Abstract
Adaptive phenotypic plasticity is particularly important to organisms with developmental cycles that undergo ontogenetic niche shifts that differentially subject individual life stages to heterogeneous and often stressful environmental conditions. The yellow fever and dengue fever vector mosquito, Aedes aegypti, typically breeds in small water-filled containers that expose the developing aquatic larvae to competition for resources with conspecifics and high probabilities for habitat drying. Here we investigated the heritability (h2) and phenotypic plasticity among Aedes aegypti laboratory populations and field populations from Trinidad, West Indies. Heritability for body size was moderate or completely eroded among the laboratory populations, while field populations contained high genetic variation among both males and females. Norms of reactions based on optimum vs. deficient larval conditions for artificial sibling families representing Trinidad field populations suggested significant gene × environment interactions influence body size and that there may be sex specific differences in allocation of resources. Individuals reared under optimum laboratory conditions were significantly larger and showed much less variability in body size plasticity than their field reared cohorts, suggesting that exposure to environmental stress may be common for Aedes aegypti larval development and would undoubtedly impact other traits, including arbovirus vector competence among adult females, in a similar fashion. Broad genetic variance in body size and other characters is likely maintained by balancing selection. Our results also suggest the need for caution in translating conclusions from experiments with laboratory colonies to natural populations. These would likely be more informative to expected phenotypes under natural conditions if conducted over a range of conditions that simulate environmental stress.
Keywords: phenotypic plasticity, genetic variance, heritability, norms of reaction, balancing selection, vector competence
1. Introduction
Organisms with complex life cycles, wherein individuals progress through multiple life stages that often reflect ontogenetic niche shifts, are subject to stage-specific selective forces. Phenotypic plasticity, the ability of a single genotype to produce more than one alternative form in response to environmental conditions, plays an important role in promoting and maintaining phenotypic diversity across heterogeneous environments (West-Eberhard, 1989; Scheiner, 1993). This allows organisms to exist across a wide range of environments but may limit adaptive selection in unpredictable habitats and yet permit divergence under environmental stability that persists over an evolutionary time scale (Via and Lande, 1985; Gotthard and Nylin, 1995; Fordyce, 2006). Further, recent studies suggest that natural selection may have a greater impact on phenotypic change under suboptimal conditions (Sangster et al., 2008a,b). Phenotypic plasticity can viewed as adaptive when a particular character state derived from that plasticity confers higher fitness under the prevailing environmental conditions in the context of a heterogeneous environment (Newman, 1992). Developmental plasticity is well-documented among invertebrate larvae (Nylin and Gotthard, 1998; Teuschl et al., 2007; Colinet et al., 2007; Kasumovic et al., 2009). Species with an aquatic larval period are of particular interest as they are subject to competition for resources and risk of habitat desiccation and, therefore, plasticity in developmental time can be highly advantageous. The ability of an individual to monitor its environment and correspondingly adjust developmental time can also result in changes in other life history traits, as selection generally does not act on single traits. For example, adult body size is often strongly correlated with multiple life history traits including fecundity (see Schluter et al., 1991; Honěk, 1993). Thus, natural selection may impose fitness constraints that might be advantageous at one life stage, but have possible negative implications at other life stages.
Aedes aegypti, the major global vector for yellow fever and dengue fever viruses, has evolved to maintain a very close association with humans. Dengue fever (DF) is an expanding global health threat with 2.5 billion people at risk and 50-100 million people infected per year that include 500,000 cases of potentially lethal dengue hemorrhagic fever or dengue shock syndrome. Efforts to limit or prevent DF are restricted by the lack of effective vaccines or drugs for disease treatment (Morens and Fauci, 2008). DF prevention has historically targeted controlling the mosquito, yet emergence of insecticide resistance and reductions in surveillance often result in epidemic outbreaks. Female mosquitoes readily oviposit in virtually any water-filled container or cavity and, therefore, development to adulthood often occurs under stress conditions that include a high probability for intra-specific competition and occasional inter-specific competition for nutritional resources, as well as habitat drying. Ae. aegypti and other container breeding mosquitoes, where larvae are subject to such habitat unpredictability, exhibit broad phenotypic variability in adult body size (Nasci, 1986; Chadee, 1993; Xue et al., 1995; Chadee and Beier, 1997; Yan et al., 1997; Schneider et al., 2004, 2007) and other life history traits including, vector competence to transmit DF to humans (Gubler et al., 1979; Bennett et al., 2002; Schneider et al., 2007).
