Abstract
Purpose
Due to the restricted expression of αvβ3 in tumours, αvβ3 is considered a suitable receptor for tumour targeting. In this study the αvβ3-binding characteristics of 68Ga-labelled monomeric, dimeric and tetrameric RGD peptides were determined and compared with their 111In-labelled counterparts.
Methods
A monomeric (E-c(RGDfK)), a dimeric (E-[c(RGDfK)]2) and a tetrameric (E{E[c(RGDfK)]2}2) RGD peptide were synthesised, conjugated with DOTA and radiolabelled with 68Ga. In vitro αvβ3-binding characteristics were determined in a competitive binding assay. In vivo αvβ3-targeting characteristics of the compounds were assessed in mice with subcutaneously growing SK-RC-52 xenografts. In addition, microPET images were acquired using a microPET/CT scanner.
Results
The IC50 values for the Ga(III)-labelled DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 were 23.9 ± 1.22, 8.99 ± 1.20 and 1.74 ± 1.18 nM, respectively, and were similar to those of the In(III)-labelled mono-, di- and tetrameric RGD peptides (26.6 ± 1.15, 3.34 ± 1.16 and 1.80 ± 1.37 nM, respectively). At 2 h post-injection, tumour uptake of the 68Ga-labelled mono-, di- and tetrameric RGD peptides (3.30 ± 0.30, 5.24 ± 0.27 and 7.11 ± 0.67%ID/g, respectively) was comparable to that of their 111In-labelled counterparts (2.70 ± 0.29, 5.61 ± 0.85 and 7.32 ± 2.45%ID/g, respectively). PET scans were in line with the biodistribution data. On all PET scans, the tumour could be clearly visualised.
Conclusion
The integrin affinity and the tumour uptake followed the order of DOTA-tetramer > DOTA-dimer > DOTA-monomer. The 68Ga-labelled tetrameric RGD peptide has excellent characteristics for imaging of αvβ3 expression with PET.
Electronic supplementary material
The online version of this article (doi:10.1007/s00259-010-1615-x) contains supplementary material, which is available to authorized users.
Keywords: αvβ3 integrin receptor, MicroPET, Multimers, Angiogenesis, 68Ga
Introduction
Angiogenesis, the formation of new blood vessels from existing ones, is an essential process if solid tumours are to grow beyond 2–3 mm3, since diffusion is no longer sufficient to supply the tissue with oxygen and nutrients [1]. Tumour-induced angiogenesis is a complex multistep process that follows a characteristic cascade of events mediated and controlled by growth factors, cellular receptors and adhesion molecules [2–4].
Activated endothelial cells express the integrin αvβ3 receptor, whereas this integrin receptor is absent on quiescent endothelial cells. In addition, αvβ3 is expressed on the cell membrane of various tumour cell types such as ovarian cancer, neuroblastoma, breast cancer and melanoma. αvβ3 Integrin expressed on endothelial cells modulates cell migration and survival during angiogenesis, whereas αvβ3 integrin expressed on carcinoma cells potentiates metastasis by facilitating invasion and movement across blood vessels. Due to this restricted expression of αvβ3 in tumours, αvβ3 is considered a suitable candidate for tumour targeting [5]. Radiolabelled ligands for this integrin could be used as tracers to noninvasively visualise αvβ3 expression in tumours. Noninvasive visualisation of αvβ3 expression might provide information about the angiogenic process and the responsiveness of a tumour to antiangiogenic drugs. Furthermore, noninvasive determination of αvβ3 expression potentially can be used to monitor the effect of antiangiogenic drugs in patients.
The αvβ3 integrin is a transmembrane protein consisting of two noncovalently bound subunits, α and β. This integrin can bind to the arginine-glycine-aspartic acid (RGD) amino acid sequence present in extracellular matrix proteins such as vitronectin, fibrinogen and laminin [6]. Based on the RGD tripeptide sequence a series of small peptides have been designed to antagonise the function of the αvβ3 integrin [7]. Especially the cyclic peptide derivatives have a relatively high affinity for the αvβ3 integrin. Radiolabelled cyclic RGD peptides have the potential for early detection of rapidly growing tumours and noninvasive visualisation of tumour metastasis and therapeutic response in cancer patients.
Over the last several years, significant progress has been made in the development of αvβ3-targeting radiotracers for the visualisation of αvβ3 expression in tumours by single photon emission computed tomography (SPECT) and positron emission tomography (PET).
Haubner and coworkers developed the first αvβ3-specific PET tracer [18F]Galacto-RGD [8], a glycosylated cyclic pentapeptide, which demonstrated that PET with [18F]Galacto-RGD enables receptor-specific monitoring of αvβ3 expression in murine tumour models. It was the first PET tracer applied in patients with cancer which could successfully image αvβ3 expression with good tumour to background ratios [9]. In addition, a strong correlation between tracer uptake and αvβ3 expression was observed [10]. In the mean time, another RGD-based PET tracer, [18F]AH111585, has been developed and evaluated in breast cancer patients [11]. [18F]AH111585 was demonstrated to be safe and metabolically stable and could visualise tumours in breast cancer patients. Although both 18F-labelled RGD monomers bind specifically to the αvβ3 integrin, their clinical translation is partly hampered by the time-consuming multistep 18F-labelling procedure and the necessity of a cyclotron facility to produce this PET isotope.
An interesting alternative is the use of the generator-produced radionuclide 68Ga. The application of 68Ga-labelled peptides has attracted considerable interest for cancer imaging, because of its physical characteristics [12]. 68Ga decays at 89% through positron emission of 1.92 MeV (max. energy) and can be eluted from an in-house 68Ge/68Ga generator (68Ge, T 1/2 = 270.8 days) which renders it independent of an on-site cyclotron. Furthermore, with a half-life of 68 min, 68Ga is also compatible with the pharmacokinetics of many peptides.
