Abstract
Purpose
Interleukin-15 (IL-15) is a promising cytokine for immunotherapy of cancer due to its ability to stimulate NK-, B- and T-cell immunity. Its effectiveness, however, may be limited by inhibitory checkpoints and pathways that can attenuate immune responses. Finding strategies to abrogate these negative regulators and enhance the efficacy of IL-15 is a critical challenge.
Experimental Design
In a pre-clinical study we evaluated IL-15 combined with antibodies to block the negative immune regulators CTLA-4 and PD-L1 in a metastatic murine CT26 colon carcinoma model.
Results
IL-15 treatment resulted in a significant prolongation of survival in mice with metastatic tumor. Administration of IL-15, however, also increased expression of PD-1 on the surface of CD8+ T-cells including CD8+CD44high memory phenotype T-cells. Moreover, IL-15 also increased the secretion of the immunosuppressive cytokine, IL-10. Combining IL-15 with anti-PD-L1 and anti-CTLA-4 (multiple immune checkpoint blockade) exhibited greater CTL killing and interferon-gamma secretion. Moreover, this combination resulted in a significant reduction in surface expression of PD-1 on CD8+ T-cells, a decrease in IL-10 secretion and lead to significantly longer survival of tumor-bearing animals compared to mice treated with IL-15 alone, or combined singularly with anti-PD-L1 or anti-CTLA-4.
Conclusions
Combining the immune stimulatory properties of IL-15 with the simultaneous removal of two critical immune system inhibitory checkpoints we demonstrated enhancement of immune responses leading to increased anti-tumor activity.
Keywords: Antibody, checkpoint blockade, cytotoxic T-lymphocyte antigen-4, interleukin-15, programmed death ligand-1
Introduction
Interleukin-15 (IL-15), a member of the 4-α-helix bundle family of cytokines is required for the generation of natural killer (NK) cells and lasting CD8+ memory T-cell responses (1). IL-15 is trans-presented by its receptor, IL-15Rα, expressed on the surface of antigen presenting cells to the IL-2Rβ and common gamma (γc) chains expressed on effector T-, B- and NK cells (2, 3). IL-15 facilitates the survival and differentiation of these cells, their activation, and the maintenance of memory T-cells (4, 5). In preclinical studies, IL-15 has been shown to enhance both humoral and cell-mediated immune responses leading to the inhibition of tumor growth (6–9).
A number of inhibitory receptors have been demonstrated to dampen or terminate immune responses in the setting of chronic viral infections and in tumor-bearing animals. These include programmed death-1 (PD-1, CD274) (10), and its ligands, PD-L1 and PD-L2 (11), and cytotoxic lymphocyte antigen-4 (CTLA-4). PD-1, a member of the CD28/CTLA-4 family of T-cell regulatory receptors that is expressed on the surface of activated B- and T-cells and is involved in the induction of immune tolerance (12). PD-L1 is constitutively expressed at low levels on hematopoietic cells, including resting T-, B-, myeloid and dendritic cells, as well as some non-hematopoietic cells in the lung, heart and other organs (13). PD-L1 is up-regulated during T-cell activation and it has been shown to interact with PD-1 and CD80. The interaction of PD-1 with its ligands results in an inhibitory signal in activated T-cells that promotes apoptosis and anergy. Similarly, the interaction of PD-L1 and CD80 also delivers an inhibitory signal to activated T-cells (14). Chronic stimulation of PD-1 may result in T-cell “exhaustion” and the attenuation of immune responses (15). Tumors may exploit these checkpoint controls to inhibit anti-tumor immune responses.
CTLA-4 (CD152), a member of the immunoglobulin superfamily is recognized as another important negative-regulator of the immune response(16) . CTLA-4 is expressed on the surface of T-cells and exhibits immunosuppressive function, down-regulating T-cell activation in response to engagement of the T-cell receptor. Blockade of the interaction of CTLA-4 with its ligands, CD80 and CD86 using monoclonal antibodies can reverse inhibition of activation and stimulate T-cell proliferation that leads to enhanced anti-tumor immunity in preclinical models and in patients (17, 18). The combined blockade of both the PD-1 and CTLA-4 pathways in conjunction with an anti-tumor vaccine was recently reported (19). The combination of anti-PD-1, anti-PD-L1 and anti-CTLA-4 antibodies along with a gene-modified tumor cell vaccine increased the numbers of tumor infiltrating T-cells in a mouse B16 melanoma model. This strategy resulted in greater tumor rejection compared to the vaccine without the blockade of the two critical checkpoints.
In the present study, we demonstrated that in addition to its immunostimulatory effect, IL-15 also increased expression of the negative T-cell regulator, PD-1 and enhanced secretion of the immunosuppressive cytokine IL-10. Blockade of multiple negative immune checkpoints by the simultaneous administration of monoclonal antibodies directed against PD-L1 and CTLA-4 reduced expression of PD-1, secretion of IL-10, and enhanced IL-15-mediated immune responses and provided greater therapeutic benefit in a metastatic murine CT26 colon carcinoma model.
