Abstract
Children are known to be particularly vulnerable to the effects of noise on speech perception, and it is commonly acknowledged that failure of central auditory processes can lead to these difficulties with speech-in-noise (SIN) perception. Still, little is known about the mechanistic relationship between central processes and the perception of speech in noise. Our aims were two-fold: to examine the effects of noise on the central encoding of speech through measurement of cortical event-related potentials (ERPs) and to examine the relationship between cortical processing and behavioral indices of SIN perception. We recorded cortical responses to the speech syllable [da] in quiet and multi-talker babble noise in 32 children with a broad range of SIN perception. Outcomes suggest inordinate effects of noise on auditory function in the bottom SIN perceivers, compared with the top perceivers. The cortical amplitudes in the top SIN group remained stable between conditions, whereas amplitudes increased significantly in the bottom SIN group, suggesting a developmental central processing impairment in the bottom perceivers that may contribute to difficulties encoding and perceiving speech in challenging listening environments.
Keywords: Speech, Evoked Responses, Children, Development, Central Auditory Processing
Introduction
Many children show considerable difficulty processing speech in background noise, and these deficits can impair communication (Cunningham et al., 2000; Cunningham et al., 2001; Bradlow et al., 2003; Ziegler et al., 2005; Ziegler et al., 2009). Understanding how sensory systems adapt to challenging listening conditions is an important concern facing auditory neuroscience research. Yet few studies have examined the mechanisms by which sensory systems adapt to challenging listening conditions. Since learning often occurs in noisy environments (e.g., a school classroom), children who have difficulty excluding background noise are particularly vulnerable to the debilitating effects of challenging listening conditions (Sperling et al., 2005). Indeed, the extent of noise in classrooms is directly related to academic achievement in school (Shield and Dockrell, 2003; Shield and Dockrell, 2008), with a 4 to 6 percent decrease in academic test scores noted with as little as 10 dB increase in ambient noise. Here, we aim to understand the impact of noise on cortical-evoked speech processing in children and to examine the neural factors that determine successful or unsuccessful speech perception in challenging listening environments.
The obligatory components of cortical potentials (P1, N1, P2, and N2) have a systematic developmental time course (Sharma et al., 1997; Ponton et al., 2000; Sussman et al., 2008; Cunningham et al., 2001). In adulthood the cortical response is dominated by the N1-P2 complex, but in childhood the P1 and N2 components dominate the response (Ceponiene et al., 2002). The P1 component serves as a central auditory developmental marker, with shorter latencies and smaller amplitudes as children mature from infancy to young adulthood (Sharma et al., 2002). Likewise, the N2 amplitude decreases while the N1 component becomes more prominent with development (Sussman et al., 2008). Furthermore, the P1 and N2 components may reflect different aspects of sound processing, with P1 encoding acoustic features of sound, such as frequency and timing, and N2 synthesizing these features into a sensory representation. (Shtyrov et al., 1998; Ceponiene et al., 2005). Ceponiene and colleagues (2001) found decreased N2 amplitude for vowels vs. complex tones and speculate that fewer neural resources may be required for representing the more familiar vowel sound.
Few studies have empirically examined the effects of background noise on the neural encoding of speech at the level of the cortex. Broad-band noise causes a reduction in speech-evoked cortical response amplitudes in young adults (Whiting et al., 1998) and children (Cunningham et al., 2001). Speech-evoked cortical responses in noise differentiate children with normal learning abilities from children with impaired learning (Cunningham et al., 2001), with P1-N1 amplitude excessively affected by background noise in the impaired group.
While cortical evoked potentials have been studied both in the context of maturation and in background noise, no study has yet examined the relationship between cortical responses and SIN perception in children. Such knowledge is necessary to understand why some children succeed more than others in challenging listening conditions. Here, we study the relationship between SIN perception and the cortical encoding of speech in noise in children. We examine cortical encoding of speech in babble noise due to its ecological validity, and also because neural mechanisms of speech-in-noise processing have been shown to differ when using speech babble versus white noise as masking (Scott et al., 2004). Given that the N2 component reflects synthesis of stimulus features into a perceptual representation as indexed by amplitude, we hypothesized that differences between children with good and poor SIN perception would correspond to differences in N2 amplitudes, with greater amplitudes reflecting more resources required to form a perceptual representation.
