Abstract
Background and Aims
While a diet rich in anti-oxidant has been favorably associated with coronary disease and hypertension, limited data have evaluated the influence of such diet on subclinical disease. Thus, we sought to examine whether chocolate consumption is associated with calcified atherosclerotic plaque in the coronary arteries (CAC).
Methods
In a cross-sectional design, we studied 2,217 participants of the NHLBI Family Heart Study. Chocolate consumption was assessed by a semi-quantitative food-frequency questionnaire and CAC was measured by cardiac CT. We defined prevalent CAC using an Agatston score of at least 100 and fitted generalized estimating equations to calculate prevalence odds ratios of CAC.
Results
There was an inverse association between frequency of chocolate consumption and prevalent CAC. Odds ratios (95% CI) for CAC were 1.0 (reference), 0.94 (0.66-1.35), 0.78 (0.53-1.13), and 0.68 (0.48-0.97) for chocolate consumption of 0, 1-3 times per month, once per week, and 2+ times per week, respectively (p for trend 0.022), adjusting for age, sex, energy intake, waist-hip ratio, education, smoking, alcohol consumption, ratio of total-to-HDL-cholesterol, non-chocolate candy, and diabetes mellitus. Controlling for additional confounders did not alter the findings. Exclusion of subjects with coronary heart disease or diabetes mellitus did not materially change the odds ratio estimates but did modestly decrease the overall significance (p = 0.07).
Conclusions
These data suggest that chocolate consumption might be inversely associated with prevalent CAC.
Keywords: Chocolate, diet, epidemiology, coronary calcium, subclinical disease
Introduction
It has been shown that the extent of calcified atherosclerotic plaque in the coronary arteries (CAC) is highly correlated with total burden of atherosclerotic plaques1,2 The degree of CAC -- measured by cardiac CT -- provides more prognostic information on coronary heart disease (CHD) incidence3-5 and CHD mortality6,7. A diet rich in fruit and vegetables, dark chocolate, or red wine – all good sources of flavonoids – has been associated with a lower risk of CHD, cardiovascular mortality, lower blood pressure, and inhibition of platelet aggregation8-12. In a cross-over study, consumption of 100 g/d of dark chocolate was associated with a statistically significant reduction in systolic blood pressure in 13 hypertensive subjects after 14 days of intervention10. Another study also demonstrated beneficial effects of dark chocolate on blood pressure in healthy subjects after 2 weeks of intervention13. However, no previous study has evaluated whether frequent chocolate consumption is associated with subclinical disease. In the present study, we used data collected on 2,217 participants of the National Heart, Lung, and Blood Institute (NHLBI) Family Heart Study (FHS) to determine whether chocolate consumption was associated with a lower prevalence of CAC.
Materials and Methods
Study population
Participants in this project were members of the NHLBI FHS in whom coronary calcified plaque was measured by cardiac gated multi-detector computed tomography (cardiac CT). The NHLBI FHS is a multi-center, population-based study designed to identify and evaluate genetic and non-genetic determinants of CHD, preclinical atherosclerosis, and cardiovascular risk factors. Detailed descriptions of the NHLBI FHS have been published.14,15 Briefly, families in the study had been chosen randomly (random group) or based on a higher than expected risk of CHD (high-risk group) from previously established population-based cohort studies. A total of 588 families were chosen at random (with 2673 subjects) and 566 families were selected based on higher than expected risk of CHD (3037 subjects). O the 5710 subjects, 265 were African-American. The high-risk group was defined based on a family risk score, which compares the family's age and sex-specific incidence of CHD to that expected in the general population.16 All members of these families were invited for a clinical evaluation (between 1993 and 1995). Between 2002 and 2003, about one-third of the families (the largest families available who also had genome-wide anonymous markers typed by the Mammalian Genotyping Service) of the NHLBI FHS – including 9 African-American participants – were invited to participate in a clinical examination that included measurement of CAC with cardiac CT. In addition to the initial NHLBI FHS study centers, an all African-American center – University of Alabama at Birmingham – was recruited from the Hypertension Genetic Epidemiology Network Study, where subjects underwent cardiac CT but did not have dietary assessments. Of the 3,370 subjects who had data on cardiac CT, 2,217 subjects also had complete data on chocolate consumption at baseline examination (1993-1995). Each participant gave informed consent and the study protocol was reviewed and approved by each of the participating institutions.