While the effects of environmental conditions (e.g., nutrients, temperature, crowding) on phenotypic plasticity in Ae aegypti body size are well-documented, the role of genetic variability in body size has received little attention. Critical to our understanding of such traits is the need to partition them into heritable versus environmental influences. Determining heritability (h2) estimates for a number of populations under a given set of environmental parameters will provide insight into the ecological and evolutionary importance of a trait. Variation in Ae. aegypti body size likely has significant sex-specific effects on fitness and, consequently, is under considerable pressure by natural selection (Bedhomme et al., 2003). Of note, while most studies with Ae. aegypti are conducted with laboratory populations, it is unknown if additive genetic variation is eroded during and following colonization or how these populations compare to natural populations. If there are significant differences in heritability estimates for body size, it is important to understand that variation in order to explain past and future responses to natural selection. Related to heritability is the norm of reaction, which provides an indication of genotype × environment interactions, and indicates the plasticity of a phenotypic response under a range of environmental conditions. In this study, we investigated the heritability of body size among two Ae. aegypti laboratory populations and a natural population from Trinidad, West Indies. In addition, we compared norms of reaction for body size among artificial families generated from the Trinidad population.
2. Materials and methods
2.1. Mosquito rearing
Rearing and adult maintenance were conducted in an environmental chamber held at 26°C, 85% relative humidity, with 16-h light/8-h dark cycles that included a 30 min crepuscular period at the beginning and end of each cycle. Similar numbers of eggs were hatched from each population (estimated number hatched per pan was approximately 300 to 500) in tepid water with a pinch of dry yeast. Larvae were reared in large pans filled with 2 L tepid water with an ad libitum solution of bovine liver powder (ICN Biomedicals, Inc., Costa Mesa, CA, USA). This ensured that the developing mosquitoes had ample nutritional resources and room for growth. Thus, the effects of environmental crowding or nutritional deprivation were adequately minimized. Pupae were transferred to 500 mL plastic cups with ~250 mL clean tepid water. Adults emerged into 20×20×30 cm mesh cages. To obtain virgin females, males and females were separated into individual 20×20×30 cm mesh cages at least every 24 hours. All adults were provided 5% sucrose solution ad libitum.
2.2. Determination of adult body size
Wing length used as a proxy for body size was determined as previously described (Schneider et al., 2007). Wings were measured with an ocular micrometer from the apical notch to the axillary margin, excluding the wing fringe. To minimize measurement errors, all wing lengths for heritability estimates, norms of reaction, and field vs laboratory rearing studies were determined by a single researcher, respectively.
2.3. Heritability estimates
2.3.1. MOYO-S and Ghana laboratory populations
MOYO-S was originally selected for susceptibility to the avian malarial parasite Plasmodium gallinaceum from the Moyo-In-Dry population originating from Mombasa, Kenya in 1974. Selection procedures for the MOYO-S population are described elsewhere (Thathy et al., 1994; Chen et al., 2004). After selection for P. gallinaceum susceptibility, large population sizes have been used to maintain random mating colonies. The MOYO-S population (including the progenitor Moyo-In-Dry) has been maintained in the laboratory for an unknown number of generations, but likely >50. The Ghana population was obtained from a field population collected from Ghana, West Africa in 2001, has not undergone any directed selection other than adaptation to laboratory colonization, and has been maintained in the laboratory for ~18 generations. More detailed information on strain origins and DENV susceptibility are provided in Schneider et al. (2007). Each laboratory population was reared and maintained under our standard conditions as previously described.
2.3.2. Trinidad field population
Our field population was derived from eggs collected from >30 ovitraps set across sites in Trinidad during March 2004, using standard ovitraps that yield ~35 eggs per positive trap (Chadee et al., 1995). Eggs from the oviposition papers were hatched and reared to adults in mass. Individual males and females were then randomly selected for establishing crosses for heritability estimates. Because rearing of the field-collected eggs occurred in the laboratory, both the P0 and the F1 generations experienced reduced environmental variability, but are likely still representative of the genetic diversity within the natural field population as this also represents a limited opportunity for selection to have occurred.
2.3.3. Half-sib crossing design
Half-sib crosses were designed to provide estimates of narrow-sense heritability (h2) as described by Lynch and Walsh (1998). For each half-sib family, one male was placed into a 500 mL paper cage with at least five virgin females. On day 3 following the establishment of the cross, females were offered a blood meal from an anesthetized rat. Our protocol for maintenance and care of experimental animals was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Notre Dame. Animals were maintained and cared for in the Freimann Life Science Center, an AAALAC accredited facility. On day 5 following the establishment of the cross, females were moved to individual oviposition cages and allowed to oviposit. Oviposition cages consisted of 5 mL scintillation vials, an added strip of textured paper towel for an oviposition substrate, and 1 mL tepid tap water. Males were frozen at - 80°C after females were removed. The females were frozen at -80°C once oviposition was completed. Following oviposition, the eggs were collected and dried for 3 days in the environmental facility at 26°C and ~85% relative humidity. Eggs were hatched and reared as described previously. Three days post-emergence, all individuals were frozen and stored at -80°C.
2.3.4. Statistical analysis
Heritability (h2) estimates were calculated with the VARCOMP procedure implemented in SAS 9.2 (SAS, Cary, NC, USA) for males and females separately.
