Abstract
Background
The profound hypogonadism that occurs with androgen-deprivation therapy (ADT) for prostate cancer (PCa) results in complications such as diabetes and metabolic syndrome that predispose to cardiovascular disease. Since phytoestrogens have been associated with an improvement in metabolic parameters, we evaluated their role in men undergoing ADT.
Objective
To evaluate the effects of high-dose isoflavones on metabolic and inflammatory parameters in men undergoing ADT.
Methods
This was a randomized, double-blind, placebo-controlled, 12-week pilot study. Participants were randomly assigned to receive 20 g of soy protein containing 160 mg of total isoflavones vs taste-matched placebo (20 g whole milk protein). The study was conducted at a tertiary care center in the United States.
Results
Thirty-three men (isoflavones=17, placebo=16) undergoing ADT for PCa completed this pilot study. Mean age in the two groups was 69 years and majority of men were Caucasians. Mean duration of ADT in both groups was approximately 2 years (P=0.70). The two groups were well-matched at baseline. After 12-weeks of intervention, there was no significant difference in either metabolic or inflammatory parameters between the two groups.
Conclusion
High-dose isoflavones over a course of 12-weeks do not improve metabolic or inflammatory parameters in androgen deprived men.
Introduction
Prostate cancer (PCa) is the most common non-cutaneous malignancy in men (Jemal et al, 2009). Androgen deprivation therapy (ADT) has traditionally been used in the treatment of locally advanced and metastatic PCa. In the former it has shown survival advantage (with radiation therapy) while in metastatic disease it has improved quality of life (Bolla et al, 1997). However, it is increasingly been used in men with early stage PCa and those experiencing biochemical recurrence, even though no survival advantage has been shown (Sharifi et al, 2005). Recent estimates suggest that more than half a million men in the United States alone are receiving ADT (Smith et al, 2007). Despite its benefits in a select group of patients, the resulting profound hypogonadism leads to adverse effects such as osteoporosis, unfavorable body composition, sexual dysfunction, metabolic perturbations, cognitive changes, hot flashes and decreased quality of life (QOL) (Basaria et al, 2002; Basaria et al, 2006; Harle et al, 2006).
Traditionally, these manifestations have been attributed to castrate levels of serum testosterone, overlooking the fact that these men also have low-to-undetectable levels of serum estradiol (Basaria et al, 2002). Hence, the relative contribution of testosterone and estradiol to these adverse effects, especially metabolic abnormalities, remains unclear. The fact that men with congenital aromatase deficiency (undetectable serum estradiol levels) have high prevalence of osteoporosis, insulin resistance and metabolic syndrome despite having normal to elevated serum testosterone levels underscores the importance of estrogen in men (Carani et al, 1997). Hence, it appears that severe deficiency of estrogen is also directly related to adverse consequences in men. Studies of estrogen replacement in men on ADT have shown some benefits, especially improvement in vasomotor symptoms (Gerber et al, 2000). Although these observations make estrogen administration in these men an attractive option, earlier studies have found an increased incidence of cardiovascular deaths in men with advanced PCa who were receiving diethylstilbestrol, a synthetic estrogen (even though the administered dose was high) (Blackard et al, 1975). Hence, the quest continues for alternative agents that may provide beneficial effects similar to estrogen while being devoid of their adverse effects.
Phyto-estrogens (plant estrogens) are non-steroidal, naturally occurring compounds that are known to exert estrogenic effects (Murkies et al, 1998). They are structurally similar to natural and synthetic estrogens and bind to estrogen receptors (especially ER-β) (Kuiper et al, 1998). The common classes of phyto-estrogens include isoflavones, lignans and coumestans. Isoflavones are present in the highest amount in soybeans, flaxseed and legumes. Genistein, daidzein and glycitein are the most common isoflavones. Soy is a staple of Asian diets where daily intake is at least 40 times higher than among western populations (de Kleijn et al, 2001). Estimates suggest that average daily intake of isoflavones among Chinese population is 100–150 mg/day compared to 1 mg/day in the United States (de Kleijn et al, 2001).