Methods
Participants
Thirty-two children (ages 8 to 13; mean, 10.41) were recruited from schools in the Chicago area. Audiometric thresholds were measured at octave intervals from 250 to 8000 Hz using air-conduction and from 500 to 4000 Hz using bone-conduction. Participants were excluded if they had pure-tone thresholds greater than 20 dB or air-bone gaps greater than 15 dB at two or more frequencies in either ear. All children had normal cognitive abilities as evidenced by standard scores of ≥ 85 on Wechsler Abbreviated Scales of Intelligence (WASI) Verbal, Performance and Overall scores (Zhu & Garcia, 1999). Informed consent was obtained from all children and their legal guardians. All procedures conformed to the protocol approved by the Institutional Review Board of Northwestern University.
Behavioral Measure of Speech Perception in Noise
Speech understanding in noise was evaluated with a commonly used clinical test, the HINT (Hearing in Noise Test, Bio-logic Systems Corporation, Mundelein, IL), developed using the Bamford-Kowal-Bench (BKB) (Bench et al., 1979) phonetically-balanced sentences. The BKB sentences are appropriate for use with children at the first-grade reading level and above. The HINT uses an adaptive paradigm that varies the level of the target sentence relative to the fixed speech-shaped noise masker until a threshold signal-to-noise ratio (SNR) is determined. For our analyses, we used the HINT-Front condition, in which the target sentences and the masking noise emanate from the same loudspeaker directly in front of the subject. Because previous studies have demonstrated a relationship between working memory and SIN performance (Pichora-Fuller & Souza, 2003; Heinrich et al., 2007; Parbery-Clark et al., 2009a), we assessed working memory using the Woodcock Johnson III Digits Reversed subtest (Mather et al., 2001). In addition, because previous findings have suggested a relationship between SIN performance and reading (Bradlow et al., 2003; Ziegler et al., 2005), we assessed reading and phonological processing using the Woodcock Johnson III Word Attack and Basic Reading (Woodcock et al., 1989–1990), Test of Oral Word Reading Efficiency (Torgesen et al., 1999), and the Comprehensive Test of Phonological Processing (Wagner et al., 1999).
Participant Groups
Top and bottom SIN groups (16 in each group) were formed from the top and bottom halves of the group distribution of the HINT-Front scores. These groups did not differ in average pure tone audiometric thresholds from 250 to 8000 Hz (p = 0.924), verbal IQ (p = 0.230), or working memory (p = 0.178). There were significant differences in age (p = 0.031) between the top SIN (mean, 11.13; S.D., 1.46) and bottom SIN (mean, 9.69; S.D., 1.62) groups. Refer to Table 1 for specific means, standard deviations, and independent t-test p-values.
Table 1.
The means and standard deviations for top and bottom SIN groups are listed for HINT-Front SNR scores, pure-tone averages (.5 to 4 kHz), age, WASI-Verbal standard scores, WJIII Digits Reversed and WJIII Reading standard scores, TOWRE Total (TOWRE-T) standard scores, and CTOPP Rapid Naming (CTOPP-RN) and Phonological Awareness (CTOPP-PA) standard scores.