2.4. Norms of reaction
2.4.1. Trinidad field population
Eggs were collected using ovitraps as previously described from sites across Trinidad during March, 2002. First instar larvae were separated into two feeding groups: 1) maintained under optimal rearing conditions, and 2) maintained under severely suboptimal conditions (nutrient deficiency). Larvae were reared in 500 mL water. Individuals in the “optimum” group were provided 0.175 g bovine liver powder (ICN Biomedicals, Inc., Costa Mesa, CA, USA) every three days until pupation. The “nutrient deficient” group was provided 0.003 g bovine liver powder every three days until pupation. Any dead larvae and/or exuviae were removed from the water every three days as well to prevent the addition of unregulated food into the system. Pupae were removed and placed into ~250 mL clean water for emergence. Adults were collected upon eclosion and stored at -80°C.
2.4.2. DNA extraction and genotyping
For norms of reaction populations, 10 single nucleotide polymorphism (SNP) markers distributed across the genome, and pre-screened for polymorphism in Trinidad natural populations, were used to genotype individuals (Supplementary Table 1). DNA extractions were performed on individual mosquitoes using our standard protocol (Severson 1997). DNA was resuspended in 50.0 μl TE and aliquots further diluted 50-fold for PCR amplification. PCR amplifications were performed on individual mosquitoes in 25 μl reactions containing 1X Taq buffer (10 mM KCl, 2 mM Tris, pH 9.0, 0.02% Triton X), 1.5 mM MgCl2, 0.4 mM each of dATP, dCTP, dGTP and dTTP, 5 pmoles of each primer, 1 unit of Taq DNA polymerase, and 5.0 μl of the diluted DNA template. PCR conditions were 94°C for five minutes, followed by 30 cycles at 94°C for one minute, a primer-specific annealing temperature for one minute, 72°C for two minutes, and a final extension at 72°C for 10 minutes. For scoring, individuals were amplified with individual SNP markers, digested with the diagnostic restriction enzyme following manufacture’s recommendations, size-fractionated on 3% agarose gels, stained with ethidium bromide and visualized under UV light.
2.4.3. Statistical analysis
To establish artificial sibling families among individuals reared from field collected eggs, genotypically similar individuals were identified using Delrious software (Stone and Björklund, 2001). This program calculates relatedness values (r) between each pair of mosquitoes using an algorithm described by Lynch and Ritland (1999) to obtain unbiased relatedness estimates with reduced sampling variances. The r-values were clustered as families using UPGMA cluster analysis with cluster cutoffs defined relative to reference full-sibling and half-sibling families constructed in the lab (Colton et al., 2003). The relative magnitudes of size differences between nutrient optimum and deficient individuals within families were analyzed using Student’s t-test (unpaired, two tailed, with equal variance).
2.5. Field vs. laboratory rearing populations
Samples were collected at two communities, Windy Hills and Bamboo, in Trinidad in March 2009. At each site, pupae were collected from natural breeding sites and transported to the laboratory and maintained until adult eclosion. In addition, eggs were collected across the same sites using ovitraps as previously described. Larvae were reared in the laboratory under optimal rearing conditions as previously described and maintained until adult eclosion.
Student’s t-test (unpaired, one tailed, with unequal variance) was used to test for body size differences among field vs. laboratory reared females and males, respectively.
3. Results
3.1. Heritability of body size
Three Ae. aegypti populations were reared under optimum environmental conditions to obtain h2 estimates. These included two long-term laboratory populations (MOYO-S and Ghana) and a field population from Trinidad collected as eggs. Variance components used for heritability (h2) estimates are listed in Supplementary Table 2. In all cases, the variance component due to dams within sires was much larger than the variance component due to sires, which suggests that common environment or dominance effects influence wing length. Given this result, we used only the sire variance component to estimate h2. These analyses demonstrated that body size among Ae. aegypti populations reflects significant additive genetic variation (Figure 1). These estimates ranged from a low of 0.00 for the highly selected MOYO-S laboratory strain, to ~0.3 for the unselected Ghana laboratory strain, to ~0.5-0.6 for females and males for the Trinidad field population. There were no consistent gender differences, indicating that h2 for body size appears to be independent of sex in Ae. aegypti.
Figure 1.
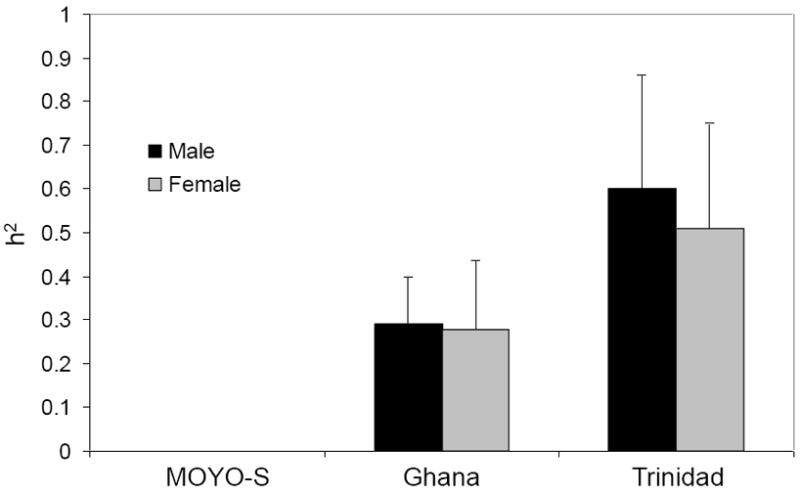
Heritability (h2 and standard error) of wing length as a proxy for body size among Aedes aegypti populations. MOYO-S, highly selected laboratory population; Ghana, unselected but long-time laboratory population; Trinidad, field population collected as eggs.