Assessment of chocolate consumption
Dietary information was collected through a staff-administered semi-quantitative food frequency questionnaire developed by Willett et al.17. The reproducibility and validity of the food frequency questionnaire has been documented elsewhere18,19. Each subject was asked the following question: “In the past year, how often on average did you consume chocolate bars or pieces, such as Hershey's Plain, M & M, Snickers, Reeses; 1 oz?” (Item # 39 in the questionnaire forms). Possible responses were: “> 6 per day, 4-6 per day, 2-3 per day, 1 per day, 5-6 per week, 2-4 per week, 1 per week, 1-3 per month, and almost never”. There were very few people consuming more than 1 per day and since the odds ratios were similar between 5-6/week and 1+/day as well as between 1/week and 2-4/week, we combined those categories to obtain stable estimates.
Measurement of calcified atherosclerotic plaque in the coronary arteries
Cardiac CT examinations were obtained using General Electric Health Systems LightSpeed Plus and LightSpeed Ultra, Siemens Volume Zoom, or Philips MX 8000 machines. Examinations were performed using the same protocol as employed in the NHLBI's Multi-Ethnic Study of Atherosclerosis.20 The scans were performed using prospective ECG gating at 50% of the cardiac cycle, 120 KV, 106 mAs, 2.5 mm slice collimation, 0.5 second gantry rotation and a partial scan reconstruction resulting in a temporal resolution of between 250-300 msec. Images were reconstructed using the standard algorithm into a 35 cm display field-of-view. All subjects were imaged with a calcium calibration standard within the imaging field (Image Analysis, Columbia, KY). The scan through the heart was repeated after a one-minute pause during the same examination, resulting in two sequential scans for measurement of CAC. The effective radiation exposure for the average participant of each coronary scan was 1.5 mSv for men and 1.9 mSv for women. CT images from all sites were sent electronically to the central CT reading center located at Wake Forest University Health Sciences, Winston Salem, NC. Trained CT analysts using dedicated hardware (GE Advantage Windows Workstation) and software (GE SmarScore) identified CAC in the epicardial coronary arteries and an Agatston score modified to account for slice thickness was calculated using a 130 CT number threshold and a minimum lesion size of 0.9 mm (i.e., 2 pixel connectivity filter). Agatston score refers to the amount of calcium detected by the scan and is based on the area and the density of the calcified plaques21. In this report, the sum of the vessel plaque is reported as the total CAC score. Total CAC scores from the first and second measured were then averaged.
For these analyses, we dichotomized the distribution of CAC score at an Agatston score of 100 or more as described previously15 and because this cut point has a reasonable sensitivity and specificity.3 Nevertheless, we conducted sensitivity analyses to assess the robustness of our findings to different cutoff points (CAC score of 0, 50, 150, 200, and 300) to define the presence of calcified atherosclerotic plaque.
Blood collection and assays
All participants were asked to fast for twelve hours before their arrival at the study center. Evacuated tubes without additives were used to collect samples for lipids. Triglyceride concentrations were measured using triglyceride GB reagent on the Roche COBAS FARA centrifugal analyzer (Boehringer Mannheim Diagnostics, Indianapolis). Serum total cholesterol was measured using a commercial cholesterol oxidase method on a Roche COBAS FARA centrifugal analyzer (Boehringer Mannheim Diagnostics, Indianapolis). HDL-cholesterol quantification was performed with the above described cholesterol method after precipitation of non-HDL-cholesterol with magnesium/dextran. For samples with triglycerides concentrations less than 4.5 mmol/L (400 mg/dL), LDL-cholesterol was calculated using the Friedewald formula.22 For subjects with higher levels of triglycerides, LDL-cholesterol quantitation was performed on EDTA plasma by ultracentrifugation.
Other variables
Information on cigarette smoking, alcohol intake, and education was obtained by interview during the clinic visit. Resting blood pressure was measured three times on seated participants after a 5-minute rest using a random zero sphygmomanometer and an appropriate cuff size. For analyses, the average systolic and diastolic blood pressures from the second and third measurements were used. We used the seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure classification to define hypertension (stages 1 or 2 – systolic blood pressure of at least 140 mm Hg or diastolic blood pressure of at least 90 mm Hg) or if the subject reported that, he/she was currently being treated for hypertension. Dietary information was obtained using a food frequency questionnaire. Level of physical activity during the previous year was estimated through self-reports. Anthropometric data were collected with participants wearing scrub suits. Diabetes mellitus was considered present if a subject was taking hypoglycemic agents, if a physician had told the subject that he/she had diabetes mellitus, or if fasting glucose was above 7 mmol/L. Individuals were defined as a case of CHD if there was a self-reported history of myocardial infarction, percutaneous transluminal coronary angioplasty, or coronary artery bypass graft. All variables used in these analyses were ascertained during the initial examination (1993-1995) except for CAC scores, which were obtained during a follow-up examination (2002-2003).
