Abstract
The anterior cingulate and a collection of other prefrontal and parietal brain regions are implicated in error processing and cognitive control. The effects of different doses of alcohol on activity within these brain regions during an fMRI task where errors are frequently committed have not been fully explored. This study examined the impact of a placebo [Breath Alcohol Concentration (BrAC) = 0.00%], moderate (BrAC = 0.05%) and high (BrAC = 0.10%) doses of alcohol on brain hemodynamic activity during a functional MRI (fMRI) Go/No-Go task in thirty-eight healthy volunteers. Alcohol increased reaction time and false alarm errors in a dose-dependent manner. FMRI analyses showed alcohol decreased activity in anterior cingulate, lateral prefrontal cortex, insula and parietal lobe regions during false alarm responses to No-Go stimuli. These findings indicate that brain regions implicated in error processing are affected by alcohol and might provide a neural basis for alcohol's effects on behavioral performance.
Keywords: functional imaging, alcohol, Go/No-Go, error monitoring, anterior cingulate
Introduction
Alcohol impairs cognitive control, leading to behavioral disinhibition, evidenced by increased aggressiveness, risky driving, risky sexual behaviors, and an increase in the likelihood of committing suicide (Steele and Southwick, 1985). The mechanisms behind this impaired control, however, are not well understood. These types of impulsive, disinhibited behavior are often examined in laboratory settings using neurocognitive tasks that provide contexts where participants respond less accurately or more rashly under the influence of alcohol. A reasonable question to ask is whether disinhibited performance results from impairment in the brain's ability to recognize, process, or adjust behavior following behavioral errors. Prior studies have found that alcohol is associated with increased errors of commission on Go/No-Go and similar neuropsychological tasks that assess ability to withhold a prepotent motor response (Dougherty et al., 2000; Easdon et al., 2005); but see (Ortner et al., 2003). However, research that uses behavioral tests and questionnaires does not address the neurobiological mechanisms underlying alcohol-related behavioral impairment. Functional neuroimaging is a useful approach for understanding how neural activity is altered in association with diminished cognitive or behavioral control. Previous research examining the neural correlates of acute alcohol effects suggests that moderate-to-high alcohol doses acutely disrupt brain activity. For example, some studies have observed that alcohol shifts activity from cortical to limbic regions (Volkow et al., 2008; Volkow et al., 1988). However, the effects of acute alcohol administration on brain activity are far from clear. Some studies report increased prefrontal cortex activation under the influence of alcohol, but only at lower doses (Sano et al., 1993), whereas decreased prefrontal and parietal activity are observed at higher doses (Levin et al., 1998; Soderlund et al., 2007; Van Horn et al., 2006). There also is evidence that task context plays an important role in determining alcohol's affects on brain activation. Gundersen and colleagues recently reported an increase in prefrontal and anterior cingulate activation when alcohol is expected, yet a decrease in those regions when alcohol is received (Gundersen et al., 2008b). Therefore, a better understanding of alcohol's effects on the brain is needed.
The Go/No-Go task, in which the participant is required to refrain from making a motor response to designated items within a series of stimuli, has frequently been used to examine cognitive control using fMRI in both healthy and psychiatric samples. The neural circuitry underlying the task has been carefully mapped in healthy populations (Konishi et al., 1998; Liddle et al., 2001; Rubia et al., 2001; Stevens et al., 2007). These prior studies have found that the anterior cingulate is a key brain region involved in response, conflict, or error monitoring (Botvinick et al., 2004; Carter et al., 1998; Kiehl et al., 2000). Other studies have supported the proposal that types of cognitive control engage a network of distributed brain regions that includes prominent anterior cingulate involvement, but also recruits local neural processing in bilateral dorsolateral prefrontal cortex, insula, inferior parietal lobule and striatal regions (Dosenbach et al., 2008; Seeley et al., 2007; Stevens et al., 2009). Error related brain activity is postulated to signal the need for increased control over cognitive processing in monitoring behavior (Botvinick et al., 2001). Supporting this idea, some reports show differences in neural activation to trials immediately preceding or following error (Garavan et al., 2002; Hester et al., 2009), which is evidence that error-related neural processing influences subsequent cognition. As such, reduced activity within these error-related cognitive control brain regions might limit the capacity or awareness of the need for behavioral adjustment, which is a plausible candidate for the negative effects of alcohol intoxication on behavior. To date, no published reports have evaluated whether decreased activity in the anterior cingulate and other error-responsive brain regions underlies the performance impairments seen under the influence of acute alcohol.
The current study used an fMRI Go/No-Go paradigm to examine how brain activity and behavior change under the influence of different alcohol doses. We specifically wished to determine whether alcohol affected the function of brain regions engaged in error processing. Because previous studies suggest alcohol's effects on the brain might be dose-dependent, the experiment included different alcohol doses (placebo, moderate, and high). Consistent with several previous behavioral findings (Ridderinkhof et al., 2002; Volkow et al., 2008), we hypothesized that alcohol would cause a dose-dependent slowing in motor performance such that higher doses of alcohol result in increased reaction time to both correct hits and false alarms. We also hypothesized that acute alcohol would cause regionally specific, dose-dependent changes in Blood Oxygenation Level Dependent (BOLD) signal in frontal and parietal lobe brain regions known to be engaged during error processing (Carter et al., 1998; Kiehl et al., 2000; Ridderinkhof et al., 2002; Volkow et al., 2008).
Methods
Subjects
We studied fifty-one healthy, right- handed adult volunteers (n = 24 males, 27 females; mean age 24.5, SD 4.7; range 19-40 years) in a repeated measures design. All participants had a high school or greater education; premorbid intelligence was estimated with the Hopkins Adult Reading Test (mean 110.2, SD 6.8). Participants were recruited via local newspaper and internet advertisement. All participants provided written informed consent approved by both the Hartford Hospital and Yale University Institutional Review Boards. Participants were moderate users of alcohol defined as individuals who consume alcohol less than two times per week and drink fewer than 3 drinks per session. Potential participants were non-smokers, had good visual acuity without correction and were screened to eliminate those with any neurologic disorder. The SCID-IV screen (First et al., 2002) was used to exclude participants with a DSM IV-TR Axis I psychiatric disorder including a history of past or current substance abuse (Janca et al., 1994). Prior to data collection on each assessment day, all participants underwent a urine toxicology screen for cocaine, opiates, marijuana, and stimulants and a baseline breathalyzer at each visit; they were excluded if either was positive. All women received a urine pregnancy test that produced negative results.