Recently, Decristoforo et al. compared the in vitro and in vivo properties of [68Ga]DOTA-RGD with that of the corresponding [111In]DOTA-RGD [13]. They found that especially in the blood and also in tumour the uptake of the 68Ga-labelled peptide was higher than the 111In-labelled counterpart which could be explained by different complex stabilities for the Ga-DOTA and the In-DOTA complexes, resulting in transmetallation of gallium to transferrin. The group in Stanford conjugated RGD monomers and multimers to p-SCN-Bn-NOTA and labelled them with 68Ga for imaging integrin expression in a U87MG glioblastoma xenograft model [14, 15]. They clearly observed by increasing RGD units an increase in αvβ3 affinity and tumour uptake. In addition, it was possible to increase the αvβ3 receptor-binding affinity of the RGD dimer by coupling the two RGD peptide units via Gly3 and PEG4 linkers.
Here, we radiolabelled mono-, di- and tetrameric RGD peptides with 68Ga and studied the tumour targeting potential of these peptides in vitro and in vivo. This is the first study in which mono-, di- and tetrameric RGD peptides labelled with 68Ga, for PET imaging of αvβ3 expression, are directly compared with their 111In-labelled counterparts.
Materials and methods
Synthesis of DOTA-conjugated RGD peptides
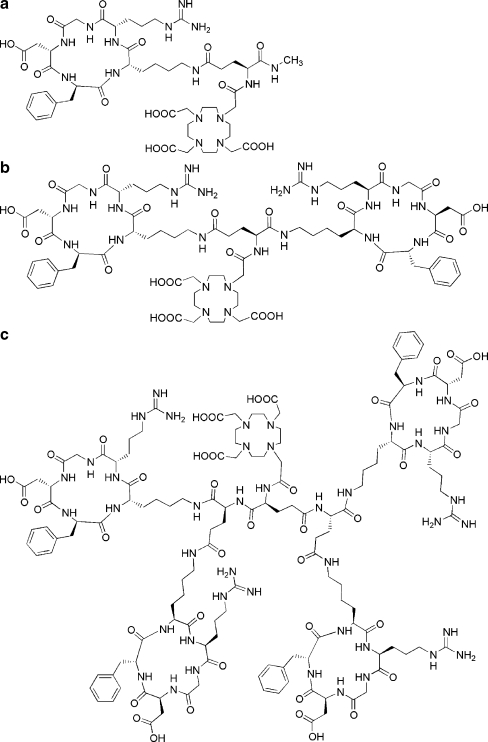
The mono-, di- and tetrameric RGD peptides were synthesised using Fmoc-based solid-phase peptide synthesis (SPPS) as described previously [16–19]. The structural formulas of DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 are shown in Fig. 1.
Fig. 1.
a Structural formula of the DOTA-conjugated monomeric RGD peptide, DOTA-E-c(RGDfK). b Structural formula of the DOTA-conjugated dimeric RGD peptide, DOTA-E-[c(RGDfK)]2. c Structural formula of the DOTA-conjugated tetrameric RGD peptide DOTA-E{E[c(RGDfK)]2}2
Radiolabelling of the RGD peptides
111In labelling
DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 were radiolabelled with 111InCl3 as described previously [17]. Briefly, 18.5 MBq 111InCl3 (Mallinckrodt, Petten, The Netherlands) was added to 5–20 nmol DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 dissolved in 300 or 500 μl ammonium acetate buffer, pH 6.0, containing 0.6 mg/ml gentisic acid. The mixtures were heated at 100°C for 15 min. For in vitro and in vivo studies, the reaction mixtures were diluted in phosphate-buffered saline (PBS).
68Ga labelling
DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 were labelled with 68GaCl3 eluted from a TiO2-based 1,110 MBq 68Ge/68Ga generator (Cyclotron Co. Ltd., Obninsk, Russia) using 0.1 M HCl (Ultrapure, J.T. Baker, Deventer, The Netherlands). Five 1-ml fractions were collected and an aliquot of the second fraction was used for labelling the peptides.
68Ga-labelled DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 were prepared by adding 250 μl 1 M HEPES, pH 7.0, solution to 10–28 μl of the peptide dissolved (1 μg/μl) in 0.25 M ammonium acetate, pH 5.5. Then, the second millilitre eluted from the generator (315–365 MBq) was added. After 20 min at 95°C, the 68Ga-labelled peptides were further purified on an Oasis® HLB (1 cm3, 30 mg) cartridge (Waters, Milford, MA, USA). After applying the sample on the cartridge, the cartridge was washed with 3 × 1 ml H2O and eluted with 200 μl 25% EtOH in H2O (v/v). For in vitro and in vivo studies, the eluate was diluted to <5% EtOH in PBS.
Analysis
The radiochemical purity was determined by reversed-phase high-performance liquid chromatography (RP-HPLC) on an Agilent 1100 system (Agilent Technologies, Palo Alto, CA, USA) using a C18 column (RX-C18, 4.6 × 250 mm, Zorbax) eluted with a gradient mobile phase [0–5 min 97% buffer A, 5-15 min 97% buffer A to 0% buffer A, buffer A = 0.1% trifluoroacetic acid (TFA) in H2O, buffer B = 0.1% TFA in acetonitrile] at 1 ml/min. The radioactivity of the eluate was monitored using an in-line NaI radiodetector (Raytest GmbH, Straubenhardt, Germany). Elution profiles were analysed using Gina Star software (version 2.18, Raytest GmbH, Straubenhardt, Germany). An additional quality control after purification on an HLB cartridge was performed by instant thin-layer chromatography (ITLC) using TEC-Control™ chromatography strips (Biodex Medical Systems, Shirley, NY, USA). The strips were developed using two different mobile phases. Mobile phase I was 0.1 M CH3COONH4/0.1 M EDTA (1:1 v/v) and mobile phase II was 0.25 M CH3COONH4/MeOH (1:1 v/v). The strips were analysed using a Fujifilm BAS-1800II Scanner (Fuji Photo Film Co., Tokyo, Japan).