Statistical analyses
We initially conducted sex-specific analyses, but since we observed similar inverse association between chocolate consumption and CAC in men (p for trend 0.02) and women (p for trend 0.03), we present combined data. We initially examined the distribution of CAC cases according to all possible responses for chocolate consumption (Never, 1-3/month, 1/week, 2-4/week, 5-6/week, 1/d, 2-3/d, 4-6/d, and >6/d). However, there were not enough cases of CAC beyond consumption of 2-4 per week to have separate categories above this level of chocolate intake. Thus, we used 4 categories of chocolate consumption (none, 1-3 times per month, once per week, and 2+ times per week) and created 3 indicator variables using never consumption of chocolate as reference. We used univariate analyses to evaluate potential confounders and used partial likelihood ratio tests to compare multivariable regression models. The initial model only adjusted for age. Additional models controlled for 1) age, sex, smoking, and energy (6 categories); 2) age, sex, smoking, energy, waist-hip ratio, education (3 groups), ratio of total-to-HDL-cholesterol, alcohol consumption, non-chocolate candy consumption, and diabetes mellitus. The full model also controlled for field center, CHD family risk group, body mass index, hypertension, triglycerides, weight loss diet, and exercise. Additional adjustment for antioxidant vitamins (E, C, multivitamins), wine, and beer consumption did not alter the results (data not shown). Because subjects were not independent, we used generalized estimating equations to control for familial clustering (exploring different correlation matrix structures). P values for linear trend were obtained by creating a new variable that was assigned values of 0, 1, 2, and 3 to chocolate consumption of never, 1-3 per month, 1 per week, and 2+ per week, respectively, and including the new variable in the regression model. We conducted sensitivity analyses by a) restricting analyses to Caucasians, b) excluding subjects with CHD, and c) using different cut points to define CAC (0, 50, 100, 150, 200, and 300). Alpha level was set at 0.05 for statistical significance. All analyses were performed using windows SAS version 9.1 (SAS Institute Inc, Cary, NC).
Results
Of the total 2,217 subjects, 44% were men and the mean age was 50.7±13.0 years (range 25.6 to 85.7 years). Table 1 shows the baseline characteristics by categories of chocolate consumption. Chocolate consumption was associated with younger age, higher body mass index, higher energy intake, higher intake of dietary linolenic acid, cholesterol, and saturated fat, lower HDL, and lower consumption of fruit and vegetables and they consumed less wine and vitamin E. In addition, subjects reporting chocolate consumption were less likely to have hypertension or clinically diagnosed CHD. There was evidence for an inverse association between chocolate consumption and prevalent CAC. Compared to subjects reporting no chocolate intake, multivariable adjusted odds ratio (95% CI) for CAC of 100 or greater were 0.95 (0.66-1.36), 0.79 (0.54-1.15), and 0.69 (0.48-0.99) among subjects reporting chocolate consumption of 1-3 times per month, once per week, and 2 or more times per week, respectively (p for linear trend 0.029, Table 2), adjusting for age, sex, smoking, energy, waist-hip ratio, education, ratio of total-to-HDL cholesterol, alcohol intake, non-chocolate candy, diabetes mellitus, study center, family CHD risk group, body mass index, triglycerides, exercise, and current weight loss diet. Additional control for fruit and vegetables, antioxidant vitamins (C, E, multivitamins), or red wine consumption (as another source of polyphenols) did not alter the findings (data not shown).
Table 1.