Recently, metabolic complications of ADT have surfaced. These include insulin resistance, diabetes, dyslipidemia and metabolic syndrome (Basaria et al, 2006; Braga-Basaria et al, 2006; Keating et al, 2006). In addition to these complications, recent reports also suggest increased coronary disease and cardiovascular mortality in this population (Keating et al, 2006; Saigal et al, 2007; D’Amico et al, 2007; Tsai et al, 2007). Isoflavones have been associated with some beneficial effects on metabolic parameters. In South East Asia, increased consumption of isoflavones has been associated with decreased incidence of diabetes and heart disease (Villegas et al, 2008; Zhang et al, 2003). In clinical trials, isoflavones have shown an improvement in glycemic control, metabolic syndrome and inflammatory profile in both men and postmenopausal women (Jayagopal et al, 2002; Azadbakht et al, 2007; Atteritano et al, 2007; Huang et al, 2005). We recently showed an increase in serum adiponectin (adipokine associated with improved insulin sensitivity) levels in postmenopausal women who were randomized to high dose (160 mg/d) isoflavones compared with placebo (Charles et al, 2009). To the best of our knowledge, no study to date has evaluated the effects of soy on metabolic or inflammatory parameters in men receiving ADT. Therefore, we conducted the current double-blind, randomized; placebo-controlled study using high dose isoflavones to answer these questions.
Methods
Participants
Participants were recruited from the Johns Hopkins Medical and Radiation Oncology Clinics. The protocol was approved by the Institutional Review Board at Johns Hopkins Hospital. English speaking men ≥21 years undergoing medical or surgical ADT for a minimum of 3 months duration were included in the study. Subjects were excluded if they had any of the following: hepatic or renal disease, untreated thyroid dysfunction, neurological or active psychiatric disorder, current use of chemotherapy or glucocorticoids, appetite stimulants or weight promoting agents, history of substance abuse or serum triglycerides >500 mg/dl. In addition, men with known allergy to soy protein or cow’s milk were also excluded. Participants who were taking soy supplements at the time of enrollment were asked to discontinue all such products for at least 3 months prior to enrollment into the study (3-month wash-out). Once enrolled, men were instructed not to consume any additional soy products throughout the 12-week study period. This was verified with a food diary.
Randomization
A personal computer generated a list of random numbers. Staffs of the clinical trials unit who were not involved in conduct of the current trial, randomly assigned men to either isoflavone or casein placebo group. The subjects and the study personnel remained blinded to the group assignment throughout the study.
Intervention
The intervention (Revival Soy®, Physicians Pharmaceuticals, Inc, Kernersville, NC) contained 20 gram of soy protein consisting of 160 mg of total isoflavones (96 mg aglycones). Isoflavones were available in the form of a powder that was mixed with a beverage. The concentrations of the individual isoflavones were as follows: genistein 64 mg, diadzein 63 mg, and glycitein 34 mg. The placebo powder (Physicians Pharmaceuticals, Inc, Kernersville, NC) contained 20 grams of whole milk protein and contained the exact same nutrients as the intervention, excluding the isoflavones. The active and the placebo powders were similar in appearance and taste, and were available in vanilla and chocolate flavors (dispensed upon patient’s preference). Supplements were taken once a day for 12 weeks and were dispensed at baseline and 6-week visit.
Laboratory methods
Blood was collected at baseline, week 6 and week 12 of the study between 8 and 10 AM after an overnight fast. Once collected, samples were centrifuged to separate the serum that was stored at −80°C until the analysis. Weight and height were measured and body mass index (BMI) was calculated.
Metabolic parameters
Quantification of serum glucose concentrations was performed by a Beckman Glucose Analyzer 2 (Beckman Instruments, Inc, Fullerton, CA). This particular analyzer uses an oxygen rate method with an oxygen electrode. Serum was injected into an enzyme reagent solution containing dissolved oxygen, from which the electronic circuitry can measure the rate of oxygen consumption from the sample, which is directly proportional to the glucose concentration. Precision for these glucose values had a standard deviation of less than 2.5 mg/dl. Insulin concentrations were determined using an enzyme-linked immunosorbent assay (ELISA) (Mercodia, Uppsala, Sweden) with intra- and interassay coefficient of variation (COV) between 2.8% and 4.0% and 2.6% and 3.6%, respectively. Insulin resistance was determined using the homeostatic model assessment of insulin resistance (HOMAIR) method (Matthews et al, 1985). C-peptide was measured using a sandwich-based ELISA (Alpco Diagnostics, Salem, NH) with intra- and interassay COV between 2.87% and 4.5%, and 6.6% and 8.7%, respectively.