Materials and Methods
Mice
Female 6–8 week-old BALB/c mice were obtained from NCI-Frederick and were maintained in the National Cancer Institute (NCI) animal holding facility. Animal use adhered to NIH Laboratory Animal Care Guidelines and was approved by the NCI Animal Care and Use Committee.
Cell lines
The CT26 cell line, a N-nitro-N-methylurethane–induced BALB/c murine colon carcinoma cell line was purchased from American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640 (Invitrogen, Gaithersburg, MD) supplemented with 10% FCS, L-glutamine, sodium pyruvate, streptomycin, and penicillin (Invitrogen).
Animal model and assessment of treatment effect
Groups of 5 or 6 mice were injected with 2 × 105 CT26 tumor cells by the tail vein on day 0. Treatment was begun one day later (day 1). The mice each received 5 µg of mIL-15 (PeproTech, Rocky Hill, NJ) intraperitoneally daily, five times a week for three weeks. Along with mIL-15, some groups of animals also received anti-mouse-PD-L1 antibody (clone 9G2, Bio-express, West Lebanon, NH), anti-CTLA-4 antibody (clone UC10-4F10-11, Bio-express), or both anti-mouse-PD-L1 and anti-CTLA-4 antibodies or as a control, an irrelevant isotype IgG (Bio-express). The dose and schedule of these antibodies was 100 µg/injection administered twice a week for two weeks. Control mice received injections of phosphate buffered saline (PBS; Biofluids, Rockville, MD). On day 21, the mice were euthanized by CO2 inhalation, their lungs harvested and the tumor nodules counted. In brief, the lungs were stained by intratracheal administration of India ink, removed, fixed in Fekete’s solution, and metastatic nodules >1 mm counted under a dissection microscope. In a matched series of experiments, similar treatment groups were followed daily for survival. Mice were humanely sacrificed using prospective ACUC-approved criteria to generate survival curves.
In vivo CD8+ T-cell depletion
Groups of mice (N = 10) were injected intravenously with 2 × 105 CT26 cells on day 0 and then received mIL-15 as described above. Groups of animals received 200 µg of either rat-anti-mouse-CD8 (clone 2.43, Bio-express) or an isotype control rat IgG (clone LTF-2, Bio-express) intraperitonelly beginning on days 0 and 1, and then three times a week for three weeks. Animal survival was followed daily.
Flow cytometry and immunophenotyping
Expression of MHC class I on CT26 cells was analyzed by staining with FITC-anti-mouse-H-2Kd. Isotype matched IgG was utilized as a control. In order to compare the effects on the immunophenotype of CD8+ T-cells of the mIL-15 and antibody combination treatments, surface expression of PD-1 on the CD8+ T-cells as well as the CD8+CD44high cell populations was evaluated using flow cytometry. Spleen cells were stained with APC-conjugated anti-mouse-CD8 (53-6.7), PE-Cy5.5-conjugated anti-mouse-CD44 (IM-7) and PE-conjugated anti-mouse-PD-1 (RPM-4) or with an isotype control, and incubated for 30 min on ice. Fc receptor binding was minimized by pre-incubation of the cells with rat anti-mouse CD16/CD32. All fluorescent-labeled antibodies were obtained from BD Biosciences (San Jose, CA). Immunofluorescence analysis was performed on a FACSCalibur (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Measurement of IFNγ and IL-10 secretion
Splenic CD8+ T-cells of animals treated with mIL-15 alone or in combination with anti-PD-L1 and/or anti-CTLA-4 were isolated using a magnetic column (Miltenyi Biotec, Auburn, CA). In brief, each spleen was processed into a single cell suspension, incubated with anti-mouse-CD8α microbeads and sorted using a LS magnetic column according to the manufacturer’s instructions (Miltenyi Biotec). The CD8+ T-cell fractions were >94% pure as assessed by flow cytometry.
Cytokine secretion by splenic CD8+ T-cells from tumor-bearing animals was assayed by incubating CD8+ T-cells (2 × 105/well) on anti-mouse CD3 (clone 2C11, BD Biosciences) (10 µg/ml) coated plates with 1 µg/ml soluble anti-mouse CD28 (clone 37.51, BD Biosciences) for 72 hours and then collecting the supernatants. Splenic CD8+ T-cells co-cultured with the same concentrations of isotype-matched antibodies were set up to control for background. IFNγ and IL-10 were measured by an ELISA (R&D Systems, Minneapolis, MN). The cytokine concentration in the supernatants was interpolated from the linear portion of the ELISA standard curve.
The ability of naïve BALB/c splenic CD8+ T-cells to respond to mIL-15, anti-PD-L1 and anti-CTLA-4 ex-vivo was also examined. Naïve BALB/c splenic CD8+ T-cells were incubated with mIL-15 at a concentration of 20 ng/mL alone or in combination with anti-mouse-PD-L1 (10 ng/mL) and/or 10 ng/mL anti-mouse-CTLA-4 or 10ng/ml isotype IgG. Meanwhile, CD8+ T cells co-cultured with different concentrations of mIL-15 were included. These cells were incubated on anti-mouse-CD3-coated plates (10 µg/mL or 4 µg/mL as indicated) with 1 µg/mL soluble anti-mouse-CD28 for 72 hours and the concentration of IL-10 secretion was determined by ELISA, as described above. Co-culture with media without mIL-15 was used as a control.