| Measure t-test p value |
HINT Front SNR (<0 .001) |
Age (years) (0.031) |
Pure Tone Average (dB) (0.941) |
Verbal IQ (WASI) (0.230) |
WJIII – Basic Reading (0.070) |
TOWRE- Total (0.223) |
CTOPP- RN (0.648) |
CTOPP- PA (0.648) |
Auditory Memory (WJIII – Rev. Digits) (0.178) |
|---|---|---|---|---|---|---|---|---|---|
|
Top SIN Mean (SD) |
−4.49 (.72) |
11.13 (1.46) |
3.31 (4.13) |
114.19 (16.68) |
110.25 (15.41) |
107.37 (21.68) |
98.88 (17.00) |
103.56 (14.21) |
103.38 (16.70) |
|
Bottom SIN Mean (SD) |
−2.30 (1.18) |
9.69 (1.62) |
3.2 (3.79) |
106.81 (17.34) |
99.75 (16.17) |
98.12 (20.33) |
95.69 (21.77) |
98.88 (15.05) |
96.94 (8.37) |
Electrophysiology
Stimulus
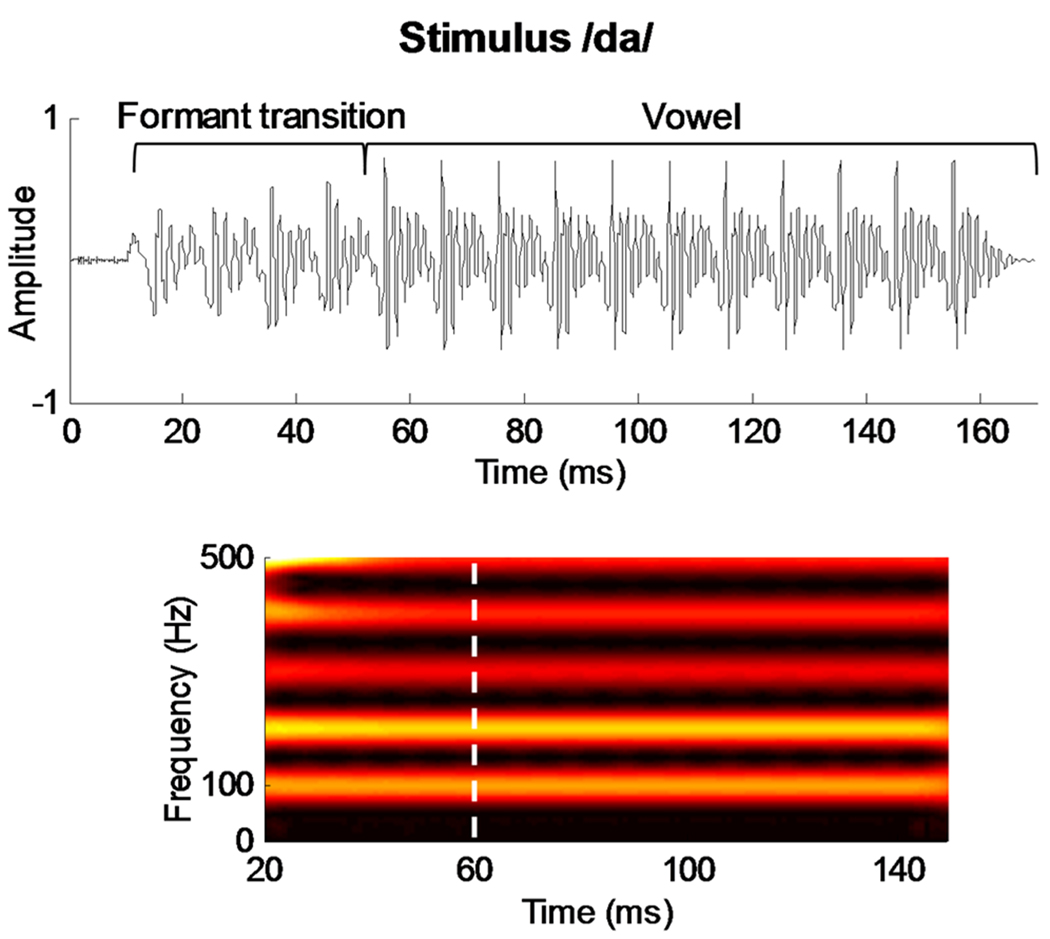
The speech syllable [da] was a six-formant syllable synthesized at a 20 kHz sampling rate using a Klatt synthesizer (Klatt, 1980). The duration of the syllable is 170 ms, the first 5 ms of which is a burst of noise, representing the initial unvoiced portion of the stop consonant preceding the voicing onset. Except for the initial noise burst, this syllable was voiced throughout with a steady fundamental frequency (F0 = 100 Hz). The formant transition from the [d] to the [a] is 50 ms in duration and is characterized by a linearly rising first formant F1 (400 to 720 Hz) and linearly falling F2 (1,700 to 1,240 Hz) and F3 (2,580 to 2,500 Hz). F4 (3,300 Hz), F5 (3,750 Hz), and F6 (4900 Hz) remain constant for the duration of the stimulus. The steady state (i.e., vowel) portion is characterized by a constant F1 (720 Hz), F2 (1,240 Hz), F3 (2,500 Hz), F4 (3,300 Hz), F5 (3,750 Hz), and F6 (4900 Hz). The [da] stimulus was presented in the right ear at 80 dB SPL through an insert earphone (ER-3, Etymotic Research, Elk Grove Village, IL) via the stimulus presentation software NeuroScan Stim2, Sound module (Compumedics Inc., Charlotte, NC). The stimulus waveform and spectrogram are shown in Figure 1.