Octanol/water partition coefficient
To an Eppendorf tube filled with 0.5 ml of the radiolabelled peptide in PBS, pH 7.4, 0.5 ml octanol was added. After the tube was vigorously vortexed for 2 min at room temperature, the two layers were separated by centrifugation (100 g, 5 min). Then, 100-μl samples were taken from each layer and radioactivity was measured in a well-type gamma counter (Wallac Wizard 3”, PerkinElmer, Waltham, MA, USA) and Log P values were calculated (n = 3).
In vitro stability
The stability of the 111In- and 68Ga-labelled RGD peptides was determined by incubating the compounds in both PBS and human serum for 2 h at 37°C. Before analysis of the serum samples the serum proteins were precipitated by adding an equal volume of MeCN to the samples. Subsequently, serum samples were centrifuged for 5 min at 13,500 g. The PBS samples were analysed without any sample preparation. An aliquot of the serum and the PBS sample were injected onto HPLC.
Protein binding
To determine their serum protein-binding properties, the 68Ga- and 111In-labelled peptides were incubated in fresh human serum at 37°C. After 2 h, the samples were analysed with fast protein liquid chromatography (FPLC), using a BioSep-Sec-S 3000 column (300 × 4.60 mm, Phenomenex, Utrecht, The Netherlands) with an isocratic mobile phase (PBS, 1 ml/min).
Solid-phase αvβ3 binding assay
The affinity of Ga(III)/In(III)-DOTA-E-c(RGDfK), Ga(III)/In(III)-DOTA-E-[c(RGDfK)]2 and Ga(III)/In(III)-DOTA-E{E[c(RGDfK)]2}2 for αvβ3 was determined using a solid-phase competitive binding assay.
For the “cold” labelling of DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 with either Ga(III) or In(III), each of the peptides was dissolved in an aqueous solution. Subsequently, a 3 M excess of InCl3 (Aldrich Chemical Company, Inc., Milwaukee, WI, USA) or Ga(NO3)3 (Sigma-Aldrich Chemie, Steinheim, Germany) was added. The Ga(III) or In(III) complexation was performed at room temperature overnight or at 40°C for 2 h, respectively.
111In-labelled DOTA-E-[c(RGDfK)]2 (3 MBq/μg) was used as the tracer in this assay. Microtiter 96-well vinyl assay plates (Corning B.V., Schiphol-Rijk, The Netherlands) were coated with 100 μl/well of a solution of purified human integrin αvβ3 (150 ng/ml) in Triton X-100 Formulation (Chemicon International, Temecula, CA, USA) in coating buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2 and 1 mM MnCl2) for 17 h at 4°C. The plates were washed twice with binding buffer [0.1% bovine serum albumin (BSA) in coating buffer]. The wells were blocked for 2 h with 200 μl blocking buffer (1% BSA in coating buffer). The plates were washed twice with binding buffer. Then, 100 μl binding buffer containing 10 kBq of 111In-DOTA-E-[c(RGDfK)]2 and appropriate dilutions (2 × 10−6–8 × 10−11 M) of Ga(III)- or In(III)-labelled DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 in binding buffer were incubated in the wells at 37°C for 1 h. After incubation, the plates were washed three times with binding buffer. The wells were cut out and counted in a gamma counter. IC50 values of the RGD peptides were calculated by nonlinear regression using GraphPad Prism (GraphPad Prism 4.0, GraphPad Software, San Diego, CA, USA). Each data point is the average of three determinations.
Biodistribution studies
In the right flank of 6- to 8-week-old female nude BALB/c mice, 0.2 ml of a cell suspension of 2 × 106 cells/ml SK-RC-52 cells was injected subcutaneously (s.c.). Two weeks after inoculation of the tumour cells, mice were injected intravenously (i.v.) with the 111In- or 68Ga-labelled RGD peptides (0.2–0.89 nmol) in 0.2 ml PBS + 0.5% BSA. Mice were killed by CO2 asphyxiation 2 h post-injection (p.i.) (five mice/group). Blood, tumour and the major organs and tissues were collected, weighed and counted in a gamma counter. The percentage injected dose per gram (%ID/g) was determined for each sample.
The receptor-mediated localisation of the radiolabelled RGD peptides was investigated by determining the biodistribution of the 111In- or 68Ga-labelled compounds in the presence of an excess (100-fold excess) unlabelled DOTA-E-[c(RGDfK)]2 (n = 3). DOTA-E-[c(RGDfK)]2 was used for these “blocking studies” as in our previous studies this compound was demonstrated to be αvβ3 specific [20, 21]. All animal experiments were approved by the local Animal Welfare Committee in accordance with the Dutch legislation and carried out in accordance with their guidelines.
MicroPET imaging
Mice with s.c. SK-RC-52 tumours were injected i.v. with 10 MBq 68Ga-labelled mono-, di- or tetrameric RGD peptide per mouse (0.89 nmol). Two hours after the injection of the peptide, mice were scanned on an animal PET/CT scanner (Inveon®, Siemens Preclinical Solutions, Knoxville, TN, USA) with an intrinsic spatial resolution of 1.5 mm [22]. The animals were placed in a supine position in the scanner. PET emission scans were acquired over 15 min. CT images were acquired for anatomical correlation directly after PET imaging (spatial resolution 113 μm, 80 kV, 500 μA, exposure time 300 ms).