Adipo-cytokines
Serum leptin and resistin were measured using ELISA. Resistin (Alpco Diagnostics, Salem, NH) had intra- and interassay COV between 2.86% and 5.17%, and 4.2% and 7.2%, respectively. The human leptin kit uses a direct sandwich-based method (Linco Research, St Charles, MO). The intra- and interassay COV were between 2.6% and 6.2%. Adiponectin (Linco Research) was measured using a radioimmunoassay (RIA) with intra- and interassay COV between 1.78% and 9.25%. The quantitative sandwich enzyme immunoassay technique was used for IL-6, spg130 (IL-6 receptor), TNF-α, sTNF RI (TNF- receptor I), and sTNF RII (TNF-α receptor II) (R&D Systems, Minneapolis, MN). The intra- and interassay precision for the assays were the following: IL-6 (6.5% and 9.6%), spg130 (5.0% and 9.8%), TNF-α (3.1% and 10.6%), sTNF RI (3.6% and 8.8%) and sTNF RII (2.6% and 5.1%). Serum C-Reactive protein was measured using a sandwich-based ELISA (Alpco Diagnostics, Salem, NH) with intra- and interassay COV between 5.5% and 6.5%, and 11.6% and 13.8%, respectively.
Testosterone
Serum testosterone (Diagnostic Systems Laboratories, Webster, TX) was measured using a radioimmunoassay (RIA) with intra- and inter-assay COV between 7.8% and 9.6%.
Statistical Analyses
Prior to testing hypotheses and modeling, normality of the continuous variables was inspected by plotting histograms and via Shapiro-Wilks test, and the need for transformation or nonparametric analysis was determined. No outliers were identified for any outcome measures or demographic variables. For comparison between treatment groups, χ2 analysis was done for the categorical demographic variables. Based on the distributional properties of the continuous demographic variables, either two-sample t-test or Wilcoxon rank sum test was used. For both metabolic and inflammatory parameters, comparison across visits within each treatment group was done via one-way Analysis of Variance (ANOVA) or the nonparametric Kruskal-Wallis test and comparison between treatment groups at each visit was done via t-test or Wilcoxon rank sum test. Data were analyzed according to a modified intent-to-treat principle, including all those who had two measurements, including baseline, in the groups to which they were randomized. All analysis was done using SAS version 9.1.3.
Results
Thirty-three men (isoflavones=17, placebo=16) undergoing ADT for PCa for at least 3 months completed this randomized, double-blind, placebo-controlled pilot study. Of the initially enrolled 39 men, 3 were excluded based on screening labs while 3 men withdrew from the study (2 due to personal reasons, 1 subject disliked the taste of the compound). None of the men had diabetes.
Baseline Data