Data were collected on three separate sessions on separate days. To avoid interfering with alcohol absorption or intoxication, participants were instructed to eat only a light meal (avoiding fatty foods) on the morning of each study and to avoid consuming alcohol for 24 hours prior to each visit. Each participant received an individually tailored dose of beverage alcohol calculated based on weight and sex following a published algorithm (Kapur, 1989) to reach breath alcohol concentration (BrAC) targets for placebo (0.0% BrAC), moderate (0.05% BrAC), and high (0.10% BrAC) alcohol conditions. Subjective ratings of intoxication and physiological measures of BrAC assessed prior to and after the fMRI scanning were significantly different in all conditions (Table 1.) Doses were administered in randomized order on separate testing occasions with an average inter-dose interval of 13.6 days (SD 16.4). All drinks were provided in identical beverage containers wrapped with an alcohol soaked cloth and with an additional small amount of alcohol floated on the surface of the drink to help disguise the contents (Hammersley et al., 1992). All drinks were mixed with either orange or cranberry juice to a constant volume of 350 ml. Subjects consumed the beverage over a 10-minute period. BrAC's were measured before drinking to assure participants were not already under the influence and immediately prior to and following MR scanning using a hand-held breathalyzer calibrated regularly using an ethanol breath standard sample dispensing device (Intoximeters, Inc., St. Louis, MO).
Table 1.
Physiological and subjective measures of intoxication throughout the test session
| Placebo Mean(SD)* | Moderate Mean(SD)* | High Mean(SD)* | |
|---|---|---|---|
| BrAC before scan | 0.00 (0.00) | 0.04 (0.01) | 0.07 (0.02) |
| BrAC after scan | 0.00 (0.00) | 0.03 (0.01) | 0.09 (0.01) |
| Subjective rating of drunk before task | 0.39 (0.83) | 3.20 (2.00) | 5.67 (2.11) |
| Subjective rating of drunk after task | 0.27 (0.75) | 2.25 (1.73) | 5.92 (2.24) |
All planned comparisons (i.e., Placebo vs Moderate, Moderate vs High, Placebo vs. High) were significant at p < 0.001.
BrAC = Breath Alcohol Concentration
Go/No-Go Task
While under the influence of alcohol, approximately 45 minutes after completion of dosing, participants underwent an event-related functional magnetic resonance imaging (fMRI) Go/No-Go task in which they were required to withhold a prepotent motor response (Kiehl et al., 2000; Stevens et al., 2007). Participants were instructed to respond by pressing a button with their right index finger as quickly and as accurately as possible every time an “X” (85% probability; n=206) appeared and not to respond to the letter “K” (15% probability; n=40). Stimuli were presented for 50 msec each with an interstimulus interval of 750, 1750 or 2750 msec. The presentation order of the “X”s and “K”s was pseudo-random with intervals between the “K” stimuli lasting between 10-15 sec. Using an LCD projector (SHARP XG-P25X), stimuli were rear-projected to a translucent screen visible via a mirror attached to the head coil of the MRI scanner. Responses were recorded with a commercially available MRI compatible fiber-optic response device (Lightwave Medical, Vancouver, BC). Hits and errors were defined as responses occurring within 1 sec of an “X” or “K” trial, respectively.
Prior to scanning, each participant performed a block of 10 practice trials to ensure that instructions were understood. Reaction time and accuracy were equally emphasized in the task instructions. If necessary, following the practice and first run of each test session, participants were encouraged to speed up or slow down as needed so that their overall performance would result in at least 15 false alarm errors to “K” No-Go stimuli per run. Normative behavioral performance on this task is between 15-25 errors (average 20), so this instructional set ensured that through practice, they were not approaching the task in an overly-cautious or overly-careless style. Participants were not given precise instructions to make a given number of errors, nor provided feedback during the task on number of errors or speed of responses which would be clear inducements to adjust behavior. Participants were encouraged to perform at this level with each alcohol dose, thereby making within-subject performance differences meaningful. Each run of the fMRI task was an event-related design in which 246 stimuli were presented over a 7 minute 21 second period. Two runs were conducted with an approximately one minute break between.
Behavioral Analyses
Behavioral data were analyzed with a repeated measures (3 doses: 0.00, 0.05, and 0.10 BrAC) analysis of variance. Four independent variables were tested: 1) the number of correct hits to the prepotent target stimulus, 2) the number of false alarms (incorrect responses to non-target stimuli) and the mean reaction time for 3) hits and 4) false alarms. Statistical significance was evaluated with F-tests with planned contrasts for a significant main effect of alcohol dose. The main effect of gender on performance and the interaction of gender x dose was examined in a supplemental analysis.
fMRI - Data Acquisition
On three separate occasions, fMRI scans were performed under sober, moderate BrAC, and high BrAC conditions in randomized order. The scans for this repeated-measures design were acquired on a Siemens Allegra 3T system (Siemens, Erlangen, Germany) located at the Olin Neuropsychiatry Research Center at the Institute of Living. Functional imaging volumes were identified using localizer images. The echo planar image (EPI) gradient-echo pulse sequence (TR/TE 1500/27ms, flip angle 65 degrees, FOV 24 cm × 24 cm, 64 × 64 matrix, 3.4 mm × 3.4 mm in plane resolution, 5 mm effective slice thickness, 30 slices), effectively covered the entire brain (150 mm) in 1.5 s. Head motion was restricted using a cushion inside the head coil. Two stimulus runs were administered. Each run consisted of 294 TRs, including a 9 second rest session at the beginning to allow for T1 effects to stabilize. These initial six images were not included in analyses.