Detection of intracellular IFNγ and cytotoxicity
Single cell suspensions of spleen cells were prepared from mice sacrificed on day 21 after CT26 tumor challenge and cultured with irradiated (100 Gy) CT26 tumor cells at a ratio of 50:1. Recombinant hIL-2 (Hoffmann-LaRoche Inc, Nutley, NJ) was added to a concentration of 10–15 U/mL. After 4 days, 1 × 106 effector cells were cultured with CT26 tumor cells at a ratio of 20:1 for 6 hours. Brefeldin A (10 µg/mL, Sigma, St. Louis, USA) was added to the cultures for the last 5 hours to prevent secretion of the intracellular protein. Cells from each group were first incubated with APC conjugated anti-mouse-CD8 Ab (clone 53-6.7, BD Biosciences) on ice for 30 minutes. The cells were fixed and permeabilized using a Cytotofix/Cytoperm kit as instructed by the manufacturer (BD Bioscience). Briefly, cells were resuspended in a fixing buffer for 20 minutes at room temperature and then washed with permeabilization buffer. Cells were then stained with anti-mouse-IFNγ-PE (clone XMG1.2, BD Bioscience) in permeabilization buffer at 4°C for 30 minutes. Cells were washed in permeabilization buffer, resuspended in FACS buffer, and analyzed on a FACScalibur flow cytometer (Becton Dickinson).
A cytotoxicity assay was performed as follows: effector cells from each of the treatment groups were cultured with 104 CT26 target cells/well in triplicate at varying E/T ratios and incubated at 37°C for 4 hours. Cytotoxic activity was measured by LDH release using the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI). The percent cytotoxicity was calculated as 100 × ([experimental release] − [effector spontaneous release] − [target spontaneous release])/([target maximum release] − [target spontaneous release]).
Statistical analysis
Kaplan–Meier non-parametric regression analysis was performed to assess the survival time of tumor-bearing animals with significance determined by the log-rank test using JMP statistical software (SAS Institute, Cary, NC). The comparison of cytokine secretion between groups was analyzed by using an unpaired Student’s t test. A value of p < 0.05 was considered significant.
Results
Characterization of tumor cells responding to mIL-15
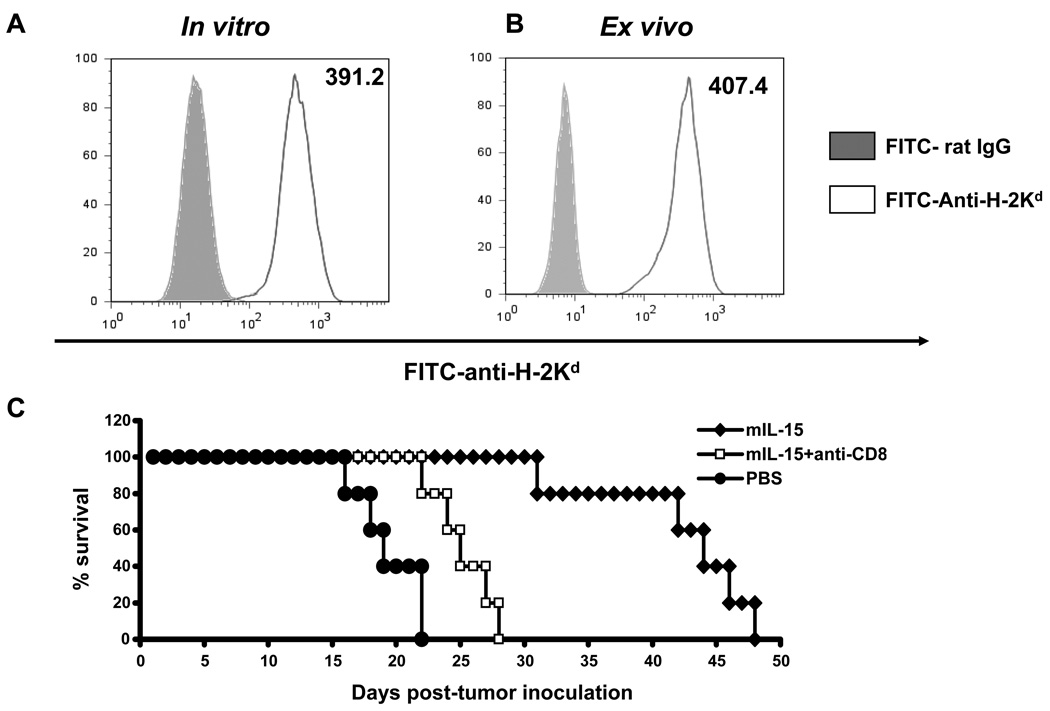
In order to examine the potential for CT26 tumors to serve as targets of CD8+ CTL we examined MHC class I expression on these cells. CT26 cells in vitro (Fig. 1A) or ex vivo from lung nodules of tumor-bearing animals (Fig. 1B) demonstrated high levels of MHC class I expression with mean fluorescence intensities (MFI) of 391.2 and 407.4, respectively suggesting that these tumors may be targeted by CD8+ cytolytic T-cells.
