet PM. Charge- and size-based separation of macromolecules using ultrathin silicon membranes. Nature. 2007;445(7129):749–753. doi: 10.1038/nature05532. [DOI] [PubMed] [Google Scholar]
- 164.Desai TA, Hansford DJ, Leoni L, Essenpreis M, Ferrari M. Nanoporous anti-fouling silicon membranes for biosensor applications. Biosens Bioelectron. 2000;15((9-10)):453–462. doi: 10.1016/s0956-5663(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 165.Sharma S, Desai TA. Nanostructured antifouling poly(ethylene glycol) films for silicon-based microsystems. J Nanosci Nanotechnol. 2005;5(2):235–243. doi: 10.1166/jnn.2005.030. [DOI] [PubMed] [Google Scholar]
- 166.Narayan RJ, Aggarwal R, Wei W, Jin C, Monteiro-Riviere NA, Crombez R, Shen W. Mechanical and biological properties of nanoporous carbon membranes. Biomed Mater. 2008;3(3):034107. doi: 10.1088/1748-6041/3/3/034107. [DOI] [PubMed] [Google Scholar]
- 167.Saha K, Pollock JF, Schaffer DV, Healy KE. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007;11(4):381–387. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.House JL, Anderson EM, Ward WK. Immobilization techniques to avoid enzyme loss from oxidase-based biosensors: a one-year study. J Diabetes Sci Technol. 2007;1(1):18–27. doi: 10.1177/193229680700100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McMahon CP, Killoran SJ, O’Neill RD. Design variations of a polymer-enzyme composite biosensor for glucose: Enhanced analyte sensitivity without increased oxygen dependence. J Electroanal Chem. 2005;580(2):193–202. [Google Scholar]
- 170.Updike SJ, Shults MC, Rhodes RK, Gilligan BJ, Luebow JO, von Heimburg D. Enzymatic glucose sensors: improved long-term performance in vitro and in vivo. ASAIO J. 1994;40(2):157–163. [PubMed] [Google Scholar]
- 171.Binyamin G, Chen T, Heller A. Sources of instability of ‘wired’ enzyme anodes in serum: Urate and transition metal ions. J Electroanal Chem. 2001;500((1-2)):604–611. [Google Scholar]
- 172.Yu B, Moussy Y, Moussy F. Coil-type implantable glucose biosensor with excess enzyme loading. Front Biosci. 2005;10:512–520. doi: 10.2741/1547. [DOI] [PubMed] [Google Scholar]
- 173.Kim J, Grate JW, Wang P. Nanostructures for enzyme stabilization. Chem Eng Sci. 2006;61(3):1017–1026. [Google Scholar]
- 174.Guto PM, Kumar CV, Rusling JF. Thermostable peroxidase-polylysine films for biocatalysis at 90 degrees C. J Phys Chem B. 2007;111(30):9125–9131. doi: 10.1021/jp071525h. [DOI] [PubMed] [Google Scholar]
- 175.Wang ZG, Wan LS, Liu ZM, Huang XJ, Xu ZK. Enzyme immobilization on electrospun polymer nanofibers: an overview. J Mol Catal B-Enzym. 2009;56(4):189–195. [Google Scholar]
- 176.Phadtare S, Vinod VP, Wadgaonkar PP, Rao M, Sastry M. Free-standing nanogold membranes as scaffolds for enzyme immobilization. Langmuir. 2004;20(9):3717–3723. doi: 10.1021/la035870j. [DOI] [PubMed] [Google Scholar]
- 177.Chen T, Barton SC, Binyamin G, Gao Z, Zhang Y, Kim HH, Heller A. A miniature biofuel cell. J Am Chem Soc. 2001;123(35):8630–8631. doi: 10.1021/ja0163164. [DOI] [PubMed] [Google Scholar]
- 178.Mano N, Mao F, Heller A. Characteristics of a miniature compartment-less glucose-O2 biofuel cell and its operation in a living plant. J Am Chem Soc. 2003;125(21):6588–6594. doi: 10.1021/ja0346328. May 28. [DOI] [PubMed] [Google Scholar]
- 179.Mano N, Mao F, Heller A. A miniature biofuel cell operating in a physiological buffer. J Am Chem Soc. 2002;124(44):12962–12963. doi: 10.1021/ja028514g. [DOI] [PubMed] [Google Scholar]
- 180.Ward WK, House JL, Birck J, Anderson EM, Jansen LB. A wire-based dual-analyte sensor for glucose and lactate: in vitro and in vivo evaluation. Diabetes Technol Ther. 2004;6(3):389–401. doi: 10.1089/152091504774198106. [DOI] [PubMed] [Google Scholar]
- 181.McMahon CP, O’Neill RD. Polymer-enzyme composite biosensor with high glutamate sensitivity and low oxygen dependence. Anal Chem. 2005;77(4):1196–1199. doi: 10.1021/ac048686r. [DOI] [PubMed] [Google Scholar]
- 182.Crespi F. In vivo voltammetric detection of neuropeptides with micro carbon fiber biosensors: Possible selective detection of somatostatin. Anal Biochem. 1991;194(1):69–76. doi: 10.1016/0003-2697(91)90152-j. [DOI] [PubMed] [Google Scholar]
- 183.Tamiya E, Sugiura Y, Takeuchi T, Suzuki M, Karube I, Akiyama A. Ultra micro glutamate sensor using platinized carbon-fiber electrode and integrated counter electrode. Sens Actuators B Chem. 1993;10(3):179–184. [Google Scholar]
- 184.Wu Z, Chen L, Shen G, Yu R. Platinum nanoparticle-modified carbon fiber ultramicroelectrodes for mediator-free biosensing. Sens Actuators B Chem. 2006;119(1):295–301. [Google Scholar]
- 185.Errachid A, Ivorra A, Aguiló J, Villa R, Zine N, Bausells J. New technology for multi-sensor silicon needles for biomedical applications. Sens Actuators B Chem. 2001;78((1-2)):279–284. [Google Scholar]
- 186.Johnson KW, Mastrototaro JJ, Howey DC, Brunelle RL, Burden-Brady PL, Bryan NA, Andrew CC, Rowe HM, Allen DJ, Noffke BW, McMahan WC, Morff RJ, Lipson D, Nevin RS. In vivo evaluation of an electroenzymatic glucose sensor implanted in subcutaneous tissue. Biosens Bioelectron. 1992;7(10):709–714. doi: 10.1016/0956-5663(92)85053-d. [DOI] [PubMed] [Google Scholar]
- 187.Renard E, Shah R, Miller M, Kolopp M, Costalat G, Bringer J. Sustained safety and accuracy of central IV glucose sensors connected to implanted insulin pumps and short-term closed-loop trials in diabetic patients. Diabetologia. 2003;46:A47. [Google Scholar]
- 188.McShane MJ. Microcapsules as “smart tattoo” glucose sensors: engineering systems with enzymes and glucose-binding sensing elements. In: Geddes CD, Lakowicz JR, editors. Topics in fluorescence spectroscopy. Vol. 11. New York: Springer; 2006. pp. 131–163. [Google Scholar]
- 189.Caduff A, Talary MS, Mueller M, Dewarrat F, Klisic J, Donath M, Heinemann L, Stahel WA. Non-invasive glucose monitoring in patients with type 1 diabetes: a multisensor system combining sensors for dielectric and optical characterisation of skin. Biosens Bioelectron. 2009;24(9):2778–2784. doi: 10.1016/j.bios.2009.02.001. [DOI] [PubMed] [Google Scholar]