fMRI – Data Processing
Functional images were reconstructed offline and each run was separately realigned using INRIAlign (Freire et al., 2002). Head motion is a frequent problem in fMRI data acquisition and analysis. Although the majority of our data met the neuroimaging field's suggested cutoff that <1 voxel length movement likely indicates clean data, a sizeable proportion of runs did not (28%). Strict adherence to this arbitrary guideline would have resulted in discarding 30 of our 51 participants due to our repeated-measures design because at least one run was possibly affected by uncorrectable head movement. We therefore wished to retain as much data as possible per subject. This approach was supported by the observation that there was no significant difference in average movement between doses [mean (SD) X: placebo 0.098 (0.041), moderate 0.076 (0.041), high 0.113 (0.041), F(2, 152) =0.212, p = 0.81; Y: placebo 0.152 (0.051), moderate 0.256 (0.051), high 0.246 (0.051), F(2, 152) = 1.256, p = 0.29; Z: placebo 0.215 (0.097), moderate 0.220 (0.097), high 0.094 (0.097), F(2, 152) = 0.540, p = 0.584]. Therefore, movement indeed appeared to be random and not related to alcohol dose. To guide our decisions as to which fMRI sessions were unusable, ArtRepair (http://cibsr.stanford.edu/tools/ArtRepair/ArtRepair.htm) was used. Infrequent artifacts (i.e., < 15% of timepoints demonstrating problems with global signal fluctuations or relatively rapid head movements) were repaired through interpolation. Data where >15% of the time points required repair (i.e., ~20% of our total dataset) were closely scrutinized to see the type of movement, its severity, and whether or not artifact repair procedures were successful. If artifact repair did not improve the results, that run was excluded as “bad.” In cases where only one run was acceptable, that one run was retained for final random effects analyses (i.e., the task typically models two identical runs to enhance SNR of the fMRI parameter estimates). However, if both runs for one or more of a given subject's scans were unacceptable, that subject's data were excluded from further analysis. Using this rational process to identify actual movement-related problems and retain valid data wherever possible, our final sample size was 38 participants.
A mean functional image volume was constructed for each session from the realigned and repaired image volumes. This mean image volume was then used to determine parameters for spatial normalization into the Montreal Neurological Institute (MNI) standardized space employed in statistical parametric mapping (SPM5). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each participant. Finally, the normalized functional images were smoothed with an 8 mm full width at half-maximum Gaussian filter.
fMRI - Data Analyses
Our analyses compared statistical parametric maps (SPMs) of brain activity during false alarm errors and between sessions defined by alcohol dose using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/) software. The SPMs were contrast images that contained weighted parameter estimates which represented brain activity (i.e., positive or negative BOLD signal change) to each condition of the Go/No-Go task. Regression contrast images were produced for each subject using GLM “first-level” SPM5 models. Each model contained terms representing the modeled response to Go stimuli “hits,” correctly rejected No-Go stimuli, and No-Go stimuli where a button press was incorrectly made. For each type of event in the paradigm, the hemodynamic response was modeled using a synthetic hemodynamic response function composed of two gamma functions and their respective temporal derivatives (Friston et al., 1998). The hit stimuli were presented frequently throughout the trial in order to maintain a sustained hemodynamic response. These models also included the six x, y, z, pitch, roll, and yaw movement correction parameters as covariates of no interest to statistically control for signal change more greatly associated with minor head motion than with task-related signal change.
The statistical parametric maps for false alarm brain activity were then examined using SPM5 in a repeated-measures full factorial ANOVA, where the three randomized alcohol dose levels were entered as the within-subject factor. We first examined the average effect of condition F test to identify regions of significant signal change during false alarm trials relative to the baseline. This analysis allowed us to confirm that our participants were generally engaging the same regions as observed in our previous reports using this paradigm and confirmed significant signal change in brain regions known to be important for error processing. We also conducted SPM5 one-sample t tests for false alarm contrast maps separately for each alcohol condition. These three analyses were done in order to confirm normal profiles of error-related activation in each condition. Reference to the stereotactic MNI template helped to identify regions of significant effects in these analyses (Brett, 2002). The significance of active brain regions was evaluated using False Discovery Rate corrections for searching the whole brain, q < .001 (Genovese et al., 2002) for the average effect of conditions F test, and q < .05 for the one-sample t tests. Although both types of analysis controlled for searching the whole brain, different critical FDR thresholds were chosen for visualization given the greater statistical power for the factorial ANOVA (i.e., 114 observations for the factorial F test vs. 36 per t test).
Next, the factorial ANOVA also was used to assess the statistical significance of any differences among dose conditions. We established which brain regions were significantly different across alcohol doses in this omnibus F test by examining voxels that survived corrections for multiple comparisons at FDR q <.05. We note that our final sample size (n=36) provided sufficient power that we were able to evaluate our a priori hypothesis for dose-related differences at statistical thresholds adequate for searching the whole brain volume. Within this subset of voxels, we then conducted planned t test comparisons (placebo vs. moderate dose, placebo vs. high dose, and moderate vs. high) to clarify patterns of specific regional dose effects within the ROIs. Because these were post hoc analyses following a significant omnibus F that confirmed the presence of a significant dose-related difference, we used a more liberal statistical threshold to ensure we did not inadvertently miss any specific difference (p < .001 uncorrected).
Results
Subjective Ratings of Intoxication
Subjective levels of intoxication and BrAC values pre- and post-fMRI scan are reported in Table 1. As expected, subjects rated themselves as significantly more intoxicated as the dose of alcohol administered increased (i.e., placebo < moderate dose < high dose).
Behavioral Performance
As hypothesized, reaction time for “hit” responses increased in a roughly dose-dependent manner. Significant differences occurred between placebo and high doses of alcohol, and between moderate and high doses of alcohol. Differences in reaction time between placebo and moderate were either not significant (“Go” hits) or reached only a trend level of significance (false alarms).
We did not predict any differences in task accuracy. This in part was because our instructional set was designed to produce a balance of speed vs. accuracy that we did not necessarily expect to produce the different numbers of errors and correct hits to Go stimuli at different alcohol doses. However, there was instead a significant dose-dependent increase in the number of false alarms and the number of omissions (Table 1).