Characteristics among 2,217 participants of the NHLBI Family Heart Study according to chocolate consumption
| TnQTable1Gender (%male) | 39.5 | 44.0 | 46.8 | 46.2 | 0.02 |
| Random sample (%) | 46.0 | 47.9 | 49.1 | 46.0 | 0.97 |
| African-Americans (%) | 0.7 | 0.6 | 0.4 | 0.1 | 0.12 |
| College education (%) | 60.5 | 65.1 | 67.3 | 66.3 | 0.05 |
| Hypertension (%) | 16.8 | 11.7 | 13.2 | 11.7 | 0.04 |
| Current drinkers (%) | 50.9 | 53.9 | 48.0 | 49.9 | 0.36 |
| Current wine consumption (%) | 16.6 | 13.1 | 12.2 | 9.8 | 0.0006 |
| Current beer consumption (%) | 17.0 | 19.7 | 18.9 | 17.2 | 0.78 |
| Current spirits consumption (%) | 16.8 | 17.0 | 14.5 | 13.8 | 0.08 |
| Current smoker (%) | 13.9 | 11.5 | 9.6 | 12.8 | 0.66 |
| Currently on weight loss diet (%) | 5.2 | 5.3 | 4.0 | 2.6 | 0.0009 |
| Current use of vitamin C (%) | 7.6 | 6.1 | 7.5 | 5.1 | 0.14 |
| Current use of vitamin E (%) | 13.7 | 12.3 | 8.8 | 8.5 | 0.0012 |
| Current use of multivitamins (%) | 25.8 | 23.4 | 21.3 | 24.0 | 0.50 |
| Non-chocolate candy consumption (%) | 32.5 | 68.6 | 80.8 | 82.3 | <0.0001 |
| Prevalent CHD (%) | 13.2 | 9.0 | 6.7 | 6.8 | 0.0002 |
P value for trend
Table 2.
Prevalence odds ratios (95% confidence intervals) of calcified atherosclerotic plaque in the coronary arteries according to chocolate consumption in 2,217 participants in the NHLBI Family Heart Study
| Frequency of chocolate intake | Cases/N | Age-adjusted | Model 1* | Model 2† | Model 3‡ |
|---|---|---|---|---|---|
| 0 | 190/446 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1-3 per month | 171/512 | 0.95 (0.70-1.29) | 0.92 (0.66-1.27) | 0.94 (0.66-1.35) | 0.95 (0.66-1.36) |
| 1 per week | 144/479 | 0.86 (0.62-1.18) | 0.79 (0.55-1.11) | 0.78 (0.53-1.13) | 0.79 (0.54-1.15) |
| 2+ per week | 203/780 | 0.74 (0.56-0.99) | 0.72 (0.53-0.98) | 0.68 (0.48-0.97) | 0.69 (0.48-0.99) |
| P for linear trend | 0.032 | 0.024 | 0.022 | 0.029 |
Adjusted for age, sex, current smoking, and energy intake (6 categories), using generalized estimating equations (GEE).
Adjusted as in model 1 plus additional adjustment for waist-to-hip ratio, education (3 groups), ratio of total-to-HDL cholesterol, alcohol consumption, non-chocolate candy (4 groups), and diabetes mellitus.
Adjusted as in model 2 plus additional adjustment for study center, CHD risk group (high vs. random), body mass index, hypertension, triglycerides, currently on weight loss diet (yes/no), and exercise.
Sensitivity analyses
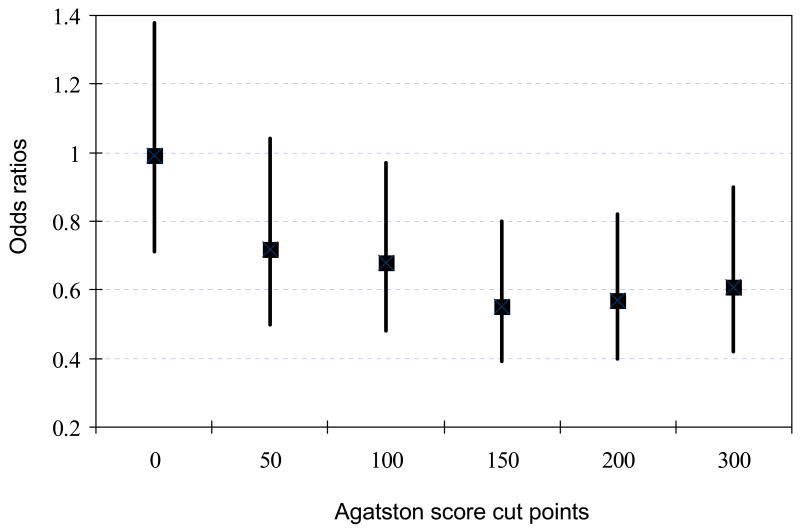
There was evidence for an inverse association between chocolate consumption and prevalent CAC when different cut points (50, 150, 200, and 300) were used to define the presence of CAC (Figure 1). However, no association was seen when using an Agatston score of 0 to define prevalent CAC (Figure 1). Inclusion of dietary cholesterol in the fully adjusted model did not attenuate the results [ORs: 1.0, 0.96 (0.67-1.37), 0.81 (0.55-1.17), and 0.68 (0.47-0.98) from the lowest to the highest category of chocolate intake, respectively (p for trend 0.023)]. When restricted to white participants, multivariable adjusted odds ratios were 1.0, 0.97 (0.68-1.39), 0.81 (0.55-1.18), and 0.70 (0.49-1.00) from the lowest to the highest category of chocolate consumption, respectively (p for linear trend 0.033). After exclusion of subjects with prevalent CHD (including prior myocardial infarction, angioplasty, or coronary bypass surgery), corresponding odds ratios were 1.0, 1.00 (0.68-1.47), 0.83 (0.54-1.27), and 0.72 (0.48-1.07), respectively, (p for trend 0.07) and after exclusion of subjects with CHD or diabetes mellitus corresponding odds ratios were 1.0, 0.99 (0.68-1.47), 0.78 (0.51-1.21), and 0.72 (0.48-1.08), respectively, (p for trend 0.07). However, using a cut point of 150 to define CAC yielded statistically significant results when subjects with prevalent CHD were excluded: multivariable odds ratios were 1.0, 0.71 (0.48-1.04), 0.63 (0.41-0.98), and 0.54 (0.36-0.82) from the lowest to the highest category of chocolate intake, respectively (p for trend 0.005).
