Abstract
The 1-aminocyclopropane-1-carboxylate oxidase gene (ACO1) was upregulated in rice (Oryza sativa L.) phyAphyBphyC mutants lacking any phytochrome and containing the GCC box element, a binding site for rice ethylene-responsive element binding protein 1 (OsEREBP1), in its promoter region. Since the OsEREBP1-like gene EBL1 (OsEREBP1-LIKE 1) was significantly downregulated in phyAphyBphyC mutants, EBL1 was suspected to repress ACO1 expression in wild-type plants. However, ACO1 was downregulated in EBL1 RNA interference plants, and the total length of these plants was slightly shorter than that of wild-type plants. This study shows that EBL1 is positively regulated by phytochrome B and associated with ACO1 upregulation.
Keywords: ACO1, Ethylene-responsive element binding protein, Internode elongation, Phytochrome, Rice
Introduction
Red and far-red light-absorbing phytochromes are major photoreceptors that regulate the expression of light-responsive genes, and thus, influence many photomorphogenic events in higher plants. Rice (Oryza sativa L.) contains three phytochrome genes, PHYA, PHYB, and PHYC, and phyAphyBphyC triple mutants have been isolated and characterized (Takano et al. 2009). Although internodes start to elongate at the reproductive stage, phyAphyBphyC mutants have morphological changes and elongated internodes, even at the vegetative stage, indicating that phytochromes play an important role in inhibition of internode elongation at the vegetative stage in wild-type plants.
1-Aminocyclopropane-1-carboxylate (ACC) oxidase (EC 1.14.17.4) catalyzes the final step of ethylene biosynthesis in which the ACC precursor is converted to ethylene. A predominant ACC oxidase gene, ACC oxidase (ACO1), has been isolated from submerged internodes of deepwater rice, and high transcript levels are associated with internode elongation during submergence (Mekhedov and Kende 1996). We have recently shown that ACO1 has an effect on internode elongation at the heading stage in rice (Iwamoto et al. 2010).
Expression of genes for ethylene-responsive element-binding proteins/factors (EREBPs/ERFs) inhibit growth (Hu et al. 2008; Wilson et al. 1996; Xu et al. 2006). We found that the upstream region of ACO1 (−1,625 to +130, cDNA start site of ACO1 being designated as +1), having promoter activity (Iwamoto et al. 2010), included a GCC box element (AGCCGCC), which is a binding site for EREBPs/ERFs. It has been reported that rice EREBP gene, OsEREBP1, binds to a GCC box element in rice (Cheong et al. 2003).
In this study, we examined an OsEREBP1-like gene, EBL1 (Os E RE B P1-L IKE 1), selected as a candidate gene associated with the regulation of ACO1 expression. RNA interference (RNAi) transgenic plants for EBL1 were generated to elucidate the relevance of EBL1 to ACO1 expression.
Materials and methods
Plant materials and growth conditions
Rice (O. sativa L. cv. Nipponbare) and its phytochrome-deficient mutants, phyA-2, phyB-1, phyA-2phyC-1, and phyA-2phyB-1phyC-1, were used in this study. Seedlings were maintained in a growth chamber at 28°C. White light (160 μmol m−2 s−1) was provided by cool-white fluorescent lamps and monochromatic red light (55 μmol m−2 s−1) was provided by a light-emitting diode panel (Model LED-R; Eyela). EBL1 RNAi transgenic plants were grown into mature plants in an isolated glasshouse at 27°C.
Plant transformation
An EBL1 fragment (441 bp) was amplified from the EBL1 cDNA clone (002-163-C07), provided by the Rice Genome Resource Center, National Institute of Agrobiological Sciences, Japan, by polymerase chain reaction (PCR) using primers EBL1Ri-F (5′-CAC CAA GTC GAT GCC GAC GAC GAG-3′) and EBL1Ri-R (5′-TGC GAT GAA CTC ATG ACT GAA CAG-3′). PCR products were inserted in the pENTR/D-TOPO cloning vector (Invitrogen) and then in the pANDA vector (Miki and Shimamoto 2004) using the pENTR Directional TOPO Cloning Kit (Invitrogen) and the Gateway LR Clonase Enzyme Mix (Invitrogen), respectively, according to manufacturer’s instructions. The resulting plasmids were introduced into Agrobacterium tumefaciens EHA105 (provided by Dr. E. Hood, ProdiGene), which was then used for transformation of rice plants according to methods of Hiei et al. (1994) and Toki (1997).