Scans were reconstructed using Inveon Acquisition Workplace software version 1.2 (Siemens Preclinical Solutions, Knoxville, TN, USA), using an ordered subset expectation maximisation 3-D/maximum a posteriori (OSEM3D/MAP) algorithm with the following parameters: matrix 256 × 256 × 159, pixel size 0.43 × 0.43 × 0.8 mm3 and a beta value of 0.1.
Statistical analysis
All mean values are given ± standard deviation (SD). Statistical analysis was performed using the one-way analysis of variance. Bonferroni corrections for multiple comparisons were applied. The level of significance was set at p < 0.05.
Results
Radiolabelling
RP-HPLC analysis indicated that the radiochemical purity of the 68Ga- or 111In-labelled DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 preparations used in these experiments ranged from 93 to 97%.
The HPLC chromatograms of 111In-DOTA-E-c(RGDfK), 111In-DOTA-E-[c(RGDfK)]2 and 111In-DOTA-E{E[c(RGDfK)]2}2 showed a single peak for each of the compounds with a retention time of 14, 26 and 15 min, respectively. Note that different gradients were used.
The ITLC profiles of 68Ga-DOTA-E-c(RGDfK) (Rf = 0.35), 68Ga-DOTA-E-[c(RGDfK)]2 (Rf = 0.53) and 68Ga-DOTA-E{E[c(RGDfK)]2}2 (Rf = 0.08) after HLB purification demonstrated a purity of 97, 98 and 99%, respectively. The maximum specific activity of the 68Ga-labelled mono-, di- and tetramer was 11.2 MBq/nmol.
Octanol/water partition coefficient
To determine the lipophilicity of the 68Ga- and 111In-labelled DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2, the octanol/water partition coefficients were determined. The Log Poctanol/water values for the 68Ga-labelled RGD mono-, di- and tetramer were −4.37 ± 0.13, −4.04 ± 0.15 and −3.76 ± 0.07, respectively. The Log P octanol/water values of the 111In-labelled RGD mono-, di- and tetramer were −4.38 ± 0.25, −3.95 ± 0.05 and −4.15 ± 0.07, respectively.
In vitro stability
Determination of the stability of the 111In- and 68Ga-labelled RGD peptides indicated high stability of the compounds. There was no evidence of release of 68Ga or 111In from the peptides or radiolysis of any of the compounds in both PBS and human serum (data not shown). After 2 h incubation at 37°C more than 95% of the activity was still associated with the DOTA-conjugated cyclic peptides and no significant reduction was observed.
Protein binding
No differences in the protein-binding properties between the 68Ga- and 111In-labelled RGD peptides were observed by FPLC (data not shown). The protein-bound activity was negligible (<5%) after 2 h incubation in human serum for the 68Ga- as well as for the 111In-labelled peptides. For each of the peptides >95% of the activity eluted as a monomeric peak at 13 min.
Solid-phase αvβ3 binding assay
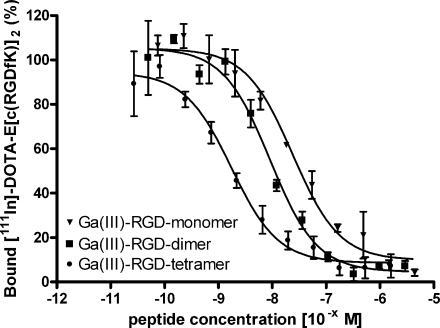
We determined the affinity of Ga(III)-DOTA-E-c(RGDfK), Ga(III)-DOTA-E-[c(RGDfK)]2 and Ga(III)-DOTA-E{E[c(RGDfK)]2}2 and their In(III)-labelled analogues for integrin αvβ3 in a competitive binding assay. The results of these assays are summarised in Fig. 2. Binding of 111In-labelled dimeric peptide, 111In-DOTA-E-[c(RGDfK)]2, to αvβ3 was competed by Ga(III)- or In(III)-labelled DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 in a concentration-dependent manner. The IC50 values were 23.9 ± 1.22 nM, 8.99 ± 1.20 nM and 1.74 ± 1.18 nM for Ga(III)-labelled DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2, respectively (Table 1). The affinities of the In(III)-labelled mono-, di- and tetrameric RGD peptides were similar: 26.6 ± 1.15 nM, 3.34 ± 1.16 nM and 1.80 ± 1.37 nM, respectively.
Fig. 2.
Competition of specific binding of 111In-DOTA-E-[c(RGDfK)]2 with Ga(III)-DOTA-E-c(RGDfK), Ga(III)-DOTA-E-[c(RGDfK)]2 and Ga(III)-DOTA-E{E[c(RGDfK)]2}2
Table 1.