Supplemental analyses found no gender differences in any aspect of behavioral performance.
fMRI-Measured Activation to False Alarm Trials
As expected based on our previous research using this Go/No-Go fMRI task, the average effect of false alarms was seen in many brain regions previously associated with error processing, including anterior cingulate (x, y, z = -3, 18, 39 F1,111 = 127.87), left middle/superior frontal gyri, BA 10 (x, y, z = -27, 45, 21 F1,111 = 63.39), right middle/superior frontal gyrus, BA 10 (x, y, z = 36, 45, 27 F1,111 = 45.73), left putamen (x, y, z = -18, 12, 0 F1,111 = 44.15), right putamen (x, y, z = 15, 6, 3 F1,111 = 42.97), and left insula (x, y, z = -36, 12, 3 F1,111 = 161.42), right insula (x, y, z = 39, 18, -6 F1,111 = 181.41), We note that the insular activations extended from the posterior aspect of orbitofrontal cortex down into anterior temporal lobe (i.e., BA 38). We report these regions as insular cortex because their predominant focus was centered in areas clearly labeled by stereotactic atlas as insula. Significant signal change to false alarms also was observed in left (x, y, z = -48, -36, 42 F1,111 = 35.56) and right (x, y, z = 51, -48, 48 F1,111 = 43.07) inferior parietal lobule/supramarginal gyri, medial frontal gyrus (x, y, z = 3, 42, -18 F1,111 = 24.99), left (x, y, z = -9, -18, 6 F1,111 = 75.43) and right (x, y, z = 6, -24, 0 F1,111 = 66.06) thalamus, left (x, y, z = -45, -81, 0 F1,111 = 39.62) and right (x, y, z = 36, -93, -6 F1,111 = 54.83) occipital cortex (BA 18), left sensoriomotor cortex (x, y, z = -39, -18, 51 F1,111 = 53.27), mid-cingulate cortex (x, y, z = 6, -18, 33 F1,111 = 58.64), and posterior cerebellum (x, y, z = 12, -63, -18 F1,111 = 49.56).
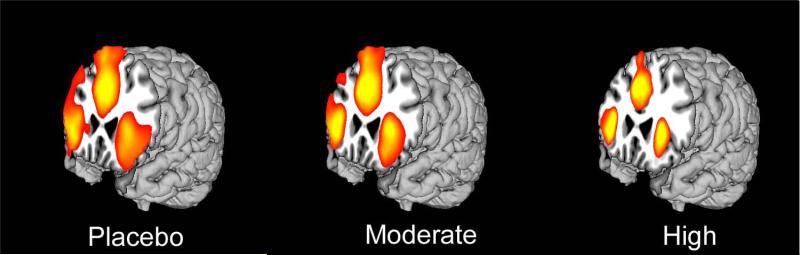
The results of the separate one-sample t tests for each alcohol dose condition of major regions of false alarm-elicited brain activity are depicted in Figure 1.
Figure 1.
Extent of activation in placebo, moderate, and high dose conditions for false alarms at q < 0.05 FDR. For placebo T = 1.82, moderate T = 2.00, high T = 2.52.
Effects of Alcohol on False Alarm Brain Activation
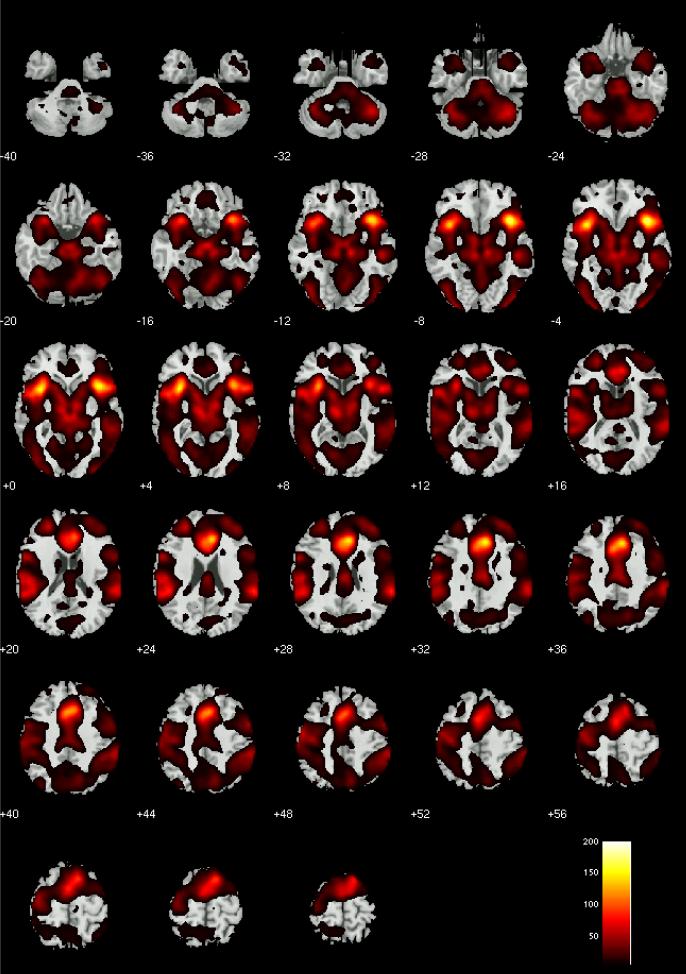
Evaluation of the omnibus F test for effects of alcohol dose condition on false alarm brain activity found numerous brain regions that differed significantly at q < .05 FDR (Figure 2). These included differences in many of the brain regions associated with error processing in previous reports (e.g., dorsal and rostral anterior cingulate, bilateral insula, right lateral prefrontal cortex). Numerous other brain regions also were noted to significantly differ by alcohol dose condition. The results of planned comparisons to characterize the patterns of dose-related effects on error-related brain activity across pairs of dose condition are listed in Tables 3-5. We note that Tables 3-5 report dose-related differences only within brain voxels that showed a significant omnibus F test for dose effects. The tables show that many brain regions known to engage during error processing showed different levels of activation between placebo vs. high alcohol doses, and between moderate and high alcohol doses. Average effect size for the differences between placebo and high alcohol dose was d = 0.88(Cohen, 1988). Average effect size for moderate vs. high was d = 0.84. Interestingly, there was no evidence at the p < .001 threshold for activity differences between placebo and a moderate dose of alcohol. However, at a more liberal p < .05 uncorrected threshold, there was evidence for dose-related differences in numerous brain regions. Although these results were not detectable using the a priori statistical thresholds chosen for this study, we note that their effect sizes for peak activated voxel within each brain region ranged from d = 0.36 to 0.66 (average 0.51) . Furthermore, they largely comprised regions known to elicit positive signal change during error processing. As such, they deserve mention. These effects were observed at p < .05 uncorrected levels in midline (x, y, z = 9, 12, 48 t111 = 2.78) and lateral (x, y, z = -36, 0, 54 t111 = 2.54) supplementary motor area cortex, rostral anterior cingulate (x, y, z = -6, 33, 6 t111 = 2.39) , medial frontal gyrus (dorsomedial) (x, y, z = -9, 51, 12 t111 = 3.01), left (x, y, z = -45, 21, -18 t111 = 2.14) and right (x, y, z = 45, 39, -6 t111 = 2.26) inferior frontal gyrus, left sensorimotor cortex (x, y, z = -45, -12, 36 t111 = 2.74), right precentral gyrus (x, y, z = 60 , 0 27 t111 = 2.38), left (x, y, z = -18, -57, 0 t111= 2.80) and right (x, y, z = 18, -60, 3 t111= 2.85) posterior cingulate cortex, precuneus (x, y, z = 6, -57, 45 t111 = 2.43), left (x, y, z = -18, 3, -24 t111 = 2.98) and right (x, y, z = 15, -6, -18 t111 = 2.64) amygdala/parahippocampal gyrus, right transverse/superior temporal gyri (x, y, z = 54, -21, 15 t111 = 1.92), left middle temporal gyrus (x, y, z = -48, 0, -15 t111 = 2.46) and (x, y, z = -48, -36, 15 t111 = 2.50), thalamus (x, y, z = -3, -21, 6 t111 = 2.19), right putamen (x, y, z = 21, -9, 6 t111 =3.30), midbrain (x, y, z = 3, -9, -12 t111 = 3.09), and cerebellum vermis (x, y, z = -3, -66, -3 t111 = 3.36) and culmen (x, y, z = -15, -39, -27 t111 = 3.45).