Reverse transcription-PCR (RT-PCR)
Total RNA was extracted from leaf blades using the RNeasy Mini Kit (Qiagen) according to manufacturer’s instructions. RT-PCR was performed using one-step reactions (Superscript One-Step RT-PCR system; Invitrogen). Total RNA (50 ng) was used as a template and RT-PCR amplification reaction mixtures contained 0.2 mM of each dNTP, 1.2 mM MgSO4, and 0.4 μM of each primer. The primer sets were ACO1-F (5′-CTG CGG CGA TGG AGC AGC TGG A-3′) and ACO1-R (5′-CAC GAA CTT GGG GTA CGC CAC GA-3′) for ACO1, OsEREBP1-F (5′-GAC GAT GAC GTC GTC GAG ATC AAG-3′) and OsEREBP1-R (5′-TGC GAG GAT CTC TGA TTT CAG CAG-3′) for OsEREBP1 (Os02g0782700), AF190770-F (5′-AGT CCG ACG CCG ACG AGG CCA AG-3′) and AF190770-R (5′-CAC CTT TGC GAG GAT CTC TGA TTT CC-3′) for Os06g0194000, AF364176-F (5′-CTT TGA GGC CGA CTT CCG CGA G-3′) and AF364176-R (5′-CAT GTT GGA CAA GCT TTG TAG TCA AC-3′) for Os09g0434500, EBL1-F (5′-GAT CAG CGA CGA CGA GGA CTT CGA G-3′) and EBL1-R (5′-CTT GAT CGA TCG ATC GCC TCA CCA TG-3′) for EBL1, and nucleotides 4,158–4,177 and 4,374–4,355 having accession number AF184280 for the polyubiquitin gene (RUBQ2). PCR amplification was performed in a DNA thermal cycler (GeneAmp PCR system 9700, Applied Biosystems). Appropriate number of PCR cycles for each gene was determined prior to RT-PCR experiments. PCR products were electrophoresed on a 1.4% (w/v) agarose gel and visualized using ethidium bromide staining.
Results
Comparison of deduced amino acid sequences of OsEREBP1 and OsEREBP1-like proteins
A homology search was performed to detect genes having a high similarity to OsEREBP1 using the cDNA sequence of OsEREBP1 as a query. We detected three OsEREBP1-like genes (Os06g0194000, Os09g0434500, and Os10g0390800), and expectation values indicating similarity to OsEREBP1 were 9e-62, 1e-17, and 1e-08 for Os06g0194000, Os09g0434500, and Os10g0390800, respectively. A multiple alignment of deduced amino acid sequences of OsEREBP1 and the three OsEREBP1-like proteins indicated that two domains in the N-terminal region (MCGGAI and EDFEADFEEFE) and an EREBP/AP2 DNA binding domain were highly conserved among the proteins (Fig. 1).
Fig. 1.
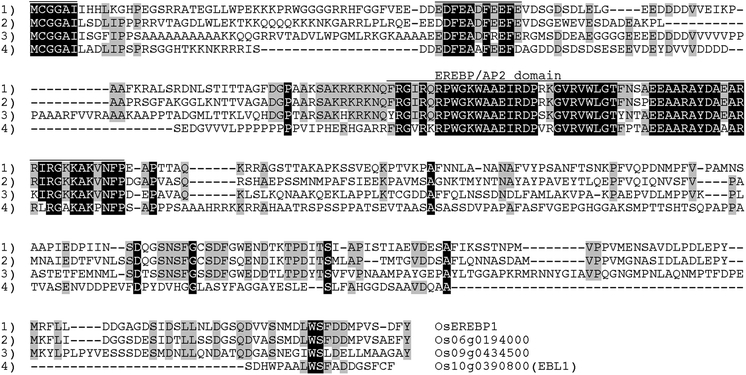
Alignment of the deduced amino acid sequences of OsEREBP1 and OsEREBP1-like proteins. The amino acids conserved among the four proteins are indicated in black and those conserved among three of the four proteins are indicated in gray. Lines above the amino acid sequences indicate the conserved domains among the four proteins
Expression of EREBP genes in phytochrome-deficient mutants
ACO1 is upregulated in phyAphyBphyC mutants (Takano et al. 2009). Expression analysis of OsEREBP1 and the three OsEREBP1-like genes was performed to confirm whether any changes were present in their expression in phyAphyBphyC mutants. It was shown that transcript levels of Os10g0390800, named EBL1, were significantly decreased in phyAphyBphyC mutants, while those of OsEREBP1, Os06g0194000, and Os09g0434500 were nearly identical between phyAphyBphyC and phyAphyC mutants (Fig. 2a). phyB is the main photoreceptor responding to red light in rice (Takano et al. 2005). Under continuous red or white light, phyB mutants demonstrated significant downregulation of EBL1expression and upregulation of ACO1 expression (Fig. 2b). On the other hand, EBL1 and ACO1 transcript levels in phyA mutants were similar to those in wild-type plants under these conditions.