Figure 1.
Odds ratios (95% CI) for CAC comparing chocolate consumption of 2+ times per week to none using different Agatston score cut points to define prevalent CAC (adjusting for age, sex, smoking, energy, waist-hip ratio, education, ratio of total-to-HDL cholesterol, alcohol intake, non-chocolate candy, diabetes mellitus, study center, CHD risk group, body mass index, triglycerides, exercise, and current weight loss diet).
Discussion
In this study, we demonstrated that chocolate consumption was inversely associated with prevalent CAC in a dose-response manner. Using other cut points to define prevalent CAC yielded similar results except when an Agatston score of 0 was used. In addition, exclusion of subjects with prevalent CHD or diabetes mellitus was suggestive of an inverse association between chocolate consumption and CAC. To our knowledge, this is the first study to examine whether chocolate consumption is associated with lower prevalent CAC as previous observational and interventional studies have focused on the effects of chocolate consumption on blood pressure, endothelial function, and platelet function. Using the same data, we identified an inverse association between chocolate consumption and prevalent CHD (manuscript under review). While health benefits from chocolate consumption might have been suspected as early as in the 17th century (mostly in Europe)23, chocolate is not viewed in the US as a healthy food, but rather as a source of fats and calories23. However, the literature suggests that moderate consumption of dark chocolate or other flavanoid-rich foods might have cardiovascular benefits12. In a cross-over design, consumption of 100 g/d of dark chocolate was associated with a 5.1 mm Hg reduction in systolic blood pressure and 1.8 mm Hg reduction in diastolic blood pressure in 13 hypertensive subjects after 14 days of intervention10. Another study also demonstrated beneficial effects of dark chocolate on blood pressure in healthy subjects after a 2-week intervention13. In the Iowa Women's Health Study24, chocolate contributed 6% of total catechins and when analyzed by catechin source, there was suggestive evidence for an inverse association between chocolate derived-catechin and CHD death [RR (95% CI): 0.88 (0.71-1.08)] in a multivariable adjusted model comparing the 3rd with the 1st tertile of catechin.
Potential physiologic mechanisms by which chocolate might lower the risk of CHD have been suggested. Besides lowering systolic and diastolic blood pressure10,13,25, dark chocolate has been shown to transiently but substantially increase nitric oxide bioactivity in human plasma and reverse endothelial dysfunction26-28. In a randomized trial of 41 diabetic subjects, a 30-day intervention with flavanol-rich cocoa resulted in a 30% increase in flow-mediated dilation of the brachial artery29. Furthermore, a prospective cohort reported a 40% lower risk of cardiovascular risk comparing the fourth to the first quartile of chocolate consumption and about 12% of that reduction was attributable to beneficial effects of chocolate on blood pressure30. In addition, dark chocolate was shown to improve insulin sensitivity and beta cell function in healthy or hypertensive subjects after 15 days of intervention13,25. Furthermore, there is evidence that chocolate might suppress epinephrine-stimulated platelet activation and platelet microparticle formation11. Other constituents of chocolate (magnesium and potassium) have been shown to exert beneficial effects on cell membrane and blood pressure. It is thus possible that flavonoids in chocolate, alone or in conjunction with other minerals, might favorably influence the development of atherosclresosis.