3.2. Norms of reaction for body size
To better understand how genotypic and environmental effects intersect to regulate phenotypic plasticity in adult Ae. aegypti body size, we investigated norms of reaction for various genotypes reared under different environmental conditions. Reaction norms were developed for body size among a total of 185 field derived mosquitoes: 104 females and 81 males. The mean wing lengths for both males and females under nutritionally optimum and deficient rearing conditions were normally distributed. Cluster analysis among all individuals grouped these genetically into 11 and 13 artificial sibling families for females and males, respectively, based on relatedness distributions observed for full-sibling and half-sibling families described elsewhere (Colton et al., 2003), with a range of 6 to 19 individuals per family. We performed a two-way ANOVA to determine the presence of significant differences among families (genotype), nutrition (environment), and their interaction (as described in Fuller et al, 2005). Results indicated that both genotype and environment were significant (P<0.001) for males and females, but the interaction term was only significant for females (P=0.01). Linear norms of reaction were constructed using the mean response of each family for the two different treatments as the data points. The reaction norms for individual families varied among females (Figure 2a) and males (Figure 2b), suggesting genotype differences in the response to environmental effects that impart wide phenotypic plasticity at the population level. In addition, the relative magnitude of the size differences observed between the optimum and deficient individuals within families was greater for females than for males (although the differences were only suggestive, P=0.075), implying a potential for differences in the allocation of resources between males and females in responding to environmental uncertainty.
Figure 2.
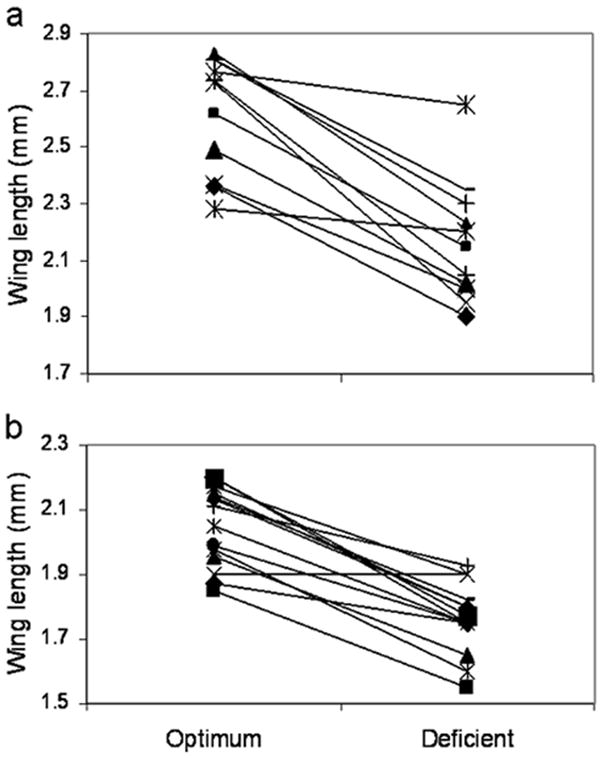
Norms of reaction for wing length as a proxy for body size among artificial sibling families identified from field-collected eggs from Trinidad and reared under nutrient optimum vs. deficient conditions. (a) Females. (b) Males.
3.3. Effects of field vs. laboratory rearing on body size
We compared body size among field vs. laboratory reared individuals collected at two communities in Trinidad: Bamboo and Windy Hill (Figure 3). Mean wing lengths were significantly different (P<0.0001) for both male and female comparisons at each site. For Bamboo, laboratory reared males and females were 9.5% and 20.2% larger than field reared individuals, respectively. For Windy Hill, laboratory reared males and females were 9.7% and 15.4% larger than field reared individuals, respectively. Further, the observed variability in body size was much greater among field reared individuals. For Bamboo, the standard deviations for body size among field reared males and females were 2.3X and 3.2X greater than among laboratory reared individuals, respectively. For Windy Hill, the standard deviations for body size among field reared males and females were 1.8X and 2.5X greater than among laboratory reared individuals, respectively.
Figure 3.