Fig. 2.
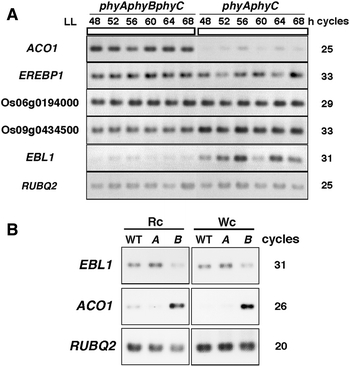
Changes in EBL1 expression in phytochrome-deficient mutants. a Expression of OsEREBP1 and OsEREBP1-like genes in phyAphyBphyC or phyAphyC mutants. Seedlings grown under light/dark conditions were transferred to continuous light (LL) conditions at the end of the dark period, and leaf blades were sampled every 4 h. The total number of amplification cycles needed to detect RT-PCR products is indicated. RT-PCR amplification of RUBQ2 was performed as a loading control. b Expression of EBL1 and ACO1 in wild-type (WT), phyA (A), or phyB (B) mutants under continuous red (Rc) or white light (Wc) conditions. The total number of amplification cycles needed to detect RT-PCR products is indicated. RT-PCR amplification of RUBQ2 was performed as a loading control
Alteration of ACO1 expression and internode elongation in EBL1 RNAi plants
From the results of Fig. 2, we deduced that EBL1 acted as a repressor to regulate ACO1 expression and that EBL1 downregulation led to high ACO1 transcript levels. To elucidate the role of EBL1 in the regulation of ACO1 expression, EBL1 RNAi transgenic plants were produced and used. All five independent lines of EBL1 RNAi plants had significantly decreased EBL1 transcript levels (Fig. 3a). However, ACO1 was unexpectedly downregulated in all of the EBL1 RNAi plants. On the other hand, OsEREBP1, Os06g0194000, and Os09g0434500 transcript levels were nearly identical between wild-type and EBL1 RNAi plants. EBL1 RNAi plants had alterations in internode elongation, and the total length of EBL1 RNAi plants was slightly shorter than that of wild-type plants (Fig. 3b). In addition, short elongation of the uppermost internode (first internode) caused panicle enclosure in the EBL1 RNAi plants (Fig. 3c).
Fig. 3.
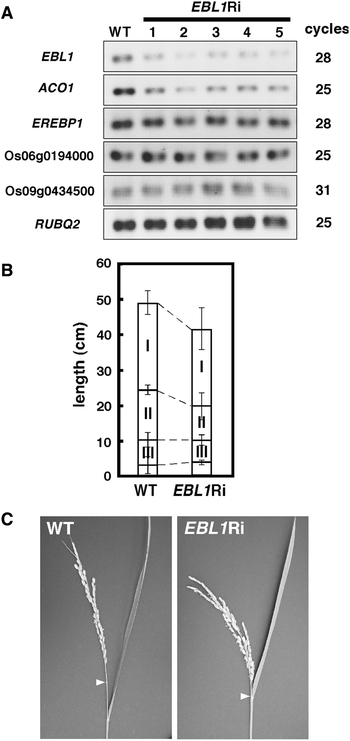
Characterization of EBL1 RNAi transgenic plants. a Expression of EBL1, ACO1, OsEREBP1, Os06g0194000, and Os09g043500 in leaf blades of wild-type (WT) and five independent lines of EBL1 RNAi plants (EBL1Ri). Plants were grown under light/dark conditions (16 h of light and 8 h of dark). The total number of amplification cycles needed to detect RT-PCR products is indicated. RT-PCR amplification of RUBQ2 was performed as a loading control. b Comparison between elongated internodes of wild-type (WT) and EBL1 RNAi plants (EBL1Ri). I to III indicate the first to third internodes, respectively. Vertical bars indicate SE of the mean (n = 4). c Panicles of wild-type (WT) and EBL1 RNAi plants (EBL1Ri). Arrowheads indicate panicle nodes
Discussion
In this study, we showed that EBL1 was a phytochrome-regulated gene that contributed to the regulation of ACO1 expression. Since EBL1 was downregulated in mutants lacking functional phyB (Fig. 2), EBL1 expression was mainly regulated by phyB. It has been reported that EREBP/ERF genes have important roles in the regulation of internode elongation in deepwater rice plants. SUBMERGENCE 1A-1 confers submergence tolerance and inhibits elongation growth during submergence (Xu et al. 2006) and SNORKEL1 and 2 contribute to flooding-induced internode elongation and promote elongation during submergence (Hattori et al. 2009). To the best of our knowledge, this is the first report on an EREBP/ERF gene associated with internode elongation at the reproductive stage in paddy field rice plants. EBL1 RNAi transgenic plants incurred panicle enclosure because of short elongation of their first internodes (Fig. 3c). Since ACO1 has effects on internode elongation (Iwamoto et al. 2010), EBL1 is believed to be associated with internode elongation by regulating ACO1 expression.