Calcium deposition in the arterial walls occurs in the early stages of atherosclerosis just after fatty streak formation31 and has been shown to correlate closely with the total burden of atheroma.1,2 In sensitivity analyses, we observed an inverse association between chocolate intake and CAC using Agatston scores other than 0 to define prevalent CAC. It is likely that with a lower Agatston score cut point such as 0, cardiac CT might not have been able to accurately discriminate the presence or absence of clinically relevant calcified atherosclerotic plaque in the coronary arteries. This might lead to effect dilution as observed in this paper. Studies have shown that the use of an Agatston score of 0 as a cut point to predict angiographic disease (at least 50% stenosis) had a relatively low specificity (up to 40%).3,32 In contrast, using a cut point of 100 had a sensitivity of 93% and a specificity of 76%.3
Our study has limitations. First, although CAC measurement was completed about 7 years after dietary assessment, we did not have baseline CAC measurement to differentiate calcification that was present at baseline from calcification that developed after assessment of chocolate consumption. Thus, our ability to determine temporality or infer causality is limited. Second, we were not able to differentiate between dark chocolate and lighter or milk chocolate. Polyphenolic content is higher in dark chocolate than it is in milk chocolate. Therefore, combining lighter and dark chocolate might have biased our results towards the null. Lastly, the possibility of confounding by indication (subjects with prevalent CAC or clinical CHD might have avoided chocolate consumption as bad foods) can not completely be excluded in this study. However, the fact that exclusion of subjects with prevalent CHD showed a similar inverse association (p for trend 0.07 when CAC of at least 100 was used as cut point and 0.005 when CAC of at least 150 was used as cut point) does not support confounding by indication as these subjects were free of symptomatic CHD. On the other hand, the large sample size, the availability of data on major CHD risk factors, and the standardized techniques used for both CAC and dietary assessment were major strengths of the study.
In conclusion, our findings indicate that consumption of chocolate might be inversely associated with prevalent CAC in a dose-response manner. Future studies are warranted to confirm these findings and elucidate physiologic mechanisms by which moderate consumption of chocolate (up to 2 servings per week) might positively influence the risk of atherosclerosis.
Acknowledgments
Appreciation is expressed to the staff of the study and especially to the study participants who volunteered for the project. The study was carried out at the following institutions: University of North Carolina; Wake Forest University; University of Alabama at Birmingham; University of Minnesota; Washington University of St. Louis; Boston University; University of Texas, Houston; University of Utah; National Heart, Lung, and Blood Institute.
Funding: This study was supported by grants from the National Heart, Lung, & Blood Institute (U01 HL56563, U01 HL56564, U01 HL56565, U01 HL56566, U01 HL56567, U01 HL56568, U01 HL56569, and K01-HL70444).
Footnotes
Author contribution: Study conception (Djoussé); data collection (Arnett, Pankow, Borecki, North, Ellison); statistical analyses (Djoussé); drafting the manuscript (Djoussé); Critical review for intellectual content (Djoussé, Arnett, Pankow, Borecki, North, Ellison); obtaining funding (Djoussé, Arnett, and Ellison); supervision of the study (Ellison). All authors read and approved the final manuscript.