Figure 2.
Omnibus F test results for the effects of alcohol dose condition on false alarm brain activity at q < .05 FDR
Table 3.
Brain regions with significant differences between placebo and a moderate dose of alcohol. Results are presented within voxels that survive an omnibus F-test for dose effects (q<.05 FDR). All results in this contrast surpass p<.001 uncorrected, activation extent 20 contiguous voxel thresholds appropriate to these planned comparisons. Brain regions are reported using reference to stereotactic MNI atlas labeling, along with peak t score for each regional activation difference and its x, y, z coordinates.
| Regions | Hemisphere | Max t | X, Y, Z |
|---|---|---|---|
| PLACEBO MINUS MODERATE DOSE | |||
| No statistically significant findings. | |||
| MODERATE MINUS PLACEBO DOSE | |||
| No statistically significant findings. | |||
Table 5.
Brain regions with significant differences between moderate and high doses of alcohol. Results are presented within voxels that survive an omnibus F-test for dose effects (q<.05 FDR). All results in this contrast surpass p<.001 uncorrected, activation extent 20 contiguous voxel thresholds appropriate to these planned comparisons. Brain regions are reported using reference to stereotactic MNI atlas labeling, along with peak t score for each regional activation difference and its x, y, z coordinates.
| Regions | Hemisphere | Max t | x, y, z |
|---|---|---|---|
| MODERATE MINUS HIGH DOSE | |||
| Superior frontal gyrus (SMA) | Midline | 3.84 | 6, 12, 63 |
| Superior/middle frontal gyrus (BA 10) | Left | 5.77 | -27, 45, 24 |
| Middle frontal gyrus (BA 10) | Right | 4.75 | 36, 45, 12 |
| Medial frontal gyrus (pre-SMA) | Midline | 3.82 | 9, 24, 48 |
| Anterior cingulate gyrus (BA 32) | Midline | 4.50 | 0, 30, 27 |
| Cingulate gyrus (mid) | Midline | 3.78 | 0, -12, 33 |
| Insula | Left | 4.46 | -36, 9, 3 |
| Right | 4.55 | 42, 18, 0 | |
| Sensoriomotor cortex | Left | 4.84 | -33, -24, 63 |
| Superior temporal gyrus | Left | 4.35 | -57, -18, 15 |
| Left | 4.54 | -57, -30, 21 | |
| Right | 3.98 | 60, -42, 24 | |
| HIGH MINUS MODERATE DOSE | |||
| No statistically significant findings. | |||
In all condition contrasts, there was no evidence for significantly greater BOLD activity at higher doses of alcohol, even at liberal p < .05 uncorrected exploratory thresholds.
Discussion
In an effort to determine whether problems with behavioral inhibition under the influence of acute alcohol is due to effects of intoxication on brain structures engaged to process performance errors, we examined the acute effects of moderate (0.05 BrAC) and high (0.10 BrAC) doses of alcohol compared to placebo (0.00 BrAC) on measures of cognitive control during a Go/No-Go task in 38 moderate users of alcohol. Replicating our previous studies of the Go/No-Go task, analyses of the placebo condition showed that false alarm trials were associated with signal change in a collection of brain regions that included anterior cingulate, insular cortex, dorsolateral prefrontal cortex and parietal lobe (Kiehl et al., 2000; Stevens et al., 2007). As hypothesized, alcohol administration influenced the degree of activation in these and other brain regions. Differences between placebo and moderate doses (average d = 0.51) were of lesser magnitude than differences between placebo and high alcohol dose (average d = 0.88) or moderate and high alcohol dose (average d = 0.74), however, each comparison has a moderate to large effect size. There was no evidence that alcohol increased the degree of BOLD signal change in any error-related brain regions. These changes in brain activity paralleled the changes in behavioral performance. Participants had greater reaction time, more errors, and less successful hits with greater doses of alcohol.