IC50 values arising from a competitive binding assay of Ga(III)- and In(III)-labelled RGD mono-, di- and tetramer
| Compound | Ga(III)-labelled ± SD (nM) | In(III)-labelled ± SD (nM) |
|---|---|---|
| Monomer | 23.9 ± 1.22 | 26.6 ± 1.15 |
| Dimer | 8.99 ± 1.20 | 3.34 ± 1.16 |
| Tetramer | 1.74 ± 1.18 | 1.80 ± 1.37 |
Biodistribution studies
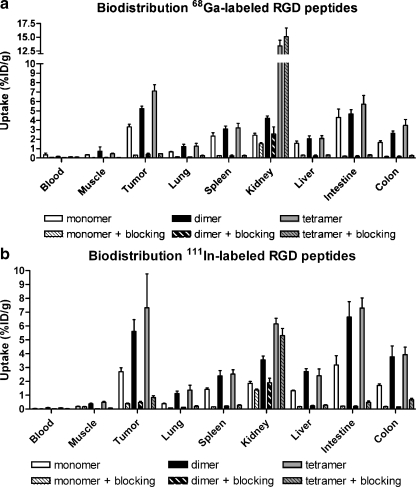
The results of the biodistribution studies of both 111In- and 68Ga-labelled DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 are summarised in Fig. 3. DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 radiolabelled with either 68Ga or 111In all cleared rapidly from the blood. At 2 h p.i. the blood level of all compounds was below 0.4%ID/g. Tumour uptakes of the 68Ga-labelled mono-, di- and tetrameric RGD peptides (3.30 ± 0.30, 5.24 ± 0.27 and 7.11 ± 0.67%ID/g, respectively) were comparable to those of their 111In-labelled counterparts (2.70 ± 0.29, 5.61 ± 0.85 and 7.32 ± 2.45%ID/g, respectively). At 2 h p.i., the tumour uptake was significantly higher for the 68Ga-labelled tetramer (7.11 ± 0.67%ID/g), compared to that of the dimer (5.24 ± 0.27%ID/g) and that of the monomer (3.30 ± 0.30%ID/g). For the 111In-labelled analogues, there was no difference in tumour uptake between the tetramer (7.32 ± 2.45%ID/g) and dimer (5.61 ± 0.85%ID/g), whereas the tumour uptake of the 111In-labelled dimer was significantly higher than that of the 111In-labelled monomer (2.70 ± 0.29%ID/g).
Fig. 3.
a Biodistribution of [68Ga]DOTA-E-c(RGDfK), [68Ga]DOTA-E-[c(RGDfK)]2 and [68Ga]DOTA-E{E[c(RGDfK)]2}2 at 2 h p.i. in athymic mice with s.c. SK-RC-52 tumours in the absence (five mice/group) or presence (three mice/group) of an excess of DOTA-E-[c(RGDfK)]2. b Biodistribution of [111In]DOTA-E-c(RGDfK), [111In]DOTA-E-[c(RGDfK)]2 and [111In]DOTA-E{E[c(RGDfK)]2}2 at 2 h p.i. in athymic mice with s.c. SK-RC-52 tumours in the absence (five mice/group) or presence (three mice/group) of an excess of DOTA-E-[c(RGDfK)]2
Coinjection of an excess unlabelled DOTA-E-[c(RGDfK)]2 (50 μg) along with 0.5 μg of 68Ga- or 111In-labelled DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 or DOTA-E{E[c(RGDfK)]2}2 resulted in a significantly reduced radioactivity concentration in the tumour, indicating that uptake of the major fraction of DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 in the tumour was αvβ3 mediated. Uptake in nontarget organs such as lung, spleen and intestine was also reduced in the presence of an excess of unlabelled RGD peptide, indicating that the uptake in these tissues was at least partly αvβ3 mediated. The kidney uptake of the 68Ga- and 111In-labelled monomer and dimer could partly be blocked. However, renal uptake of the 68Ga- and 111In-labelled tetramer could not be blocked. Kidney uptake was remarkably high for the 68Ga-labelled tetramer compared to its 111In-labelled analogue.
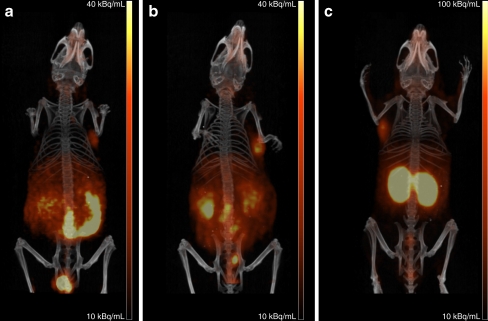
Fused PET and CT scans are shown in Fig. 4. PET scans were in line with the biodistribution data. On all PET scans, the tumour could be clearly visualised. The 68Ga-labelled tetramer showed the highest tumour uptake compared to the monomer and dimer. All three tracers showed some uptake in the kidneys, especially the tetramer. On the other hand, the monomer demonstrated relatively high intestinal uptake. The 68Ga-labelled tetramer showed the highest tumour to background ratio and therefore this tracer is the most suitable for imaging αvβ3 expression by PET.
Fig. 4.
Anterior 3-D volume rendering projections of fused PET and CT scans of mice with a s.c. growing SK-RC-52 tumour after i.v. injection of [68Ga]DOTA-E-c(RGDfK) (a), [68Ga]DOTA-E-[c(RGDfK)]2 (b) or [68Ga]DOTA-E{E[c(RGDfK)]2}2 (c). Scans were recorded at 2 h p.i.
Discussion
In this study, the feasibility of using 68Ga-labelled multimeric RGD peptides for radionuclide imaging of the αvβ3 integrin expression with PET was investigated. The radiolabelled mono-, di and tetrameric RGD peptides were very hydrophilic as demonstrated by their partition coefficients (Log Poctanol/water). The Log P values varied between −4.38 ± 0.25 for the 111In-labelled monomer and −3.76 ± 0.07 for the 68Ga-labelled tetramer. These values are even lower than the value found for [18F]Galacto-RGD (Log P = −3.2) which is cleared almost exclusively via the kidneys [23]. The hydrophilicity of the 68Ga-labelled monomer and dimer labelled with either 68Ga or 111In was not different. Only for the tetramer was the Log P value lower for the 111In-labelled variant compared to its 68Ga-labelled counterpart. Heppeler et al. demonstrated that for the complexation of gallium by DOTA four nitrogen atoms of the macrocycle and two oxygen atoms from the carboxylate groups are involved [24]. Thus, one carboxylic acid group of the DOTA chelator is not involved in complexation of 68Ga. However, for 111In it is assumed that the four nitrogen atoms of the DOTA macrocycle and the four oxygen atoms of the carboxylic groups are involved in the complexation. Despite the fact that the 68Ga-DOTA complex has one more carboxylic acid group that is not involved in the complexation compared to the 111In-DOTA complex, the 68Ga-labelled analogue does not have an increased hydrophilicity.