Mean age of the subjects was 69 years and 80% were caucasian (Table 1). Mean duration of ADT in both groups was approximately 2 years (P=0.70). The majority of men were undergoing medical ADT and >80% also received radiation therapy. Only four subjects were receiving combined androgen blockade. The two groups were well-matched in terms of age, weight, BMI, TSH or comorbidities. Men in the placebo group had higher mean PSA values, however, they were not significantly different than the isoflavone group (p = 0.30). This high mean value in the placebo group was driven by four subjects who had PSA values ranging between 100–600 ng/ml. During the course of the study, there were no significant changes in PSA, weight or BMI in any of the groups (data not shown).
Table 1.
Baseline comparison
| Variable | Placebo (n=16) | Treatment (n=17) | P - value |
|---|---|---|---|
| Age (year) | 69.0 (2.2) * | 69.2(2.5) * | 0.94 |
| Race—White/Black (n) | 11/5 | 15/2 | |
| Weight (Kg) | 97.39 (4.73)* | 90.20 (4.01)* | 0.25 |
| BMI (Kg/m2) | 30.05 (1.44)* | 28.71 (1.24)* | 0.48 |
| PSA (ng/ml) | 45.05 (39.54)* | 3.90 (2.58)* | 0.30 |
| TSH (mIU/ml) | 1.98 (0.22)* | 1.81 (0.24)* | 0.60 |
| Duration of ADT to baseline (year) | 1.96 (0.64) * | 2.37 (0.37) * | 0.70 |
| GnRH Analogues/Orchiectomy (n) | 16/0 | 16/1 | |
| AR Antagonist (n) | 1 | 3 | |
| Radiation Therapy (n) | 11 | 13 | |
| History of Metastases (n) | 7 | 7 | |
| Number of abnormalities in system review | 3.19 (0.42) * | 3.41 (0.52) * | 0.74 |
Mean (standard error)
Lipids & Glycemic Parameters
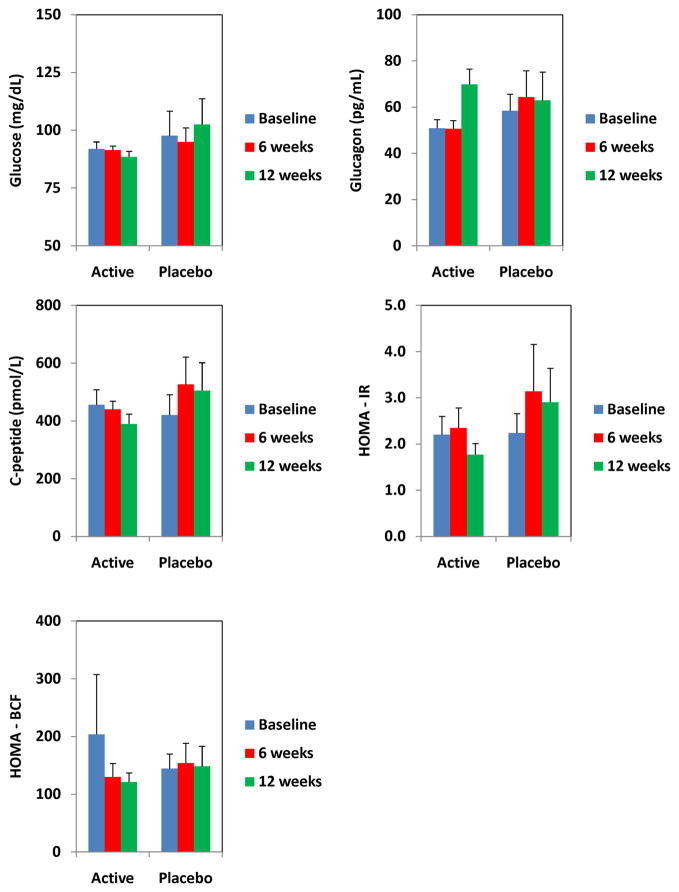
At 6 weeks, and 12 weeks, there were no significant differences in any of the lipid parameters between the two groups (Table 2). Similarly, there were no significant differences in the glycemic parameters (Figure 1). Insulin resistance, as determined by HOMAIR also did not change.
Table 2.
Changes in the lipid profiles during treatment with isoflavones.
| Variable | Baseline | 6 weeks | 12 weeks | p-values | |
|---|---|---|---|---|---|
| Total cholesterol (mg/dl) | Placebo | 186.8 (13.08) | 193.93 (10.74) | 190.69 (11.66) | 0.91 |
| Active | 207.33 (12.45) | 206.2 (12.64) | 211.6 (11.91) | 0.948 | |
| p-value | 0.265 | 0.466 | 0.224 | ||
| Triglycerides (mg/dl) | Placebo | 129.33 (16.85) | 119.4 (16.36) | 130.15 (23.74) | 0.903 |
| Active | 123.6 (7.97) | 128.93 (9.63) | 119.87 (11.55) | 0.807 | |
| p-value | 0.761 | 0.619 | 0.688 | ||
| HDL cholesterol (mg/dl) | Placebo | 55.47 (3.46) | 56.4 (3.37) | 56.92 (4.15) | 0.96 |
| Active | 55.33 (2.93) | 56 (3.06) | 57.93 (3.46) | 0.833 | |
| p-value | 0.977 | 0.931 | 0.852 | ||
| LDL cholesterol (mg/dl) | Placebo | 114.27 (9.35) | 113.67 (8.51) | 107.77 (8.54) | 0.856 |
| Active | 127.2 (10.65) | 124.33 (11.13) | 129.67 (10.09) | 0.939 | |
| p-value | 0.369 | 0.453 | 0.116 | ||
| Total Cholesterol/HDL ratio | Placebo | 3.6 (0.22) | 3.57 (0.23) | 3.52 (0.31) | 0.978 |
| Active | 3.81 (0.22) | 3.71 (0.2) | 3.77 (0.25) | 0.95 | |
| p-value | 0.51 | 0.65 | 0.54 | ||
Figure 1.
Changes in glycemic parameters in men on isoflavones and placebo.
Adipokines & Inflammatory Cytokines
There were no significant improvements in any of the inflammatory parameters or adipokines in the isoflavone group (Table 3).
Table 3.