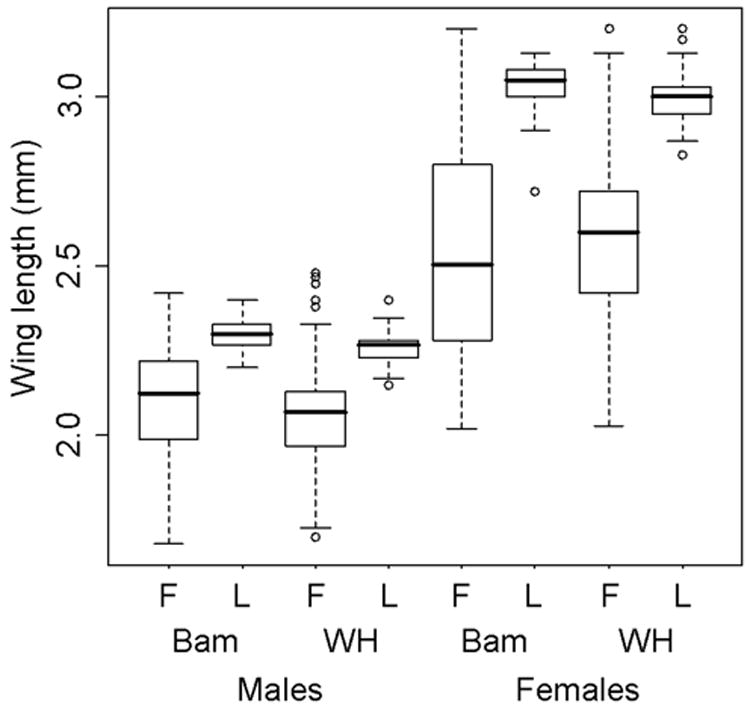
Comparisons of wing length as a proxy for body size among field (F) vs. laboratory (L) reared adult Aedes aegypti populations collected in Bamboo (Bam) and Windy Hills (WH), Trinidad. Box plots identify medians and quartiles, with whiskers representing the 10th and 90th percentiles. Open circles indicate outliers.
4. Discussion
Here we investigated the genetic and environmental constraints on body size in the dengue and yellow fever vector mosquito, Ae. aegypti among long-time laboratory populations as well as field populations from Trinidad. Understanding the quantitative genetics of adult body size, potential gene × environment interactions, and the role of adaptive phenotypic plasticity on selection for body size and other life history traits is key to fully elucidating the relationship between fitness characters, including body size and adult female competence to vector arboviruses.
Body size is recognized as a fitness trait and likely be subject to significant pressure by natural selection, which should rapidly deplete genetic variation. We identified limited additive genetic variation (h2) remaining in the highly selected MOYO-S laboratory population. This population was rigorously selected for high susceptibility to the avian malaria parasite, P. gallinaceum (Thathy et al., 1994; Chen et al., 2004), but in the process also apparently inadvertently selected for maximum body size (Yan et al., 1997; Schneider et al., 2007). Thus the combination of effects due to laboratory colonization and stringent artificial selection undoubtedly led to the complete erosion of genetic body size variability in this population. The long-term but unselected Ghana laboratory population still showed moderate additive genetic variation (h2 = ~0.3 for both males and females), while the Trinidad field population contained high genetic variation (h2 ranged from 0.51-0.6 for females and males, respectively). Laboratory populations undoubtedly retain reduced genetic variation for most characters because they are consistently reared under optimum insectary conditions. Therefore, both larvae and adults are not subjected to the negative selection effects of environmental variation or competition. Although impossible to determine in retrospect, it seems likely the Ghana population also experienced some loss of genetic body size variability in concert with laboratory colonization, perhaps even up to 50% if the Trinidad population is typical of Ae. aegypti populations globally. Conversely, h2 estimates for the Trinidad field population are likely near the maximum for Ae. aegypti. That is, because natural populations are subject to highly variable environmental conditions (further enhanced by ontogenetic niche shifts), broad genetic variance in body size is likely maintained by balancing selection, wherein larvae maximize their growth potential relative to environmental conditions as opposed to adults whose fitness including fecundity is strongly impacted by body size. From previous collections and genetic analyses, we know that Ae. aegypti populations from Trinidad (Yan et al., 1998) are highly heterogeneous.
Our simplified norms of reaction for Ae. aegypti field populations from Trinidad also provide insight into the development of body size under different potential environmental conditions, and provide further demonstration of the broad genetic continuum in body size among the Trinidad populations. Of note, we observed a significant gene × environment interaction effect on body size only in females. Although additional studies are needed, the observed differences in relative magnitude for body size among individuals provided optimum vs. deficient rearing conditions are therefore suggestive that there may be sex specific differences in the allocation of resources as has been observed for other organisms (Stillwell et al., 2010). Laboratory rearing and prolonged exposure to consistent and optimum environmental conditions may select for larger individuals with higher fitness, including, for example, individuals with higher mating success, greater fecundity, and longer survivorship (Blackmore and Lord, 2000). A high quality laboratory environment in which mosquitoes are reared may also allow for the gradual shift of resources away from traits needed in the field such as immunity and/or different feeding tactics, and more toward growth, development, and reproduction (Koella and Boëte, 2002).
Adaptive phenotypic plasticity is likely fundamental to mosquito evolutionary success, and especially among species that breed in small containers often with limited nutrient resources and transient water availability. Periodic environmental stress has potential to influence evolutionary rates by maintaining or even increasing genetic variation and thus overcoming adaptation limits that can effect changes in trait distributions within populations (Hoffmann and Hercus, 2000; Badyaev, 2005). The field reared populations may be significantly smaller than the laboratory reared populations, but retain high variability in the trait, which allows populations to adapt to dynamic field conditions. That is, sub-optimal environmental conditions and competition may result in selection for smaller body size, yet alleles for larger body size would still be maintained in populations by periodic exposure to optimal conditions often associated with seasonal effects (e.g., wet vs. dry seasons).