Figure 1. CD8+ T-cells are involved in the anti-tumor response to CT26 mediated by IL-15.
Expression of MHC class I on CT26 cells was analyzed by flow cytometry using FITC-anti-mouse-H-2Kd (open area). An antibody of the same isotype was used as a negative control (filled area). MHC class I mean fluorescence intensity (MFI) of CT26 cells either A; in vitro or B; obtained ex vivo from lung nodules of tumor-bearing animals treated with PBS is shown. C; Survival of CT26 tumor-bearing mice treated with mIL-15 with and without CD8+ T-cells depletion is shown. One day after tumor inoculation, mice received 5 µg/day of mIL-15 injected five times a week for three weeks (mIL-15, diamonds). One group of mice were depleted of CD8+ T-cells (mIL-15 + anti-CD8, squares) using anti-mouse-CD8 antibody administered on days 0 and 1, and then three time a week for three weeks. PBS treated animals served as controls (PBS, dots). The data represent three independent experiments.
To see if IL-15 could induce CD8+ CTL-mediated protection against tumor, mIL-15 was administered beginning one day after intravenous injection of 2 × 105 CT26 tumor cells and the mice followed for survival (Fig. 1C). Animals treated with mIL-15 demonstrated longer survivals compared to control mice receiving PBS control (P<0.05). The median survival of mIL-15 treated mice was 44 days while those treated with PBS survived a median of 19 days. To determine whether CD8+ T-cells played a role in this effect, we used anti-CD8 antibody to deplete CD8+ T-cells. The survival advantage afforded by mIL-15 was largely abrogated by the depletion of CD8+ T-cells indicating that they play a major role in protection from tumor in this model (Fig. 1C). Animals administered IL-15 along with an IgG control exhibited no significant difference in survival when compared to IL-15 alone (data not shown).
IL-15 increased PD-1 expression on CD8+ T-cells in vivo
As increased PD-1 expression on CD8+ T-cells has been shown to attenuate immune responses (9–11), we examined the effects of mIL-15 on the expression of PD-1 on CD8+ T-cells from tumor-bearing animals. Mice treated with mIL-15 showed increased PD-1 expression on CD8+ T-cells (Fig. 2A and 2C, compared to PBS group, P<0.05) as well as on CD8+CD44high cells in the spleen (Fig. 2B and 2D, compared to PBS group, P<0.05). When anti-PD-L1 was administered, PD-1 expression decreased compared to that seen with mIL-15 treatment alone. This was seen in both the CD8+ T-cells and the CD8+CD44high T-cell population (Fig. 2C and 2D, P<0.05). Anti-CTLA-4 when used alone did not reduce PD-1 expression on CD8+ T cells (data not shown), however when anti-CTLA-4 and anti-PD-L1 were combined a further decrease in the expression of PD-1, most notably in the CD8+CD44high population was noted (Fig. 2D, P<0.05).
Figure 2. Negative immune checkpoint blockade combined with mIL-15 down-regulated surface PD-1 expression on CD8+ T-cells and CD8+CD44high T-cells that had been mediated by mIL-15.
Flow cytometry was used to analyze the PD-1 expression on splenic CD8+ T-cells as well as on the CD8+CD44high population from the CT26-bearing mice following treatment with PBS, mIL-15, mIL-15 and anti-PD-L1, or mIL-15, anti-PD-L1 and anti-CTLA-4 on day 10 after CT26 tumor inoculation. Surface expression of PD-1 on CD8+ T-cells was detected using APC-anti-CD8 and PE-anti-PD-1 (open area, Fig. 2A). The CD8+CD44high population was stained with APC-anti-CD8 and PE-Cy5.5-anti-CD44 antibodies (open area, Fig. 2B). An isotype matched IgG was used as a negative control (filled area). All the samples were analyzed on gated CD8+ T-cells or CD8+CD44high cells as indicated. MFI is shown. The figure is representative of two independent experiments wherein each group contained three to five animals. Statistical analysis was performed based on PD-1 expression on CD8+ T cells (Fig. 2C) and CD8+ CD44high population (Fig. 2D). * P<0.05 and ** P<0.01.