There were a few specific dose-related findings that might have implications for how the brain is influenced by different amounts of alcohol. Though we urge some caution interpreting the placebo vs. moderate alcohol dose differences due to the comparatively liberal statistical threshold at which these results were observed, we note that error-elicited brain activity did not differ even at p < .05 uncorrected in dorsal anterior cingulate or bilateral anterior insular cortex. These regions are key structures that respond to errors (Botvinick et al., 2004; Carter et al., 1998; Kiehl et al., 2000). For example, in our previous work examining distributed error-processing neural circuits, these regions were prominent in a network that appeared to serve a generalized performance monitoring function throughout the task, but whose activation significantly increased during commission of an error (Stevens et al., 2009). This is consistent with others’ interpretation of the role of these brain regions in maintaining overall cognitive set throughout lengthy tasks (Dosenbach et al., 2008). The presence of some activation differences only at high alcohol doses suggests that certain cognitive processes that rely on this intact neural processing might not be affected at moderate doses. For instance, there were deficits in bilateral ventrolateral prefrontal cortex to moderate doses not seen in the other alcohol dose planned contrasts. Right ventrolateral prefrontal cortex has been associated with successful response inhibition (Aron, 2007), arguing that an early effect of alcohol might be to reduce activity in regions known to be important to preventing impulsive actions. Although our focus in this study was on errors, we believe it will be fruitful to also test the effects of alcohol on response inhibition-related neural activation directly in future work in order to gain a fuller appreciation for association of neural impairment and various cognitive processes involved with complex cognitive and behavioral control. We also observed that hemodynamic activity was further reduced by high doses of alcohol in only a subset of regions previously found to be sensitive to the effects of alcohol (Levin et al., 1998; Newlin et al., 1982; Seifritz et al., 2000). There were differences between moderate and high alcohol doses only in dorsal anterior cingulate, bilateral anterior insula, supplementary motor area, dorsolateral prefrontal cortex, left sensorimotor cortex, and some temporal lobe regions. Both doses of alcohol compromised cerebellar activation, but there were no differences between moderate and high alcohol doses. The cerebellum is implicated in a variety of limbic, motor and sensory functions (Schmahmann, 1997). Its decreased activation under acute alcohol conditions has previously been found (Gundersen et al., 2008a; Van Horn et al., 2006) and may underlie impairments seen in mood, behavior, cognition and motor activity (Volkow et al., 2008). Similarly, there was evidence for greater reduction of medial frontal gyrus, posterior cingulate, and precuneus seen between alcohol doses and placebo, but not additionally observed between moderate and high dose alcohol. In our previous work with this task and in many other paradigms, these brain regions typically have negative signal change relative to baseline (Broyd et al., 2009). One interpretation of this profile of negative signal change is that regions with high baseline activity are reduced when active cognitive demands are employed, leading some to suggest the degree of change indexes cognitive effort (Broyd et al., 2009). In this context, the relatively greater negative signal change in these regions seen with active alcohol doses might denote greater difficulty in maintaining effective cognitive control when intoxicated. However, this intriguing possibility is best explored in future experiments using fMRI tasks directly designed to disentangle the influences of effort and alcohol on brain activity.
There are several potential explanations for the neural activity changes that accompany error (Botvinick et al., 2001). According to the conflict monitoring theory, the anterior cingulate monitors for the presence of an error (i.e., conflict) and then signals the lateral prefrontal cortex to increase cognitive control. Our findings show both the anterior cingulate and aspects of prefrontal cortex exhibit decreased activation to the presence of errors while under the influence of alcohol. Our findings are consistent with those reported by Ridderinkhof and colleagues (Ridderinkhof et al., 2002). They reported that flanker interference was reduced after errors in the placebo condition, but not after alcohol suggesting participants did not realize they made an error. Although the current paradigm was not constructed to differentiate between the effects of conflict monitoring, response selection or any other theoretical accounts of error-related neural processing, it would certainly be instructive if future neuroimaging research employed tasks that specifically test various theories of error processing in placebo controlled alcohol challenge studies. This would shed more light on exactly how the neural deficits we observed here might translate to specific forms of impaired behavior. For instance, tasks could alter the salience of the stimuli to differentiate if participants are unable to detect the errors (implying deficits in error awareness), test if varying degrees of response selection or conflict alters how errors are processed while intoxicated, or examine the degree to which neural deficits in specific brain regions during error processing relate to any subsequent performance adjustments.
The study hypotheses were predicated on the assumption of regional specificity in brain function. However, we note that brain regions are interconnected and alcohol may affect connectivity both up and/or downstream from the site of change visible on fMRI. As we have described in previous reports (Stevens et al., 2007), the brain's response to error commission involves a network of brain regions that presumably coordinate the contributions of localized neural processing across a distributed network, where signals ultimately are used to adjust behavior to optimize performance. With this in mind, we note that our analyses also found an alcohol-related decrease in brain activation within the basal ganglia and cerebellum, which comprise parts of networks know to be important to movement and cognition (Middleton and Strick, 2001). In particular, a decrease in cerebellar activity following alcohol dosing has previously been reported (Gundersen et al., 2008a; Van Horn et al., 2006) and may be directly linked with the poor motor coordination often associated with alcohol intoxication. Analyzing the functional connectivity of these networks is beyond the scope of this paper. We can, however, speculate that the fMRI results present here might represent the net effect of changes to how brain inter-regional communication is differentially hindered by different doses of alcohol. Future studies examining the effects of alcohol on networks associated with response inhibition and error monitoring would supplement information gathered in the approach presented here.