Our comparisons of field reared vs. laboratory reared progeny from two independent communities in Trinidad clearly reflect the extreme phenotypic plasticity in Ae. aegypti body size among natural populations. Individuals reared under optimum laboratory conditions were significantly larger and showed much less variability in body size than their cohorts reared under field conditions. This suggests that exposure to environmental stress may be common for Ae. aegypti larval development and would undoubtedly impact other traits in a similar fashion. Mosquitoes and other organisms that exist in highly unpredictable environments must be capable of detecting environmental cues and adjusting their developmental patterns appropriately (Aubin-Horth and Renn, 2009). Previous studies suggested that mosquito larvae actively monitor container water volume and are able to accelerate development in response to habitat deterioration (Juliano and Stoffregen, 1994; Schäfer and Lundström, 2006).
The genetic and physiological intersection between plasticity for body size and arbovirus vector competence in Ae. aegypti remains unclear as some studies have shown increased vector competence with large body size while others implicated smaller body size with vector competence; this inconsistency applies to other life history traits as well (see Morales-Vargas et al., 2010). Small mosquitoes are known to take smaller and multiple blood meals during a gonotropic cycle, and to even probe more often during a blood meal, thus increasing the likelihood for dengue transmission (Xue et al., 1995; Schneider et al., 2004).
Adaptive selection during colonization, accompanied by genetic drift associated with small effective population sizes, has been shown to result in reduced polymorphism and apparently inadvertent selection for diverse phenotypes among various mosquito species. Recently derived field populations of Culex pipiens reflected significant changes in vector competence and mating behavior (Gargan et al., 1983). Lima et al. (2004) reported significant effects of colonization in the malaria vector, Anopheles albitarsis, wherein males exhibited significantly increased rates of insemination compared to feral males after ~120 generations in the laboratory. Further, Lorenz et al. (1984) found significant changes in both the genetics and the susceptibility of Ae. aegypti to yellow fever virus following colonization. These studies and our present results also emphasize the need for caution in translating conclusions from experiments with laboratory colonies to natural populations. Laboratory experiments would likely be more informative to expected phenotypes under natural conditions if conducted over a range of conditions that simulate environmental stress.
The maintenance of extreme phenotype and genotype plasticity in body size among Ae. aegypti populations suggests that they are readily able to respond and adapt to the ever changing extremes in environmental conditions. Of note, there is evidence for seasonal variability in vector competence for DENV in Ae. aegypti in Cambodia, wherein DENV susceptibility was significantly lower during the dry season (Paupy et al., 2003). In addition, Morales Vargas et al. (2010) reported evidence for seasonal variability in Ae. aegypti body size in Thailand as well, where adults were significantly larger during November and February compared those in June and August, and body size showed a significant negative correlation with seasonal relative humidity; of note, these periods generally correspond to the wet vs. dry seasons, respectively, in Thailand. The rainy season in Trinidad and other tropical habitats creates significantly more breeding sites and theoretically more high quality sites as well. This may result not only in increased overall abundance, but also larger body size as well as influencing other life history traits, including vector competence for DENV.
Supplementary Material
Acknowledgments
This research was funded by grant AI059342 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, U.S.A.
Footnotes
Appendix A. Supplementary data Supplementary Information is available at the IGE website
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aubin-Horth N, Renn SC. Genomic reaction norms: using integrative biology to understand molecular mechanisms of phenotypic plasticity. Mol Ecol. 2009;18:3763–3780. doi: 10.1111/j.1365-294X.2009.04313.x. [DOI] [PubMed] [Google Scholar]
- Bedhomme S, Agnew P, Sidobre C, Michalakis Y. Sex-specific reaction norms to intraspecific larval competition in the mosquito Aedes aegypti. J Evol Biol. 2003;16:721–730. doi: 10.1046/j.1420-9101.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- Bennett KE, Olson KE, Muñoz-deLourdes M, Fernandez-Salas I, Farfan-Ale JA, Higgs S, Black WC, IV, Beaty BJ. Variation in vector competence for dengue-2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am J Trop Med Hyg. 2002;67:85–92. doi: 10.4269/ajtmh.2002.67.85. [DOI] [PubMed] [Google Scholar]