Changes in the adipokines and inflammatory cytokines during treatment with isoflavones.
| Variable | Baseline | 6 weeks | 12 weeks | p-values | |
|---|---|---|---|---|---|
| Leptin (ng/ml) | Placebo | 23.5 (3.6) | 24.2 (4.5) | 25.2 (4.7) | 0.965 |
| Active | 19.7 (2.9) | 19.1 (3) | 19 (3.7) | 0.985 | |
| p-value | 0.407 | 0.345 | 0.307 | ||
| Resistin (ng/ml) | Placebo | 13.8 (2) | 14.4 (2.1) | 12.4 (2.6) | 0.827 |
| Active | 12.4 (1.4) | 12.6 (2.2) | 12.8 (2.1) | 0.991 | |
| p-value | 0.586 | 0.566 | 0.912 | ||
| Adiponectin (mg/ml) | Placebo | 13 (2.1) | 9.3 (1.1) | 10.7 (1.4) | 0.253 |
| Active | 14.3 (1.7) | 16.5 (2.3) | 13.9 (2.5) | 0.673 | |
| p-value | 0.646 | 0.011 | 0.305 | ||
| TNFα (pg/ml) | Placebo | 1.4 (0.3) | 1.4 (0.2) | 1.5 (0.3) | 0.98 |
| Active | 1.5 (0.2) | 1.3 (0.1) | 1.4 (0.1) | 0.641 | |
| p-value | 0.839 | 0.635 | 0.834 | ||
| TNFα RI (pg/ml) | Placebo | 1623.5 (141) | 1537.8 (133.3) | 1550.5 (120.5) | 0.884 |
| Active | 1670.1 (131.7) | 1697.3 (121.5) | 1687.4 (113.7) | 0.988 | |
| p-value | 0.811 | 0.384 | 0.418 | ||
| TNFα RII (pg/ml) | Placebo | 2795.6 (241.3) | 2677.8 (216.9) | 2672.7 (189.4) | 0.903 |
| Active | 2844 (266.2) | 2893.1 (227.5) | 2970.3 (228.1) | 0.932 | |
| p-value | 0.895 | 0.501 | 0.339 | ||
| spg130 (ng/ml) | Placebo | 329 (11.7) | 332 (10.8) | 363.2 (29.3) | 0.376 |
| Active | 360.4 (19.8) | 370 (26.9) | 392.2 (35.3) | 0.712 | |
| p-value | 0.2 | 0.212 | 0.545 | ||
| CRP (ng/ml) | Placebo | 2304.2 (801.2) | 2950 (1271.4) | 2120.1 (853.1) | 0.829 |
| Active | 3005.8 (1275) | 2599.6 (693.1) | 5110.8 (1734) | 0.363 | |
| p-value | 0.656 | 0.807 | 0.161 | ||
| IL6 (pg/ml) | Placebo | 1.6 (0.2) | 3.2 (1.2) | 1.4 (0.2) | 0.148 |
| Active | 2.5 (0.8) | 3.1 (1.2) | 2.1 (0.3) | 0.695 | |
| p-value | 0.27 | 0.953 | 0.112 | ||
Discussion
In this double blind, randomized, placebo controlled pilot study; administration of high-dose isoflavones to men with PCa undergoing ADT did not demonstrate any beneficial effects on metabolic or inflammatory parameters. To the best of our knowledge, this is the first study in the English literature using high-dose isoflavones in this patient population.
In recent years there has been a significant increase in the use of ADT (3.7% in 1991 to 30.9% in 1999) and it is estimated that more than half a million Americans are on androgen deprivation (Smith et al, 2007). Recently, in addition to traditional complications of ADT such as sexual dysfunction, osteoporosis and vasomotor symptoms, metabolic complications such as insulin resistance, diabetes and metabolic syndrome have emerged. Recent literature also suggests that men on ADT also experience higher cardiovascular mortality (D’Amico et al, 2007; Tsai et al, 2007). Indeed, epidemiological studies have shown that male hypogonadism is an independent risk factor for diabetes, metabolic syndrome and cardiovascular mortality (Laaksonen et al, 2004, Laughlin et al, 2008). Although castrate levels of serum testosterone have been implicated in the pathophysiology of these complications, these men also have low to undetectable levels of serum estradiol (Basaria et al, 2002). Hence, the relative contribution of testosterone or estradiol to these adverse effects remains unclear. A few studies using estrogen therapy in these men have shown improvement in parameters such as hot flashes (Gerber et al, 2000), however, its use has been associated with increased cardiovascular mortality (Blackard et al, 1975). Since isoflavones have slightly different mechanism of action (via ER-β) and their use has resulted in an improvement in metabolic parameters in postmenopausal women (Jayagopal et al, 2002; Azadbakht et al, 2007; Atteritano et al, 2007; Huang et al, 2005; Charles et al, 2009), the hope has been that isoflavones would be beneficial in men on ADT. Soy is consumed in large amounts in Asian countries compared to western population (Chen et al, 1999), and a few epidemiological studies have attributed lower incidence of diabetes in such populations to soy consumption (Villegas et al, 2008). We recently showed in a clinical trial that high-dose isoflavones (concentration similar to that consumed by Asian population) in postmenopausal women increased adiponectin (insulin sensitizing adipokine) levels in postmenopausal women compared to placebo (Charles et al, 2009). Hence, we used the same dose of isoflavones to evaluate metabolic parameters in men on ADT.