There are several limitations of this study worth noting. First, we restricted our focus to trials in which an error was committed. However, it is reasonable to ask the question of whether alcohol also influences brain regions engaged for the successful inhibition of behavior. Because many brain regions we see affected by alcohol in this study are engaged on both error and correctly-rejected No-Go trials, we might find similar effects of alcohol on the neural correlates of response inhibition (Aron, 2007). We plan to explore this in future work. Second, it is possible that a participants’ brain activity was subtly different as a consequence of making more or less errors (i.e., a contextual effect that has implications for interoceptive and conscious monitoring/adjustment of performance following awareness of errors). These intriguing questions are beyond the scope of the current study, but it might be instructive to examine them in future research. Third, our participants were tested at the same chronological time point following dosing. While we do not have precise measures of which phase of the BrAC curve participants were in, it is likely they were in the descending limb of the BrAC curve in the moderate dose and the ascending limb in the high dose. This interaction limits the generalizability of our findings. Fourth, , it has been observed that alcohol might alter the BOLD fMRI signal through its vasoactive effects (Levin et al., 1998; Newlin et al., 1982; Seifritz et al., 2000), which raises the question of whether the current fMRI BOLD results reflect alcohol's effect on the signal measured rather than brain activity itself. Although our review of the literature suggests there is as yet insufficient information to fully confirm all the possible physiological effects of alcohol on the BOLD signal measured in fMRI, there are several factors that raise our confidence in the current results. Volkow has reported that blood flow (a key component contributing to the BOLD response during fMRI) is not especially influenced by alcohol in low or moderate concentrations similar to the moderate dose used in this study (Volkow et al., 1988). Our results should be considered with caution, however, as at higher doses, she did find blood flow changes in frontal, temporal and cerebellar regions. Second, Hine et al (Hine et al., 1952) found no changes in cerebral metabolic rate for oxygen (CMRO2) at moderate doses. This was confirmed for moderate doses of alcohol by Battey et al (Battey et al., 1953). However, high doses of alcohol produced an increase in CBF and a decrease in CMRO2. Thus we cannot completely rule out the possibility that our high alcohol dose results might not be directly comparable to the placebo and moderate dose. Because alcohol-related increases and decreases in rCBF may be found in different brain regions (e.g., alcohol may depress cerebellar rCBF and increase prefrontal rCBF), if signal were artifactually altered, we would expect to see depression of BOLD signal throughout the brain. This was not evident in our study. Although it remains possible that physiological effects on the BOLD signal influenced the degree of alcohol-induced decrease, this selectivity strongly suggests that we are measuring true regional effects of alcohol on brain activation. We also note that many of our fMRI effects directly paralleled our behavioral effects. That is, performance deficits increased to statistically significant levels with the high alcohol dose, consistent with even greater activity deficits in many brain regions we examined. A final limitation of this study is that fMRI does not provide absolute measures of neural activity and instead relies on quantifying degree of change between conditions. FMRI is not typically suited to disentangling whether alcohol alters the degree of activation, results in greater or lesser regional brain activation, or both. Future research using positron emission tomography or similar techniques that provide absolute quantification of brain activity, or complex mixed event/block fMRI designs that might disentangle these complex effects would no doubt help to address this important question.
Conclusions
This study shows that high doses of alcohol increase not only the amount of time needed to respond to a stimulus, but also increases the frequency of inappropriate error responses. No statistically significant reaction time impairments were visible at a moderate dose of alcohol, consistent with the choice of a BrAC of 0.08% as the legal driving limit in many states in the United States of America, but errors did increase. Moreover, performance generally declined in a dose-dependent manner. Functional imaging suggests there is a significant decrease in brain activity involved in the commission of an error for both BrAC of 0.05 that were stronger in many regions at a BrAC of 0.10%. Overall these findings suggest that acute alcohol use impairs cognitive control through a dose dependent decrease in cortical activation.
Table 2.
Behavioral performance on the Go/No-Go task
| Placebo Dose Mean (SD) | Moderate Dose Mean (SD) | High Dose Mean (SD) | Placebo vs Moderate Df = 37 | Moderate vs High Df = 37 | Placebo vs High Df = 37 | Overall Df = 2, 74 | |
|---|---|---|---|---|---|---|---|
| Number of False Alarms | 13.3 (4.9) | 15.3 (4.9) | 17.8 (5.9) | T = 3.03 P = 0.004 |
T = 3.06 P = 0.004 |
T = 4.97 P < 0.001 |
F = 15.83 P < 0.001 |
| Number of Hits | 204.6 (2.06) | 201.5 (7.9) | 195.6 (16.5) | T = 2.35 P = 0.024 |
T = 2.29 P = 0.028 |
T = 3.30 P = 0.002 |
F = 8.01 P = 0.001 |
| Mean False Alarm Reaction Time (msec) | 335.7 (24.3) | 340.00 (25.0) | 374.4 (39.0) | T = 1.27 P = 0.21 |
T = 6.56 P < 0.001 |
T = 7.55 P < 0.001 |
F= 41.58 P < 0.001 |
| Mean ‘Go’ Reaction Time (msec) | 366.5 (34.4) | 368.6 (35.0) | 386.8 (54.0) | T = 0.49 P = 0.63 |
T = 2.46 P = 0.019 |
T = 2.98 P = 0.005 |
F = 6.22 P = 0.003 |
Table 4.
Brain regions with significant differences between placebo and a high dose of alcohol. Results are presented within voxels that survive an omnibus F-test for dose effects (q<.05 FDR). All results in this contrast surpass p<.001 uncorrected, activation extent 20 contiguous voxel thresholds appropriate to these planned comparisons. Brain regions are reported using reference to stereotactic MNI atlas labeling, along with peak t score for each regional activation difference and its x, y, z coordinates.
| Regions | Hemisphere | Max T | X, Y, Z |
|---|---|---|---|
| PLACEBO MINUS HIGH DOSE | |||
| Superior frontal gyrus (SMA) | Midline | 6.05 | 6, 3, 66 |
| Left | 4.15 | -30, -9, 66 | |
| Right | 3.72 | 33, 3, 63 | |
| Medial frontal gyrus (pre-SMA) | Midline | 5.23 | 3, 12, 48 |
| Anterior cingulate gyrus (BA 32) | Midline | 5.07 | -3, 27, 27 |
| Anterior cingulate gyrus (BA 24) | Midline | 5.09 | -6, 36, 15 |
| Medial frontal gyrus (dorsomedial) | Midline | 5.65 | -6, 54, 21 |
| Middle frontal gyrus (lat orbitofrontal) | Left | 4.68 | -39, 30, -6 |
| Right | 4.51 | 51, 24, -3 | |
| Insula | Left | 4.94 | -42, 12, -12 |
| Right | 5.35 | 39, 9, -9 | |
| Sensoriomotor cortex | Left | 4.70 | -45, -9, 57 |
| Left | 5.14 | -54, -15, 24 | |
| Precentral gyrus | Right | 4.04 | 45, 0, 57 |
| Superior temporal gyrus | Left | 5.75 | -63, -39, 21 |
| Left | 3.70 | -48, -60, 12 | |
| Right | 4.20 | 51, -27, -12 | |
| Right | 4.56 | 51, -54, 9 | |
| Posterior cingulate gyrus | Left | 3.67 | -12, -69, 9 |
| Right | 4.38 | 18, -69, 9 | |
| Precuneus | Midline | 4.45 | 9, -78, 51 |
| Thalamus | Left | 4.46 | -15, -15, 3 |
| Left | 4.61 | -6, -21, 9 | |
| Right | 4.59 | 18, -12, 6 | |
| Cerebellum (vermis) | Midline | 3.27 | 0, -75, -9 |
| Brainstem | 4.24 | 3, -15, -15 | |
| HIGH DOSE MINUS PLACEBO | |||
| No statistically significant findings. | |||
Acknowledgements
This work was supported by NIAAA grant # R01AA015615 awarded to Dr. Godfrey Pearlson.