IL-15 modulation of cytokine secretion and the effect of checkpoint blockade
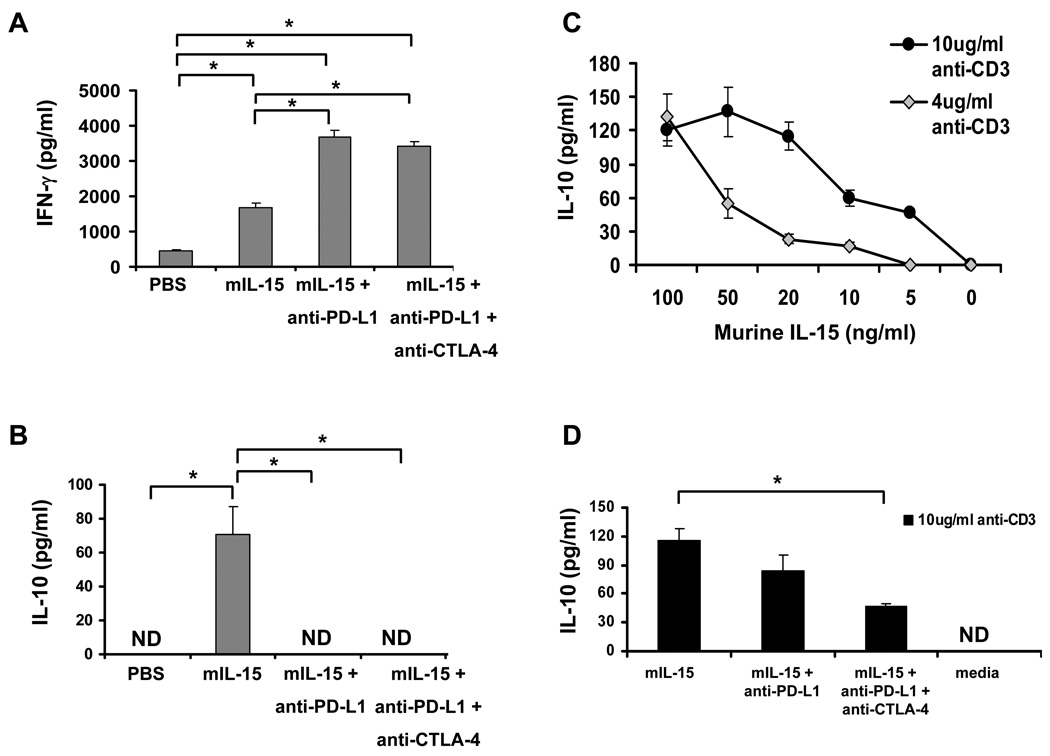
On day 14 after tumor inoculation, CD8+ T-cells obtained from the spleens of mice in the different treatment groups were stimulated with anti-mouse-CD3 and anti-mouse-CD28, and IFNγ and IL-10 secretion were measured. CD8+ T-cells isolated from animals treated with mIL-15 demonstrated increased IFNγ secretion compared to the PBS control group (P<0.05) (Fig. 3A). The addition of anti-PD-L1, or the combination of anti-PD-L1 and anti-CTLA-4 to treatment with mIL-15 resulted in further increases in IFNγ secretion.
Figure 3. The combination of anti-PD-L1 and anti-CTLA-4 antibodies that block negative checkpoints enhanced mIL-15 induced IFNγ secretion as well as down-regulated IL-10 secretion by CD8+ T-cells.
A and B; Splenic CD8+ T-cells obtained ex vivo from CT26 tumor-bearing mice treated with PBS, mIL-15, mIL-15 and anti-PD-L1, or mIL-15 combined with anti-PD-L1 and anti-CTLA-4 were stimulated with 10 µg/ml plate-bound anti-CD3 plus 1 µg/mL of a soluble anti-CD28 antibody and the supernatants were collected after 72 hours. IFNγ and IL-10 secretion was quantitated by ELISA. Cells were co-cultured with the same concentration of isotype-matched antibodies as a background control; *P<0.05; C, Naïve splenic CD8+ T-cells were stimulated with anti-CD3 (10 µg/mL, black dots; 4 µg/mL, grey diamonds) plus 1 µg/mL of a soluble anti-CD28 antibody in the presence of different amounts of mIL-15 as indicated. IL-10 secretion was quantitated by ELISA. Cells cultured with the same concentration of an isotype control antibody were set up as controls. D, anti-PD-L1 and anti-CTLA-4 (10 ng/mL) were added with 20 ng/mL mIL-15 present in wells at the same stimulation conditions as above (black bars). IL-10 secretion in the supernatants was quantitated by ELISA. Media alone with no mIL-15 was set up as a control, *P<0.05. ND: non-detectable (>16pg/ml). The data is representative of three independent experiments.
Splenic CD8+ T-cells isolated from animals treated with mIL-15 also secreted higher levels of IL-10 compared to those isolated from PBS treated animals (P<0.05) (Fig. 3B). Co-administration of antibodies to PD-L1 and CTLA-4 in the mIL-15 treated mice inhibited the secretion of IL-10 below detectable levels (<16 pg/ml). Anti-CTLA-4 alone had no significant effect on either IFNγ or IL-10 secretion compared to PBS treated control animals (data not shown).