- Blackmore MS, Lord CC. The relationship between size and fecundity in Aedes albopictus. J Vector Ecol. 2000;25:212–217. [PubMed] [Google Scholar]
- Chadee DD. Size of emerging and host-seeking Aedes aegypti and the relationship to containers and blood-feeding success in Trinidad, West Indies. Bull Soc Vector Ecol. 1993;18:105–108. [Google Scholar]
- Chadee DD, Beier JC. Factors influencing the duration of blood-feeding by laboratory-reared and wild Aedes aegypti (Diptera: Culicidae) from Trinidad, West Indies. Ann Trop Med Parasitol. 1997;91:199–207. doi: 10.1080/00034983.1997.11813130. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Corbet PS, Talbot H. Proportions of eggs laid by Aedes aegypti on different substrates within an ovitrap in Trinidad, West Indies. Med Vet Entomol. 1995;9:66–70. doi: 10.1111/j.1365-2915.1995.tb00118.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang J, Liang P, Karsay-Klein M, James AA, Brazeau D, Yan G. Microarray analysis for identification of Plasmodium-refractoriness candidate genes in mosquitoes. Genome. 2004;47:1061–1070. doi: 10.1139/g04-056. [DOI] [PubMed] [Google Scholar]
- Colinet H, Boivin G, Hance T. Manipulation of parasitoid size using the temperature-size rule: fitness consequences. Oecologia. 2007;152:425–433. doi: 10.1007/s00442-007-0674-6. [DOI] [PubMed] [Google Scholar]
- Colton YM, Chadee DD, Severson DW. Natural skip oviposition of the mosquito Aedes aegypti indicated by codominant genetic markers. Med Vet Entomol. 2003;17:195–204. doi: 10.1046/j.1365-2915.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- Fordyce JA. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. J Exp Biol. 2006;209:2377–2383. doi: 10.1242/jeb.02271. [DOI] [PubMed] [Google Scholar]
- Fuller T, Sarkar S, Crews D. The use of norms of reaction to analyze genotypic and environmental influences on behavior in mice and rats. Neurosci Biobehav Rev. 2005;29:445–456. doi: 10.1016/j.neubiorev.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Gargan TP, Bailey CL, Higbee GA, Gad A, El Said S. The effect of laboratory colonization on the vector-pathogen interactions of Egyptian Culex pipiens and Rift Valley fever virus. Am J Trop Med Hyg. 1983;32:1154–1163. doi: 10.4269/ajtmh.1983.32.1154. [DOI] [PubMed] [Google Scholar]
- Gotthard K, Nylin S. Adaptive plasticity and plasticity as an adaptation; a selective review of plasticity in animal morphology and life history. Oikos. 1995;74:3–17. [Google Scholar]
- Gubler DJ, Nalim S, Tan R, Saipan H, Sarosos JS. Variation in susceptibility to oral infection with dengue viruses among geographic strains of Aedes aegypti. Am J Trop Med Hyg. 1979;28:1045–1052. doi: 10.4269/ajtmh.1979.28.1045. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Hercus MJ. Environmental stress as an evolutionary force. BioSci. 2000;50:217–226. [Google Scholar]
- Honěk A. Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos. 1993;66:483–492. [Google Scholar]
- Juliano SA, Stoffregen TL. Effects of habitat drying on size at and time to metamorphosis in the tree hole mosquito Aedes triseriatus. Oecologia. 1994;97:369–376. doi: 10.1007/BF00317327. [DOI] [PubMed] [Google Scholar]
- Kasumovic MM, Bruce MJ, Herberstein ME, Andrade CB. Evidence for developmental plasticity in response to demographic variation in nature. Ecology. 2009;90:2287–2296. doi: 10.1890/08-1540.1. [DOI] [PubMed] [Google Scholar]
- Koella JC, Boëte C. A genetic correlation between age at pupation and melanization immune response of the yellow fever mosquito Aedes aegypti. Evolution. 2002;56:1074–1079. doi: 10.1111/j.0014-3820.2002.tb01419.x. [DOI] [PubMed] [Google Scholar]
- Lima JBP, Valle D, Peixoto AA. Adaptation of a South American malaria vector to laboratory colonization suggests faster-male evolution for mating ability. BMC Evol Biol. 2004;4:12. doi: 10.1186/1471-2148-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz L, Beaty BJ, Aitken TH, Wallis GP, Tabachnick WJ. The effect of colonization upon Aedes aegypti susceptibility to oral infection with yellow fever virus. Am J Trop Med Hyg. 1984;33:690–694. doi: 10.4269/ajtmh.1984.33.690. [DOI] [PubMed] [Google Scholar]
- Lynch M, Ritland K. Estimation of pairwise relatedness with molecular markers. Genetics. 1999;152:1753–1766. doi: 10.1093/genetics/152.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. Sinauer Associates, Inc.; Sunderland, MA: 1998. [Google Scholar]
- Morales Vargas RE, Ya-umphan P, Phumala-Morales N, Komalamisra N, Dujardin J-P. Climate associated size and shape changes in Aedes aegypti (Diptera : Culicidae) populations from Thailand. Inf Genet Evol. 2010;10:580–585. doi: 10.1016/j.meegid.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Morens DM, Fauci AS. Dengue and hemorrhagic fever. J Am Med Asso. 2008;299:214–216. doi: 10.1001/jama.2007.31-a. [DOI] [PubMed] [Google Scholar]