Footnotes
Disclosure/Conflict of Interest
No authors report a conflict of interest.
References
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13(3):214–28. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Battey LL, Heyman A, Patterson JL., Jr. Effects of ethyl alcohol on cerebral blood flow and metabolism. Journal of the American Medical Association. 1953;152(1):6–10. doi: 10.1001/jama.1953.03690010012002. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8(12):539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brett M. The MNI brain and the Talairach atlas. 2002.
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chikazoe J. Localizing performance of go/no-go tasks to prefrontal cortical subregions. Curr Opin Psychiatry. doi: 10.1097/YCO.0b013e3283387a9f. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. L. Erlbaum Associates; Hillsdale, N.J.: 1988. [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Moeller FG, Chokshi RV, Rosen VC. Effects of moderate and high doses of alcohol on attention, impulsivity, discriminability, and response bias in immediate and delayed memory task performance. Alcohol Clin Exp Res. 2000;24(11):1702–11. [PubMed] [Google Scholar]
- Easdon C, Izenberg A, Armilio ML, Yu H, Alain C. Alcohol consumption impairs stimulus- and error-related processing during a Go/No-Go Task. Brain Research. Cognitve Brain Research. 2005;25(3):873–83. doi: 10.1016/j.cogbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axiws I Disorders, Research Version, Non-patient Edition (SCIDI/NP) Biometrics Research, New York State Psychiatric INistitute; New York: 2002. [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21(5):470–84. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7(1):30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gundersen H, Gruner R, Specht K, Hugdahl K. The effects of alcohol intoxication on neuronal activation at different levels of cognitive load. Open Neuroimaging Journal. 2008a;2:65–72. doi: 10.2174/1874440000802010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H, Specht K, Gruner R, Ersland L, Hugdahl K. Separating the effects of alcohol and expectancy on brain activation: an fMRI working memory study. Neuroimage. 2008b;42(4):1587–96. doi: 10.1016/j.neuroimage.2008.05.037. [DOI] [PubMed] [Google Scholar]
- Hammersley R, Finnigan F, Millar K. Alcohol placebos: you can only fool some of the people all of the time. Br J Addict. 1992;87(10):1477–80. doi: 10.1111/j.1360-0443.1992.tb01926.x. [DOI] [PubMed] [Google Scholar]
- Hester R, Madeley J, Murphy K, Mattingley JB. Learning from errors: error-related neural activity predicts improvements in future inhibitory control performance. J Neurosci. 2009;29(22):7158–65. doi: 10.1523/JNEUROSCI.4337-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine CH, Shick AF, Margolis L, Burbridge TN, Simon A. Effects of alcohol in small doses and tetraethylthiuramdisulphide (antabus) on the cerebral blood flow and cerebral metabolism. J Pharmacol Exp Ther. 1952;106(3):253–60. [PubMed] [Google Scholar]
- Janca A, Ustun TB, Sartorius N. New versions of World Health Organization instruments for the assessment of mental disorders. Acta Psychiatr Scand. 1994;90(2):73–83. doi: 10.1111/j.1600-0447.1994.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Kapur BM. Computer Blood Alcohol Calculator v1.20 ARF Software. Addiction Research Foundation; Toronto, Canada: 1989. [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37(2):216–23. [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci. 1998;10(3):1209–13. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Levin JM, Ross MH, Mendelson JH, Kaufman MJ, Lange N, Maas LC, Mello NK, Cohen BM, Renshaw PF. Reduction in BOLD fMRI response to primary visual stimulation following alcohol ingestion. Psychiatry Res. 1998;82(3):135–46. doi: 10.1016/s0925-4927(98)00022-5. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12(2):100–9. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21(2):700–12. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, Golden CJ, Quaife M, Graber B. Effect of alcohol ingestion on regional cerebral blood flow. Int J Neurosci. 1982;17(3):145–50. doi: 10.3109/00207458208985916. [DOI] [PubMed] [Google Scholar]
- Ortner CN, MacDonald TK, Olmstead MC. Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol Alcohol. 2003;38(2):151–6. doi: 10.1093/alcalc/agg041. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GP. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298(5601):2209–11. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13(2):250–61. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Sano M, Wendt PE, Wirsen A, Stenberg G, Risberg J, Ingvar DH. Acute effects of alcohol on regional cerebral blood flow in man. J Stud Alcohol. 1993;54(3):369–76. doi: 10.15288/jsa.1993.54.369. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifritz E, Bilecen D, Hanggi D, Haselhorst R, Radu EW, Wetzel S, Seelig J, Scheffler K. Effect of ethanol on BOLD response to acoustic stimulation: implications for neuropharmacological fMRI. Psychiatry Res. 2000;99(1):1–13. doi: 10.1016/s0925-4927(00)00054-8. [DOI] [PubMed] [Google Scholar]
- Soderlund H, Grady CL, Easdon C, Tulving E. Acute effects of alcohol on neural correlates of episodic memory encoding. Neuroimage. 2007;35(2):928–39. doi: 10.1016/j.neuroimage.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Steele CM, Southwick L. Alcohol and social behavior I: the psychology of drunken excess. J Pers Soc Psychol. 1985;48:18–34. doi: 10.1037//0022-3514.48.1.18. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181(1):12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Brain network dynamics during error commission. Hum Brain Mapp. 2009;30:24–37. doi: 10.1002/hbm.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn JD, Yanos M, Schmitt PJ, Grafton ST. Alcohol-induced suppression of BOLD activity during goal-directed visuomotor performance. Neuroimage. 2006;31(3):1209–21. doi: 10.1016/j.neuroimage.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ma Y, Zhu W, Fowler JS, Li J, Rao M, Mueller K, Pradhan K, Wong C, Wang GJ. Moderate doses of alcohol disrupt the functional organization of the human brain. Psychiatry Res. 2008;162(3):205–13. doi: 10.1016/j.pscychresns.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24(2):201–9. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]