To clarify the relationship between IL-15 and IL-10 secretion, splenic CD8+ T-cells were isolated from naïve mice and stimulated with anti-mouse-CD3 and anti-mouse-CD28 as described. Different concentrations of mIL-15 were provided to the cultures, supernatants were collected and IL-10 was quantitated (Fig. 3C). There was a dose-dependent relationship between IL-10 secretion and the concentration of mIL-15. Moreover, if both anti-PD-L1 and anti-CTLA-4 antibodies were added to the culture system along with mIL-15, they significantly reduced IL-10 secretion by CD8+ T-cells compared to mIL-15 alone (P<0.05, Fig. 3D) No statistical difference was observed between mIL-15 (20ng/ml) alone treated cells and mIL-15 (20ng/ml) combined with isotype IgGs (10ng/ml) treated cells (data not shown).
IL-15 induced CD8+ T-cell CT26 cell-specific IFNγ secretion and lytic activity
Tumor-specific IFNγ secretion was detected by intracellular staining of splenic CD8+ T-cells. There was no obvious IFNγ secretion by PBS treated CD8+ T-cells, but mIL-15 treatment as well as mIL-15 combined with anti-PD-L1 and anti-CTLA-4 treated tumor-bearing animals showed a significantly higher number of IFNγ secreting CD8+ T-cells in the spleen (Fig. 4A) compared to the PBS control group (P<0.01). The ability of CD8+ T-cells isolated from tumor-bearing mice to induce CT-26-specific cell lysis was examined (Fig. 4B). CD8+ T-cells isolated from PBS treated animals did not demonstrate any lytic activity against CT26 cells, whereas CD8+ T-cells isolated from mice treated with mIL-15 alone or in combination with anti-PD-L1 and/or anti-CTLA-4 showed increased CD8+ T-cell lytic activity against CT26 cells in an effector:tumor cell ratio-dependent manner.
Figure 4. mIL-15 induced tumor-specific IFNγ secretion and lytic activity.
On day 21, CT26-bearing mouse splenocytes were harvested and incubated with irradiated CT26 cells at a ratio of 50:1. Cultures were incubated for 4 days. A, 1 × 106 effector cells were cultured with CT26-tumor cells at a ratio of 20:1 for 6 h. Brefeldin A (10 µg/mL) was added to the cultures for the last 5 h. Cells from each group were first stained with APC conjugated anti-CD8, then intracellularly stained with PE-anti-IFNγ. Five hundred thousand events were collected for each sample by a flow cytometer. B; tumor-specific CD8+ T-cell target cell killing activities were detected by a non-radioactive cytotoxicity assay. PBS (open triangles), mIL-15 (open squares), mIL-15 + anti-PD-L1 (filled triangles) and mIL-15 + anti-PD-L1 + anti-CTLA4 (filled diamonds) groups are shown in panel B. The figures are representative of three independent experiments.
IL-15 reduced the number of tumor lung nodules
Treatment with IL-15 significantly reduced the number of tumor nodules in the lungs of mice injected with CT26 cells compared to animals receiving PBS, P<0.05 (Fig. 5A). Co-administration of anti-PD-L1 and mIL-15 also lead to a reduction of the number of tumor nodules compared to PBS treatment, P<0.05 (Fig. 5A). The combination of mIL-15, anti-PD-L1 and anti-CTLA-4 led to a further decrease in the number of pulmonary metastases relative to that observed in the mIL-15 treated group (P < 0.05). Representative lungs are shown in Fig. 5B.
Figure 5. IL-15 treatment reduced the number of tumor nodules in the lungs.
A; On day 21, pulmonary metastases were counted; each group included 3 to 5 mice, *P<0.05. The data represents three independent experiments. B; Representative lung samples showing pulmonary metastases at day 21.
IL-15 increased the survival of CT26 tumor-bearing animals
We next examined if mIL-15 in combination with anti-PD-L1 and anti-CTLA-4 could improve the survival of mice with metastatic CT26 tumors (Fig. 6). Mice treated with PBS survived a median of 21 days (range, 19–23 days). Animals treated with anti-CTLA-4 did not show a survival advantage over animals treated with PBS (P = 0.075). In contrast, animals treated with anti-PD-L1 alone did exhibit a survival advantage over those treated with PBS (P<0.05); however, when compared to the mIL-15 treated group, their survival was significantly less (P<0.05). Mice receiving mIL-15 demonstrated a significant prolongation of survival when compared with the PBS treated group (P<0.01). Combining mIL-15 with an irrelevant IgG provided no survival advantage compared to mIL-15 alone (data not shown). Combining mIL-15 and anti-CTLA-4 we found that there was no survival advantage compared to treatment with mIL-15 alone (median survival- 46 vs. 45 days, P = 0.399). However, when mIL-15 was combined with anti-PD-L1, there was a significant survival benefit with this addition over mIL-15 alone, (median survival- 59 vs. 45 days, P<0.01). The addition of anti-CTLA-4 to mIL-15 and anti-PD-L1 treatment gave the greatest survival benefit compared to mIL-15 treatment alone or treatment with mIL-15 in combination with anti-PD-L1 alone, (median survival- 74 vs. 59 days, P<0.05).
Figure 6. IL-15 treatment with blockade of multiple inhibitory checkpoints prolonged the survival of CT26 tumor-bearing animals.