- Nasci RS. The size of emerging and host-seeking Aedes aegypti and the relationship of size to blood feeding success in the field. J Am Mosq Control Assoc. 1986;2:61–62. [PubMed] [Google Scholar]
- Newman RA. Adaptive plasticity in amphibian metamorphosis. BioSci. 1992;42:671–678. [Google Scholar]
- Nylin S, Gotthard K. Plasticity in life-history traits. Ann Rev Entomol. 1998;43:63–83. doi: 10.1146/annurev.ento.43.1.63. [DOI] [PubMed] [Google Scholar]
- Paupy C, Chantha N, Vazeille M, Reynes J-M, Rodhain F, Failloux A-B. Variation over space and time of Aedes aegypti in Phnom Penh (Cambodia): genetic structure and oral susceptibility to a dengue virus. Genet Res Camb. 2003;82:171–182. doi: 10.1017/s0016672303006463. [DOI] [PubMed] [Google Scholar]
- Sangster TA, Salathia N, Undurrage S, Milo R, Schellenberg K, Lindquist S, Queitsch C. HSP90 affects the expression of genetic variation and developmental stability in quantitative traits. Proc Natl Acad Sci USA. 2008a;105:2963–2968. doi: 10.1073/pnas.0712200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster TA, Salathia N, Lee HN, Watanabe E, Schellenberg K, Morneau K, Wang H, Undurranga S, Queitsch C, Lindquist S. HSP90-buffered genetic variation is common in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2008b;105:2969–2974. doi: 10.1073/pnas.0712210105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer ML, Lundström JO. Different responses of two floodwater mosquito species, Aedes vexans and Ochlerotatus sticticus (Diptera: Culicidae), to larval habitat drying. J Vector Ecol. 2006;31:123–128. doi: 10.3376/1081-1710(2006)31[123:drotfm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Scheiner SM. Genetics and evolution of phenotypic plasticity. Ann Rev Ecol Syst. 1993;24:35–68. [Google Scholar]
- Schluter D, Price TD, Rowe L. Conflicting selection pressures and life history trade-offs. Proc R Soc Lond B. 1991;246:11–17. [Google Scholar]
- Schneider JR, Morrison AC, Astete H, Scott TW, Wilson ML. Adult size and distribution of Aedes aegypti (Diptera: Culicidae) associated with larval habitats in Iquitos, Peru. J Med Entomol. 2004;41:634–642. doi: 10.1603/0022-2585-41.4.634. [DOI] [PubMed] [Google Scholar]
- Schneider JR, Mori A, Romero-Severson J, Chadee DD, Severson DW. Investigations of dengue-2 susceptibility and body size among Aedes aegypti populations. Med Vet Entomol. 2007;21:370–376. doi: 10.1111/j.1365-2915.2007.00699.x. [DOI] [PubMed] [Google Scholar]
- Severson DW. RFLP analysis of insect genomes. In: Crampton JM, Beard CB, Louis C, editors. The Molecular Biology of Insect Disease Vectors: A Methods Manual. Chapman & Hall; London: 1997. pp. 309–320. [Google Scholar]
- Stillwell RC, Blanckenhorn WU, Teder T, Davidowitz G, Fox CW. Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: from physiology to evolution. Ann Rev Entomol. 2010;55:227–245. doi: 10.1146/annurev-ento-112408-085500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Björklund M. Delrious: a computer program designed to analyze molecular marker data and calculate relatedness and delta estimates with confidence. Mol Ecol Notes. 2001;1:209–212. [Google Scholar]
- Teuschl Y, Reim C, Blanckenhorn WU. Correlated responses to artificial body size selection in growth, development, phenotypic plasticity and juvenile viability in yellow dung flies. J Evol Biol. 2007;20:87–103. doi: 10.1111/j.1420-9101.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- Thathy V, Severson DW, Christensen BM. Reinterpretation of the genetics of susceptibility of Aedes aegypti to Plasmodium gallinaceum. J Parasitol. 1994;80:705–712. [PubMed] [Google Scholar]
- Via S, Lande R. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution. 1985;39:505–522. doi: 10.1111/j.1558-5646.1985.tb00391.x. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Phenotypic plasticity and the origins of diversity. Ann Rev Ecol Syst. 1989;20:249–278. [Google Scholar]
- Xue R-D, Edman JD, Scott TW. Age and body size effects on blood meal size and multiple blood feeding by Aedes aegypti (Diptera: Culicidae) J Med Entomol. 1995;32:471–474. doi: 10.1093/jmedent/32.4.471. [DOI] [PubMed] [Google Scholar]
- Yan G, Severson DW, Christensen BM. Costs and benefits of mosquito refractoriness to malaria parasites: implications for genetic variability of mosquitoes and genetic control of malaria. Evolution. 1997;51:441–450. doi: 10.1111/j.1558-5646.1997.tb02431.x. [DOI] [PubMed] [Google Scholar]
- Yan G, Chadee DD, Severson DW. Molecular population genetics of the yellow fever mosquito: evidence for genetic hitchhiking effects associated with insecticide resistance. Genetics. 1998;148:793–800. doi: 10.1093/genetics/148.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