Several studies have shown potential advantageous effect of soy on metabolic parameters. Soy is a staple of Asian diet. Epidemiological data indicates that Japanese-Americans residing in Seattle, Washington have four times higher prevalence of type 2 diabetes than those living in Tokyo (Fujimoto et al, 1994; Fujimoto et al, 1991). Clinical trials of isoflavones have also shown beneficial effects on glucose homeostasis in postmenopausal women who have metabolic syndrome and type 2 diabetes (Jayagopal et al, 2002; Azadbakht et al). One study showed that 132 mg of isoflavones improved both insulin resistance and hemoglobin A1c in postemenopausal women with type 2 diabetes (Jayagopal et al, 2002) The mechanisms by which isoflavones exert anti-diabetic effects include inhibition of intestinal brush border uptake of glucose and binding to both PPARγ and PPARα (Vedavanam et al, 1999; Dang et al, 2003; Wagner et al, 2008). Even though we used concentrations of isoflavones that are consumed by the Asian population, we found no significant improvement in glycemic parameters or insulin resistance in men undergoing ADT.
Inflammatory cytokines have been implicated in the pathogenesis of insulin resistance (Hotamisligil & Spiegelman, 1994). Our use of isoflavones to target inflammation has sound basis since isoflavones have been shown in animals models to reduce tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) (Ito et al, 2001; Miyamoto et al, 1999; Pfeilschifter et al, 2002). Tumor necrosis factor-α causes insulin resistance by: i) down-regulating insulin receptor substrate-1 (IRS-1), ii) inducing serine phosphorylation of IRS-1, and iii) decreasing glucose transport protein-1 (GLUT-1) (Arnr et al, 2005). On the other hand, IL-6 causes insulin resistance by decreasing the activation of IRS-1 (Arnr et al, 2005). In postmenopausal women low-dose isoflavones have been shown to decrease serum TNF-α level (Huang et al, 2005). These findings were not confirmed by our study.
The efficacy of isoflavones in improving lipid parameters remain controversial. In a frequently cited meta-analysis of 38 controlled clinical trials, 34 studies showed significant improvement in lipid parameters (Anderson et al, 1995). The reductions in total cholesterol, LDL-cholesterol and triglycerides were by 9%, 13% and 10%, respectively. However, further subanalyses showed that patients with the highest pretreatment total cholesterol (>335 mg/dl) and LDL-cholesterol (highest quartile) got the most benefit, while it was not beneficial in subjects with mild hyperlipidemia. Since then, some trials have failed to show any benefit of soy on serum lipids (Dewell et al, 2002; Gardner et al, 2001; Nestel et al, 1999; Simons et al, 2000), while a few have shown some improvement (Crouse et al, 1999; Bairey Merz et al, 2006; Allen et al, 1990). The likely explanation for the lack of response in our men may be the fact that these subjects at baseline only had mild hypercholesterolemia (both total and LDL) and normal triglyceride levels.
Our study has a few limitations. First, we did not measure serum or urine levels of isoflavones, hence, the absorption of isoflavones could not be confirmed. These should be measured in future studies. Second, we did not measure body composition of the subjects. Lastly, the serum levels of adipokines and cytokines do not equate to the actual tissue concentrations. Therefore, any influence of isoflavones on tissue levels of these markers cannot be ruled out. Our study also has a few strengths. First, this was a double blind, randomized, placebo-controlled study. Second, we implemented a washout period of 3 months in those subjects who were on soy supplements and ensured based on food diary that they did not consume soy in any other form. Third, we measured sophisticated inflammatory markers such as adipokines/cytokines/soluble receptors in our study. Lastly, our study had a reasonably large sample size for a pilot study.
In conclusion, administering isoflavones at a concentration comparable to that consumed by the Asian populations did not produce any significant improvements in markers of glucose homeostasis or inflammation. However, we suggest future studies using variable doses of isoflavones for a longer duration before ruling out any beneficial effects of isoflavones in this population.
Footnotes
The authors have nothing to disclose.