Kaplan-Meier survival by treatment illustrating tumor bearing animal survival after the different treatments. PBS control (open diamonds), anti-CTLA-4 (open squares), mIL-15 and anti-CTLA-4 (grey squares), mIL-15 (black triangles), anti-PD-L1 (black diamond), mIL-15 and anti-PD-L1 (grey diamonds) and mIL-15 combined with anti-PD-L1 and anti-CTLA-4 (grey circles). The data represent three independent experiments.
Discussion
Many immunotherapeutic strategies directed against cancer focus on enhancing effector cell function in an attempt to increase tumor killing. Despite attempts to augment anti-tumor immunity by a variety of approaches, the enthusiasm for many of these approaches has been diminished by the fact that the generation of measurable increases in anti-tumor effector responses have not often correlated with clinical responses. It is now recognized that at least part of this failure is attributable to the presence of numerous naturally occurring negative regulatory pathways that act to limit immunological responses.
IL-15 is a cytokine that is important in the development and homeostasis of several lymphocyte populations including NK, NK/T and CD8+ T-cells (20–25). In addition, IL-15 can also stimulate proliferation; enhance cytotoxicity and up-regulate production of IFNγ by NK and T-cells (20, 21). In the current study, we showed that IL-15 up-regulated IFNγ secretion by CD8+ T-cells and prolonged the survival of tumor-bearing animals. However, IL-15 as monotherapy also suffers from the potential limitations imposed by up-regulation of negative regulatory checkpoints. In this study, we showed that treatment with IL-15 lead to increased expression of PD-1 on CD8+ T-cells, most notably on CD8+CD44high memory phenotype T-cells. Further, we demonstrated that IL-15 also induced the production of IL-10, a negative regulatory cytokine (26), suggesting that IL-15 may work as a "double-edged sword.” In an attempt to address this issue, we studied the effects of IL-15 combined with the removal of a number of these negative immune regulatory checkpoints.
PD-1 is a surface molecule that is over-expressed on apoptotic cells (27). It moved into the immunologists’ sights as a molecule that is also expressed on ‘exhausted’ T-cells from chronic virus-infected patients and in patients with advanced cancer that contributed to poor T-cell proliferative capacity and incompetent effector function (28–30). One study demonstrated that the numbers of T-cells expressing PD-1 were increased in patients with high-risk renal cell carcinoma (31). Patients with increased numbers of PD-1+ T-cells were at greater risk of cancer-specific death compared with patients whose T-cells exhibited a low expression of PD-1. In addition, one of the PD-1’s ligands, PD-L1 has also become a focus of interest due to its broad expression and negative-regulatory function. Blockade of the PD-1/PD-L1 negative checkpoint has been demonstrated to restore effector CD8+ T-cell responses in chronic infections such as HIV, hepatitis C and lymphocytic choriomeningitis virus (32–34). In addition, monoclonal antibodies to PD-1 have produced tumor responses in clinical trials in patients with advanced cancer (35). In this study we utilized an anti-PD-L1 antibody to block the PD-1/PD-L1 pathway in a murine metastatic colon cancer model. We found that administration of anti-PD-L1 antibody was able to reduce PD-1 expression on CD8+ T-cells following treatment with IL-15 in vivo. Mice treated with the combination of IL-15 with anti-PD-L1 also had CD8+ T-cells that secreted lesser amounts of the immune inhibitory cytokine, IL-10. Moreover, this combination also enhanced IFNγ secretion and prolonged the survival of tumor-bearing animals.
In order to examine if we could further increase the therapeutic benefit provided by eliminating negative checkpoints, we also inhibited CTLA-4. Antibody-mediated CTLA-4 blockade has been shown to improve the outcome of tumor-bearing animals and patients with advanced malignant melanoma (18, 36). Blockade of CTLA-4 has also been shown to inhibit the secretion of IL-10 (37). The removal of CTLA-4 function, in our system, when used alone, compared to PBS, or in combination with IL-15, compared to IL-15 alone, did not lead to a statistically significant survival advantage. However, when used in combination with PD-L1 inhibition and IL-15, showed a significantly prolonged survival when compared to any of these agents used individually or in single combination with IL-15. Both PD-1 and CTLA-4 ligation inhibit Akt activation and block T-cell responses by targeting distinct signaling molecules (38). PD-1 engagement inhibits an upstream proximal step blocking phosphatidylinositol-3-kinase (PI3K) activation. In contrast, signaling by CTLA-4 preserves PI3K activity, instead it functions through its binding to phosphatase PP2A leading to the inhibition of Akt phosphorylation and ultimately the inhibition of T-cell activation (38). By blocking both PD-L1 and CTLA-4 we are able to target two pathways leading to Akt activation, the result being a likely synergistic response allowing effective T-cell activation and ultimately an increased therapeutic effect. A recent study Curran et al, found that the combination of CTLA-4 and PD-1/PD-L1 blockade may also have effects on the tumor microenvironment (19). They showed that this combination resulted in increased tumor infiltrating T-cells with a reduction in the T-regulatory cell populations within the tumor. Whether immune-stimulation through IL-15 in combination CTLA-4 and PD-1/PD-L1 blockade leads to similar changes in tumor infiltrating cells is yet to be determined. While Curran and colleagues also used a whole cell anti-tumor vaccine, their results in terms of increased animal survival were similar to ours highlighting the benefit of multiple immune checkpoints removal.