The binding affinity of Ga(III)-DOTA-tetramer (IC50 = 1.74 ± 1.18 nM), as determined in a solid-phase competitive binding assay, was about 5 times higher compared to Ga(III)-DOTA-dimer (IC50 = 8.99 ± 1.20 nM) and about 13 times higher compared to Ga(III)-DOTA-monomer (IC50 = 23.9 ± 1.22 nM). The binding affinity of the In(III)-labelled monomeric (26.6 ± 1.15 nM), dimeric (3.34 ± 1.16 nM) and tetrameric RGD peptides (1.80 ± 1.37 nM) was comparable.
In the s.c. SK-RC-25 renal cell carcinoma xenograft model, the tetrameric RGD peptide, labelled with either 68Ga or 111In, showed the highest tumour uptake. Thus, there is a relation between the binding affinity for αvβ3 and the accumulation of the compound in αvβ3-expressing tumours. All three RGD peptides of this study labelled with either 68Ga or 111In showed specific tumour uptake in athymic mice with s.c. SK-RC-52 tumours: in the presence of an excess of unlabelled DOTA-E-[c(RGDfK)]2, the specificity of the tumour targeting of the monomeric, dimeric, and tetrameric RGD peptides was evident.
Several research groups have applied the multivalent concept to prepare cyclic RGD peptides with an enhanced binding affinity and demonstrated that the multimeric RGD peptides have an enhanced localisation in αvβ3-expressing tumours [17, 19, 25–30]. Although the advantages of multimeric RGD peptides as targeting molecules are universally accepted, the cause of the enhanced affinity of the multimeric RGD analogues for integrin αvβ3 is still a matter of debate [31]. Cells can form a cluster of many monovalent receptors on the cell surface [32], and particularly multimeric ligands with a spacer between the binding moieties could span the required distance between binding sites and could then bind multiple receptors simultaneously. On the other hand, multimeric compounds could have enhanced affinity due to statistical rebinding: the receptor binding of one RGD unit will significantly enhance the local concentration of the second RGD unit in the vicinity of the receptor. This could lead to an enhanced αvβ3-binding rate or a reduced αvβ3-dissociation rate of the RGD multimer [19]. The distance between the RGD units of the multimers used in this study is relatively short and therefore statistical rebinding might be the most likely explanation for the increased affinity in the series tetramer > dimer > monomer.
The three 68Ga-labelled RGD peptides showed a remarkable difference in kidney uptake. The uptake of the 68Ga-labelled tetrameric RGD peptide was at 2 h p.i. significantly higher than that of the 68Ga-labelled dimer and monomeric RGD peptides. In the integrin specificity experiment, the excess of nonradiolabelled RGD peptide partly inhibited the kidney uptake of the radiolabelled monomer and dimer, but not the kidney uptake of the radiolabelled tetramer. This indicates that different mechanisms cause the relatively high uptake of the RGD peptides in the kidneys. Wu and coworkers have recently shown by immunohistochemistry that the endothelial cells of the glomeruli vessels in the kidneys express β3 integrins [33], which could explain the partly specific kidney uptake of the RGD peptide. Furthermore, the difference in charge between the three peptides could cause the difference in tubular reabsorption. A trend has been observed that positively charged peptides are more efficiently reabsorbed by the proximal renal tubular cell than neutral peptides [34]. Due to the presence of more guanidine groups, the tetrameric RGD peptide is more positively charged than the dimeric and monomeric RGD peptides. Remarkably, the 68Ga-labelled tetramer demonstrated a much higher kidney uptake than the 111In-labelled tetramer which may hamper its clinical application.
Other nontumour tissues such as lung, liver and colon also showed specific uptake of the mono-, di- and tetrameric RGD peptides, suggesting αvβ3 expression in these tissues. Indeed, β3 expression in these tissues has been described for rodents as well as for humans [33, 35]. Decristoforo and coworkers compared the biodistribution of [68Ga]DOTA-RGD, [111In]DOTA-RGD and [18F]Galacto-RGD and found that [68Ga]DOTA-RGD had the highest tracer uptake in all organs [13]. Especially, the radioactivity concentration in the blood was significantly higher for [68Ga]DOTA-RGD compared with [111In]DOTA-RGD. The authors hypothesised that the lower complex stability of the 68Ga-DOTA complex could result in transchelation of gallium to transferrin. In our study, the 68Ga-labelled peptides, especially the dimer and tetramer, did not show enhanced blood levels as compared to the 111In-labelled counterparts. In addition, in our in vitro studies no evidence of instability of the 68Ga-DOTA complex or protein-binding activity was observed. This is in line with the recent observation of Haukkala and coworkers who found that there was no evidence of dissociation of 68Ga from DOTA in the blood [36]. Although DOTA has a larger cavity than NOTA and the log stability constants are in favour of the Ga-NOTA complex compared with the Ga-DOTA complex [37, 38], the 68Ga-DOTA complex is stable enough for in vitro and in vivo studies.