References
- 1.Allen JK, Fitzgerald ST, Swank RT, Becker DM. Functional status after coronary artery bypass grafting and percutaneous transluminal coronary angioplasty. Am J Cardiol. 1990;66:921–5. doi: 10.1016/0002-9149(90)90926-r. [DOI] [PubMed] [Google Scholar]; Am J Cardiol. 2000;85:1297–301. doi: 10.1016/s0002-9149(00)00759-1. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333:276–82. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 3.Arnr P. Insulin resistance in type 2 diabetes—role of the adipokines. Curr Mol Med. 2005;5:333–339. doi: 10.2174/1566524053766022. [DOI] [PubMed] [Google Scholar]
- 4.Atteritano M, Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Mazzaferro S, D’Anna R, Cannata ML, Gaudio A, Frisina A, Frisina N, Corrado F, Cancellieri F, Lubrano C, Bonaiuto M, Adamo EB, Squadrito F. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a two-year randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007;92:3068–75. doi: 10.1210/jc.2006-2295. [DOI] [PubMed] [Google Scholar]
- 5.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Padyab M, Hu FB, Willett WC. Soy inclusion in the diet improves features of the metabolic syndrome: a randomized crossover study in postmenopausal women. Am J Clin Nutr. 2007;85:735–741. doi: 10.1093/ajcn/85.3.735. [DOI] [PubMed] [Google Scholar]
- 6.Bairey Merz CN, Johnson BD, Braunstein GD, Pepine CJ, Reis SE, Paul-Labrador M, Hale G, Sharaf BL, Bittner V, Sopko G, Kelsey SF. Phytoestrogens and lipoproteins in women. J Clin Endocrinol Metab. 2006;91:2209–13. doi: 10.1210/jc.2005-1853. [DOI] [PubMed] [Google Scholar]
- 7.Basaria S, Lieb J, 2nd, Tang AM, DeWeese T, Carducci M, Eisenberger M, Dobs AS. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 2002;56:779–86. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 8.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–8. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 9.Blackard CE. The Veterans’ Administration Cooperative Urological Research Group studies of carcinoma of the prostate: a review. Cancer Chemother Rep. 1975;59:225–7. [PubMed] [Google Scholar]
- 10.Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Gil T, Collette L, Pierart M. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 11.Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, Basaria S. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–83. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 12.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337:91–5. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 13.Charles C, Yuskavage J, Carlson O, John M, Tagalicud AS, Maggio M, Muller DC, Egan J, Basaria S. Effects of high-dose isoflavones on metabolic and inflammatory markers in healthy postmenopausal women. Menopause. 2009;16:395–400. doi: 10.1097/gme.0b013e3181857979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Zheng W, Custer LJ, Dai Q, Shu XO, Jin F, Franke AA. Usual dietary consumption of soy foods and its correlation with the excretion rate of isoflavonoids in overnight urine samples among Chinese women in Shanghai. Nutr Cancer. 1999;33:82–7. doi: 10.1080/01635589909514752. [DOI] [PubMed] [Google Scholar]
- 15.Crouse JR, 3rd, Morgan T, Terry JG, Ellis J, Vitolins M, Burke GL. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch Intern Med. 1999;159:2070–6. doi: 10.1001/archinte.159.17.2070. [DOI] [PubMed] [Google Scholar]
- 16.D’Amico AV, Denham JW, Crook J, Chen MH, Goldhaber SZ, Lamb DS, Joseph D, Tai KH, Malone S, Ludgate C, Steigler A, Kantoff PW. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–5. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 17.Dang ZC, Audinot V, Papapoulos SE, Boutin JA, Löwik CW. Peroxisome proliferator-activated receptor gamma (PPARgamma) as a molecular target for the soy phytoestrogen genistein. J Biol Chem. 2003;278:962–7. doi: 10.1074/jbc.M209483200. [DOI] [PubMed] [Google Scholar]
- 18.de Kleijn MJ, van der Schouw YT, Wilson PW, Adlercreutz H, Mazur W, Grobbee DE, Jacques PF. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the Framingham study. J Nutr. 2001;131:1826–32. doi: 10.1093/jn/131.6.1826. [DOI] [PubMed] [Google Scholar]
- 19.Dewell A, Hollenbeck CB, Bruce B. The effects of soy-derived phytoestrogens on serum lipids and lipoproteins in moderately hypercholesterolemic postmenopausal women. J Clin Endocrinol Metab. 2002;87:118–21. doi: 10.1210/jcem.87.1.8155. [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto WY, Bergstrom RW, Boyko EJ, Kinyoun JL, Leonetti DL, Newell-Morris LL, Robinson LR, Shuman WP, Stolov WC, Tsunehara CH. Diabetes and risk factors in second- and third-generation Japanese Americans in Seattle, Washington. Diabetes Res Clin Pract. 1994;24:S43–S52. doi: 10.1016/0168-8227(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto WY, Leonetti DL, Bergstrom RW, Kinyoun JL, Stolov WC, Wahl PW. Glucose intolerance and diabetes complications among Japanese-American women. Diabetes Res Clin Pract. 1991;13:119–129. doi: 10.1016/0168-8227(91)90042-c. [DOI] [PubMed] [Google Scholar]