In the present study, we found that to achieve an effective immune response to a tumor not only is activation of the immune system by IL-15 required but also the simultaneous inhibition of two independent negative immune regulatory checkpoints (CTLA-4 and PD-l/PD-Ll) that act to attenuate the immune response. While IL-15 effectively activated the immune system against tumor, it also activated negative regulators such as IL-10 and PD-1, potentially limiting its therapeutic efficacy. By using a strategy that combines IL-15 with the simultaneous blockade of both CTLA-4 and PD-1 pathways, we reduced the expression of IL-10 and PD-1 allowing a stronger immune response and an increased animal survival.
Translational Relevance
Interleukin-15 has recently entered clinical trials for treatment of cancer. IL-15 stimulates natural killer cells, and CD8+ effector and memory T-cell immunity. However, IL-15 as monotherapy may not be optimal. We found that IL-15 also increased the expression of the PD-1 inhibitory molecule on the surface of CD8+ T-cells and also stimulated the secretion of the immunosuppressive cytokine, IL-10. To maximize the clinical efficacy of IL-15, the removal of immune inhibitory checkpoints may be necessary. In the present study we accomplished this through the simultaneous use of anti-PD-L1 and anti-CTLA-4 antibodies. We show that blockade antibodies combined with IL-15 lead to decreased expression of PD-1, reduced IL-10 secretion, and increased survival of mice with metastatic CT26 colon cancer. The present study supports a clinical trial combining IL-15 immunotherapy with the simultaneous blockade of CTLA-4 and the PD-1 T-cell inhibitory regulators.
Acknowledgement
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviations
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- ELISA
enzyme-linked immunosorbent assay
- IL-10
interleukin-10
- mIL-15
murine interleukin-15
- IL-15Rα
interleukin-15 receptor-alpha
- IFNγ
interferon-gamma
- MHC
major histocompatability complex
- MFI
mean fluorescence intensity
- PD-1
programmed death-1
- PD-L1
programmed death ligand-1
Footnotes
Disclosure of potential conflicts of Interest
The authors disclosed no potential conflicts of Interest.
References
- 1.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 2.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15R alpha recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 3.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett. 2010;127:85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diab A, Cohen AD, Alpdogan O, Perales MA. IL-15: targeting CD8+ T cells for immunotherapy. Cytotherapy. 2005;7:23–35. doi: 10.1080/14653240510018037. [DOI] [PubMed] [Google Scholar]
- 5.Carson WE, Fehniger TA, Haldar S, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steel JC, Ramlogan CA, Yu P, et al. Interleukin-15 and its receptor augment dendritic cell vaccination against the neu oncogene through the induction of antibodies partially independent of CD4 help. Cancer Res. 2010;70:1072–1081. doi: 10.1158/0008-5472.CAN-09-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi H, Dubois S, Sato N, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105:721–727. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Yao Z, Dubois S, Ju W, Müller JR, Waldmann TA. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci U S A. 2009;106:7513–7518. doi: 10.1073/pnas.0902637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD1 deficient mice: implication of PD-1 as a negative regulator for T, B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 11.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodig N, Ryan T, Alie JA, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 14.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T Cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Lenschow Deborah J., Walunas Theresa L., Bluestone Jeffrey A. CD28/B7 system of T cells costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 17.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Jun 14; doi: 10.1056/NEJMoa1003466. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fehniger TA, Caligiuri MA. IL-15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 21.Carson WE, Giri JG, Lindemann MJ, et al. IL-15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15 deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Judge A, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 Controls both Proliferation and Survival of a Subset of Memory-Phenotype CD8+ T Cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 25.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore KW, Malefyt RW, Coffman RL, O'Garra A. Interleukin -10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 27.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 31.Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 32.Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1: PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol. 2007;178:2714–2720. doi: 10.4049/jimmunol.178.5.2714. [DOI] [PubMed] [Google Scholar]
- 33.Lukens JR, Cruise MW, Lassen MG, Hahn YS. Blockade of PD-1/B7-H1 interaction restores effector CD8+ T cell responses in a hepatitis C virus core murine model. J Immunol. 2008;180:4875–4884. doi: 10.4049/jimmunol.180.7.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 35.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korman A, Yellin M, Keler T. Tumor immunotherapy: Preclinical and clinical activity of anti-CTLA4 antibodies. Curr Opin Invest Drugs. 2005;6:582–591. [PubMed] [Google Scholar]
- 37.Jovasevic VM, Gorelik L, Bluestone JA, Mokyr MB. Importance of IL-10 for CTLA-4 mediated inhibition of tumor-eradicating immunity. J Immunol. 2004;172:1449–1454. doi: 10.4049/jimmunol.172.3.1449. [DOI] [PubMed] [Google Scholar]
- 38.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA4 and PD1 receptors inhibit T- cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9545–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]