In conclusion, the tetrameric RGD peptide demonstrated improved tumour targeting compared to the dimeric RGD peptide. Analogously, the dimeric RGD peptide exhibits improved tumour targeting compared to the monomeric RGD peptide. The results of the biodistribution study of the 68Ga- and 111In-labelled dimer and tetramer are rather comparable. The 68Ga-labelled tetrameric RGD peptide is a suitable ligand for the noninvasive visualisation of αvβ3 expression in vivo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 317 kb)
(DOC 322 kb)
(PPT 476 kb)
Acknowledgments
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res. 2000;55:15–35. [PubMed] [Google Scholar]
- 2.Kuwano M, Fukushi J, Okamoto M, Nishie A, Goto H, Ishibashi T, et al. Angiogenesis factors. Intern Med. 2001;40:565–572. doi: 10.2169/internalmedicine.40.565. [DOI] [PubMed] [Google Scholar]
- 3.Ellis LM, Liu W, Ahmad SA, Fan F, Jung YD, Shaheen RM, et al. Overview of angiogenesis: biologic implications for antiangiogenic therapy. Semin Oncol. 2001;28:94–104. doi: 10.1016/S0093-7754(01)90287-8. [DOI] [PubMed] [Google Scholar]
- 4.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 6.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligands binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 7.Haubner R, Finsinger D, Kessler H. Stereoisomeric peptide libraries and peptidomimetics for designing selective inhibitors of the αvβ3 integrin for a new cancer therapy. Angew Chem Int Ed Engl. 1997;36:1374–1389. doi: 10.1002/anie.199713741. [DOI] [Google Scholar]
- 8.Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, et al. Noninvasive imaging of alpha(v)beta3 integrin expression using 18F-labeled RGD-containing glycopeptides and positron emission tomography. Cancer Res. 2001;61:1781–1785. [PubMed] [Google Scholar]
- 9.Haubner R, Weber WA, Beer AJ, Vabuliene E, Reim D, Sarbia M, et al. Noninvasive visualization of the activated alphavbeta3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med. 2005;2:e70. doi: 10.1371/journal.pmed.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beer AJ, Haubner R, Sarbia M, Goebel M, Luderschmidt S, Grosu AL, et al. Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin alpha(v)beta3 expression in man. Clin Cancer Res. 2006;12:3942–3949. doi: 10.1158/1078-0432.CCR-06-0266. [DOI] [PubMed] [Google Scholar]
- 11.Kenny LM, Coombes RC, Oulie I, Contractor KB, Miller M, Spinks TJ, et al. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–886. doi: 10.2967/jnumed.107.049452. [DOI] [PubMed] [Google Scholar]
- 12.Maecke HR, Hofmann M, Haberkorn U. (68)Ga-labeled peptides in tumor imaging. J Nucl Med. 2005;46(Suppl 1):172S–178S. [PubMed] [Google Scholar]
- 13.Decristoforo C, Hernandez Gonzalez I, Carlsen J, Rupprich M, Huisman M, Virgolini I, et al. 68Ga- and 111In-labelled DOTA-RGD peptides for imaging of alphavbeta3 integrin expression. Eur J Nucl Med Mol Imaging. 2008;35:1507–1515. doi: 10.1007/s00259-008-0757-6. [DOI] [PubMed] [Google Scholar]
- 14.Li ZB, Chen K, Chen X. (68)Ga-labeled multimeric RGD peptides for microPET imaging of integrin alpha(v)beta(3) expression. Eur J Nucl Med Mol Imaging. 2008;35:1100–1108. doi: 10.1007/s00259-007-0692-y. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Niu G, Shi J, Liu S, Wang F, Liu S, et al. (68)Ga-labeled cyclic RGD dimers with Gly3 and PEG4 linkers: promising agents for tumor integrin alphavbeta3 PET imaging. Eur J Nucl Med Mol Imaging. 2009;36:947–957. doi: 10.1007/s00259-008-1045-1. [DOI] [PubMed] [Google Scholar]
- 16.Dijkgraaf I, Kruijtzer JA, Frielink C, Soede AC, Hilbers HW, Oyen WJ, et al. Synthesis and biological evaluation of potent alphavbeta3-integrin receptor antagonists. Nucl Med Biol. 2006;33:953–961. doi: 10.1016/j.nucmedbio.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Dijkgraaf I, Kruijtzer JA, Liu S, Soede AC, Oyen WJ, Corstens FH, et al. Improved targeting of the alpha(v)beta(3) integrin by multimerisation of RGD peptides. Eur J Nucl Med Mol Imaging. 2007;34:267–273. doi: 10.1007/s00259-006-0180-9. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Cheung E, Ziegler M, Rajopadhye M, Edwards DS. (90)Y and (177)Lu labeling of a DOTA-conjugated vitronectin receptor antagonist useful for tumor therapy. Bioconjug Chem. 2001;12:559–568. doi: 10.1021/bc000146n. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, et al. microPET imaging of glioma integrin {alpha}v{beta}3 expression using (64)Cu-labeled tetrameric RGD peptide. J Nucl Med. 2005;46:1707–1718. [PubMed] [Google Scholar]
- 20.Janssen M, Frielink C, Dijkgraaf I, Oyen W, Edwards DS, Liu S, et al. Improved tumor targeting of radiolabeled RGD peptides using rapid dose fractionation. Cancer Biother Radiopharm. 2004;19:399–404. doi: 10.1089/cbr.2004.19.399. [DOI] [PubMed] [Google Scholar]