- 22.Gardner CD, Newell KA, Cherin R, Haskell WL. The effect of soy protein with or without isoflavones relative to milk protein on plasma lipids in hypercholesterolemic postmenopausal women. Am J Clin Nutr. 2001;73:728–35. doi: 10.1093/ajcn/73.4.728. [DOI] [PubMed] [Google Scholar]
- 23.Gerber GS, Zagaja GP, Ray PS, Rukstalis DB. Transdermal estrogen in the treatment of hot flashes in men with prostate cancer. Urology. 2000;55:97–101. doi: 10.1016/s0090-4295(99)00370-2. [DOI] [PubMed] [Google Scholar]
- 24.Harle LK, Maggio M, Shahani S, Braga-Basaria M, Basaria S. Endocrine complications of androgen-deprivation therapy in men with prostate cancer. Clin Adv Hematol Oncol. 2006;4:687–96. [PubMed] [Google Scholar]
- 25.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–8. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Cao S, Nagamani M, Anderson KE, Grady JJ, Lu LJ. Decreased circulating levels of tumor necrosis factor-alpha in postmenopausal women during consumption of soy-containing isoflavones. J Clin Endocrinol Metab. 2005;90:3956–62. doi: 10.1210/jc.2005-0161. [DOI] [PubMed] [Google Scholar]
- 27.Ito A, Bebo BF, Jr, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, Offner H. Estrogen treatment down-regulates TNF-alpha production production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. 2001;167:542–552. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- 28.Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25:1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- 29.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 30.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 31.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 32.Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–41. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 33.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto N, Mandai M, Suzuma I, Suzuma K, Koyayashi K, Honda Y. Estrogen protects against cellular infiltration by reducing the expressions of E-slectin and IL-6 in endotoxin-induced uveitis. J Immunol. 1999;163:374–379. [PubMed] [Google Scholar]
- 36.Murkies AL, Wilcox G, Davis SR. Clinical review 92: Phytoestrogens. J Clin Endocrinol Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- 37.Nestel PJ, Pomeroy S, Kay S, Komesaroff P, Behrsing J, Cameron JD, West L. Isoflavones from red clover improve systemic arterial compliance but not plasma lipids in menopausal women. J Clin Endocrinol Metab. 1999;84:895–8. doi: 10.1210/jcem.84.3.5561. [DOI] [PubMed] [Google Scholar]
- 38.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 39.Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 40.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 41.Simons LA, von Konigsmark M, Simons J, Celermajer DS. Phytoestrogens do not influence lipoprotein levels or endothelial function in healthy, postmenopausal women. doi: 10.1016/s0002-9149(00)00759-1. [DOI] [PubMed] [Google Scholar]
- 42.Smith MR. Androgen deprivation therapy for prostate cancer: new concepts and concerns. Curr Opin Endocrinol Diabetes Obes. 2007;14:247–254. doi: 10.1097/MED.0b013e32814db88c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai HK, D’Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–24. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 44.Vedavanam K, Srijayanta S, O’Reilly J, Raman, Wiseman H. Antioxidant, action and potential antidiabetic properities of an isoflavonoid-containing soyabean phytochemical extract (SPE) Phytother Res. 1999;13:601–608. doi: 10.1002/(sici)1099-1573(199911)13:7<601::aid-ptr550>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 45.Villegas R, Gao YT, Yang G, Li HL, Elasy TA, Zheng W, Shu XO. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am J Clin Nutr. 2008;87:162–7. doi: 10.1093/ajcn/87.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner JD, Zhang L, Shadoan MK, Kavanagh K, Chen H, Tresnasari K, Kaplan JR, Adams MR. Effects of soy protein and isoflavones on insulin resistance and adiponectin in male monkeys. Metabolism Clinical and Experimental. 2008;57:S24–S31. doi: 10.1016/j.metabol.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Shu XO, Gao YT, Yang G, Li Q, Li H, Jin F, Zheng W. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 2003;133:2874–8. doi: 10.1093/jn/133.9.2874. [DOI] [PubMed] [Google Scholar]