EREBPs/ERFs act as transcriptional activators or repressors of GCC box-mediated gene expression (Fujimoto et al. 2000). Prior to expression analysis using EBL1 RNAi plants, we assumed that EBL1 functioned as a repressor for ACO1 downregulation in wild-type plants; however, EBL1 contributed to ACO1 upregulation, similar to an activator (Fig. 3a). These results indicate that two mechanisms regulate ACO1 expression under the control of phyB: EBL1-dependent and -independent pathways (Fig. 4). The EBL1-dependent pathway plays a role in ACO1 upregulation. The total length of EBL1 RNAi plants was slightly shorter than that of wild-type plants (Fig. 3b), similar to ACO1-deficient plants (Iwamoto et al. 2010). On the other hand, the EBL1-independent pathway is necessary for ACO1 downregulation, and unknown transcriptional factor(s) are believed to regulate ACO1 expression. The EBL1-dependent pathway has a small effect on ACO1 expression compared to the EBL1-independent pathway, since ACO1 transcript levels in plants lacking phyB were higher than those in plants with phyB (Fig. 2). Further experiments are required to understand the transcriptional factor(s) associated with ACO1 downregulation under the control of phyB.
Fig. 4.
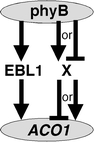
Model for phyB-regulated ACO1 expression in rice. EBL1 contributes to ACO1 upregulation under the positive control of phyB. X represents an unknown transcription factor. The two possibilities of X-mediated ACO1 regulation are as follows: X downregulates ACO1 under positive control of phyB or upregulates ACO1 under negative control of phyB
Acknowledgments
We thank Dr. D. Miki (Nara Institute of Science and Technology, Japan) for providing the pANDA vector. The EBL1 cDNA clone (002-163-C07) was created in the Rice Genome Project and obtained from the Rice Genome Resource Center, National Institute of Agrobiological Sciences, Japan. We are grateful to S. Ohashi for technical assistance. This work was supported in part by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Cheong YH, Moon BC, Kim JK, et al. BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol. 2003;132:1961–1972. doi: 10.1104/pp.103.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell. 2000;12:393–404. doi: 10.1105/tpc.12.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, Matsuoka M, Mori H, Ashikari M. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–1030. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994;6:271–282. doi: 10.1046/j.1365-313X.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhao L, Chong K, Wang T. Overexpression of OsERF1, a novel rice ERF gene, up-regulates ethylene-responsive genes expression besides affects growth and development in Arabidopsis. J Plant Physiol. 2008;165:1717–1725. doi: 10.1016/j.jplph.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Baba-Kasai A, Kiyota S, Hara N, Takano M. ACO1, a gene for aminocyclopropane-1-carboxylate oxidase: effects on internode elongation at the heading stage in rice. Plant Cell Environ. 2010;33:805–815. doi: 10.1111/j.1365-3040.2009.02106.x. [DOI] [PubMed] [Google Scholar]
- Mekhedov SL, Kende H. Submergence enhances expression of a gene encoding 1-aminocyclopropane-1-carboxylate oxidase in deepwater rice. Plant Cell Physiol. 1996;37:531–537. doi: 10.1093/oxfordjournals.pcp.a028976. [DOI] [PubMed] [Google Scholar]
- Miki D, Shimamoto K. Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol. 2004;45:490–495. doi: 10.1093/pcp/pch048. [DOI] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie X, Yuzurihara N, Hihara F, Ishizuka T, Yano M, Nishimura M, Miyao A, Hirochika H, Shinomura T. Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell. 2005;17:3311–3325. doi: 10.1105/tpc.105.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie X, Kiyota S, Baba-Kasai A, Tanabata T, Shinomura T. Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice. Proc Natl Acad Sci USA. 2009;106:14705–14710. doi: 10.1073/pnas.0907378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki S. Rapid and efficient Agrobacterium-mediated transformation in rice. Plant Mol Biol Rep. 1997;15:16–21. doi: 10.1007/BF02772109. [DOI] [Google Scholar]
- Wilson K, Long D, Swinburne J, Coupland G. A dissociation insertion causes a semidominant mutation that increases expression of TINY, an Arabidopsis gene related to APETALA2. Plant Cell. 1996;8:659–671. doi: 10.1105/tpc.8.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]