- 21.Janssen ML, Oyen WJ, Dijkgraaf I, Massuger LF, Frielink C, Edwards DS, et al. Tumor targeting with radiolabeled alpha(v)beta(3) integrin binding peptides in a nude mouse model. Cancer Res. 2002;62:6146–6151. [PubMed] [Google Scholar]
- 22.Visser EP, Disselhorst JA, Brom M, Laverman P, Gotthardt M, Oyen WJ, et al. Spatial resolution and sensitivity of the Inveon small-animal PET scanner. J Nucl Med. 2009;50:139–147. doi: 10.2967/jnumed.108.055152. [DOI] [PubMed] [Google Scholar]
- 23.Haubner R, Kuhnast B, Mang C, Weber WA, Kessler H, Wester HJ, et al. [18F]Galacto-RGD: synthesis, radiolabeling, metabolic stability, and radiation dose estimates. Bioconjug Chem. 2004;15:61–69. doi: 10.1021/bc034170n. [DOI] [PubMed] [Google Scholar]
- 24.Heppeler A, Froidevaux S, Mäcke H, Jermann E, Behe M, Powell P, et al. Radiometal-labelled macrocyclic chelator-derivatised somatostatin analogue with superb tumour-targeting properties and potential for receptor-mediated internal radiotherapy. Chem Eur J. 1999;5:1974–1981. doi: 10.1002/(SICI)1521-3765(19990702)5:7<1974::AID-CHEM1974>3.0.CO;2-X. [DOI] [Google Scholar]
- 25.Boturyn D, Coll JL, Garanger E, Favrot MC, Dumy P. Template assembled cyclopeptides as multimeric system for integrin targeting and endocytosis. J Am Chem Soc. 2004;126:5730–5739. doi: 10.1021/ja049926n. [DOI] [PubMed] [Google Scholar]
- 26.Poethko T, Thumshirn G, Hersel U, Rau F, Haubner R, Schwaiger M, et al. Improved tumor uptake, tumor retention and tumor/background ratios of pegylated RGD multimers. J Nucl Med. 2003;44:46P. [Google Scholar]
- 27.Poethko T, Schottelius M, Thumshirn G, Herz M, Haubner R, Henriksen G, et al. Chemoselective pre-conjugate radiohalogenation of unprotected mono- and multimeric peptides via oxime formation. Radiochim Acta. 2004;92:317–327. doi: 10.1524/ract.92.4.317.35591. [DOI] [Google Scholar]
- 28.Thumshirn G, Hersel U, Goodman SL, Kessler H. Multimeric cyclic RGD peptides as potential tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation. Chem Eur J. 2003;9:2717–2725. doi: 10.1002/chem.200204304. [DOI] [PubMed] [Google Scholar]
- 29.Poethko T, Schottelius M, Thumshirn G, Hersel U, Herz M, Henriksen G, et al. Two-step methodology for high-yield routine radiohalogenation of peptides: (18)F-labeled RGD and octreotide analogs. J Nucl Med. 2004;45:892–902. [PubMed] [Google Scholar]
- 30.Liu S, Hsieh WY, Jiang Y, Kim YS, Sreerama SG, Chen X, et al. Evaluation of a (99m)Tc-labeled cyclic RGD tetramer for noninvasive imaging integrin alpha(v)beta3-positive breast cancer. Bioconjug Chem. 2007;18:438–446. doi: 10.1021/bc0603081. [DOI] [PubMed] [Google Scholar]
- 31.Vrasidas I, André S, Valentini P, Böck C, Lensch M, Kaltner H, et al. Rigidified multivalent lactose molecules and their interactions with mammalian galectins: a route to selective inhibitors. Org Biomol Chem. 2003;1:803–810. doi: 10.1039/b210923a. [DOI] [PubMed] [Google Scholar]
- 32.Kiessling LL, Pohl NL. Strength in numbers: non-natural polyvalent carbohydrate derivatives. Chem Biol. 1996;3:71–77. doi: 10.1016/S1074-5521(96)90280-X. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z, Li ZB, Chen K, Cai W, He L, Chin FT, et al. microPET of tumor integrin alphavbeta3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4) J Nucl Med. 2007;48:1536–1544. doi: 10.2967/jnumed.107.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behr TM, Goldenberg DM, Becker W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: present status, future prospects and limitations. Eur J Nucl Med. 1998;25:201–212. doi: 10.1007/s002590050216. [DOI] [PubMed] [Google Scholar]
- 35.Max R, Gerritsen RR, Nooijen PT, Goodman SL, Sutter A, Keilholz U, et al. Immunohistochemical analysis of integrin alphavbeta3 expression on tumor-associated vessels of human carcinomas. Int J Cancer. 1997;71:320–324. doi: 10.1002/(SICI)1097-0215(19970502)71:3<320::AID-IJC2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Haukkala J, Laitinen I, Luoto P, Iveson P, Wilson I, Karlsen H, et al. (68)Ga-DOTA-RGD peptide: biodistribution and binding into atherosclerotic plaques in mice. Eur J Nucl Med Mol Imaging. 2009;36:2058–2067. doi: 10.1007/s00259-009-1220-z. [DOI] [PubMed] [Google Scholar]
- 37.Clarke ET, Martell AE. Stabilities of trivalent metal ion complexes of the tetraacetate derivatives of 12-, 13- and 14-membered tetraazamacrocycles. Inorganica Chim Acta. 1991;190:37–46. doi: 10.1016/S0020-1693(00)80229-7. [DOI] [Google Scholar]
- 38.Clarke ET, Martell AE. Stabilities of the Fe(III), Ga(III), and In(III) chelates of N,N′,N″-triazacyclononanetriacetic acid. Inorganica Chim Acta. 1991;181:273–280. doi: 10.1016/S0020-1693(00)86821-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 317 kb)
(DOC 322 kb)
(PPT 476 kb)