Conflict of interest: None to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 2.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 3.Haberl R, Becker A, Leber A, Knez A, Becker C, Lang C, et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: results of 1,764 patients. J Am Coll Cardiol. 2001;37:451–457. doi: 10.1016/s0735-1097(00)01119-0. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 5.Park R, Detrano R, Xiang M, Fu P, Ibrahim Y, LaBree L, et al. Combined use of computed tomography coronary calcium scores and C-reactive protein levels in predicting cardiovascular events in nondiabetic individuals. Circulation. 2002;106:2073–2077. doi: 10.1161/01.cir.0000033819.29662.09. [DOI] [PubMed] [Google Scholar]
- 6.Raggi P, Shaw LJ, Berman DS, Callister TQ. Gender-based differences in the prognostic value of coronary calcification. J Womens Health (Larchmt) 2004;13:273–283. doi: 10.1089/154099904323016437. [DOI] [PubMed] [Google Scholar]
- 7.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 8.Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Archives of Internal Medicine. 1995;155:381–386. published erratum appears in Arch Intern Med 1995 Jun 12;155(11):1184. [PubMed] [Google Scholar]
- 9.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156:637–642. [PubMed] [Google Scholar]
- 10.Taubert D, Berkels R, Roesen R, Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA. 2003;290:1029–1030. doi: 10.1001/jama.290.8.1029. [DOI] [PubMed] [Google Scholar]
- 11.Rein D, Paglieroni TG, Wun T, Pearson DA, Schmitz HH, Gosselin R, et al. Cocoa inhibits platelet activation and function. Am J Clin Nutr. 2000;72:30–35. doi: 10.1093/ajcn/72.1.30. [DOI] [PubMed] [Google Scholar]
- 12.Grassi D, Desideri G, Croce G, Tiberti S, Aggio A, Ferri C. Flavonoids, vascular function and cardiovascular protection. Curr Pharm Des. 2009;15:1072–1084. doi: 10.2174/138161209787846982. [DOI] [PubMed] [Google Scholar]
- 13.Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 14.Higgins M, Province M, Heiss G, Eckfeldt J, Ellison RC, Folsom AR, et al. NHLBI Family Heart Study: objectives and design. Am J Epidemiol. 1996;143:1219–1228. doi: 10.1093/oxfordjournals.aje.a008709. [DOI] [PubMed] [Google Scholar]
- 15.Djousse L, Arnett DK, Carr JJ, Eckfeldt JH, Hopkins PN, Province MA, et al. Dietary linolenic acid is inversely associated with calcified atherosclerotic plaque in the coronary arteries: the NHLBI Family Heart Study. Circulation. 2005;111:2921–2926. doi: 10.1161/CIRCULATIONAHA.104.489534. [DOI] [PubMed] [Google Scholar]
- 16.Hunt SC, Williams RR, Barlow GK. A comparison of positive family history definitions for defining risk of future disease. J Chronic Dis. 1986;39:809–821. doi: 10.1016/0021-9681(86)90083-4. [DOI] [PubMed] [Google Scholar]
- 17.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 19.Stein AD, Shea S, Basch CE, Contento IR, Zybert P. Consistency of the Willett semiquantitative food frequency questionnaire and 24-hour dietary recalls in estimating nutrient intakes of preschool children. Am J Epidemiol. 1992;135:667–677. doi: 10.1093/oxfordjournals.aje.a116346. [DOI] [PubMed] [Google Scholar]
- 20.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann U, Brady TJ, Muller J. Cardiology patient page. Use of new imaging techniques to screen for coronary artery disease. Circulation. 2003;108:e50–e53. doi: 10.1161/01.CIR.0000085363.88377.F2. [DOI] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Steinberg FM, Bearden MM, Keen CL. Cocoa and chocolate flavonoids: implications for cardiovascular health. J Am Diet Assoc. 2003;103:215–223. doi: 10.1053/jada.2003.50028. [DOI] [PubMed] [Google Scholar]
- 24.Arts IC, Jacobs DR, Jr, Harnack LJ, Gross M, Folsom AR. Dietary catechins in relation to coronary heart disease death among postmenopausal women. Epidemiology. 2001;12:668–675. doi: 10.1097/00001648-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, et al. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008;138:1671–1676. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 26.Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H, Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA. 2003;290:1030–1031. doi: 10.1001/jama.290.8.1030. [DOI] [PubMed] [Google Scholar]
- 27.Engler MB, Engler MM, Chen CY, Malloy MJ, Browne A, Chiu EY, et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 28.Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens. 2003;21:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Balzer J, Rassaf T, Heiss C, Kleinbongard P, Lauer T, Merx M, et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J Am Coll Cardiol. 2008;51:2141–2149. doi: 10.1016/j.jacc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 30.Buijsse B, Weikert C, Drogan D, Bergmann M, Boeing H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur Heart J. 2010 doi: 10.1093/eurheartj/ehq068. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Wexler L, Brundage B, Crouse J, Detrano R, Fuster V, Maddahi J, et al. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation. 1996;94:1175–1192. doi: 10.1161/01.cir.94.5.1175. [DOI] [PubMed] [Google Scholar]
- 32.Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis. 2003;167:237–242. doi: 10.1016/s0021-9150(02)00427-6. [DOI] [PubMed] [Google Scholar]