Figure 1.
Top: The acoustic waveform of the target stimulus [da]. The formant transition and vowel regions are bracketed. Bottom: The spectrogram (stronger amplitudes represented with brighter colors). The boundary between the consonant-vowel formant transition and the steady-state vowel portion of the syllable is marked by a dashed white line.
Recording
Cortical responses were recorded using NeuroScan Acquire 4.3 with a 32-channel electrode cap that incorporates a subset of the International 10–20 system (Jasper, 1958). Reference electrodes were placed on the right and left earlobes. Additional electrodes were placed above the left eye and adjacent to the outer canthus of the left eye. Impedances were maintained < 5 kΩ at each electrode site. The [da] syllable was presented to the right ear with a 1 s inter-stimulus interval. The left ear was unoccluded and participants were told they were not required to pay attention to the stimuli in the right ear. They watched movies of their choice with the soundtrack presented in sound field at < 40 dB SPL. The use of movies ensured participant cooperation, minimized movement-related artifacts, and enabled the participants to sit quietly for 90-minute sessions (total time required for cap application and ERP recording).
ERPs were recorded to the stimulus in two conditions: quiet and six-talker babble background noise. The six-talker babble, created by mixing six tracks of semantically anomalous but grammatically correct sentences in Cool Edit Pro 2.1 (Syntrillium Software, 2003), combined four female and two male voices. The 4.7 second babble track was presented at a signal-to-noise ratio of +10 dB in a continuous loop. The Stim2 Sound program automatically tracks the level of the babble relative to the [da] syllable, keeping the signal-to-noise (SNR) constant at +10 dB. The quiet and noise conditions were presented in blocked presentation mode.
Data Reduction
Data processing and analysis were performed in Matlab (The MathWorks, Inc., Natick, MA). In order to remove the vertical electrooculographic (VEOG) blink artifact, a spatial filtering algorithm in Neuroscan 4.3 Edit was used to perform a principal components analysis decomposition of the blink while retaining the EEG activity of interest. The file was then bandpass filtered from 1 to 40 Hz (24dB/octave) and averaged over a window of −100 to 500 ms, with time 0 corresponding to the stimulus onset. After the EEG epochs were baseline corrected (shifted so that their mean voltage was zero), an artifact rejection criterion of +/− 100 µV was applied to remove sweeps which contained large myogenic (muscle) noise. The remaining sweeps were each correlated with an average of all artifact-free sweeps. The sweeps were then ranked according to how well they correlated with the average, and only the top 70% of sweeps with the highest correlations were included in the final average response, which included approximately 500 sweeps. Global Field Power (GFP) was then computed using the root of the mean of the squared potential differences between all possible electrode pairs within the field. The GFP provided a reference-free measure of cortical activity that was free of experimental bias in selecting a single electrode or set of electrodes (Skrandies, 2005; Sussman et al., 2008).
Data Analysis
P1 and N2 were identified on the grand average GFP and Fz electrode and 50 ms windows (65 to 115 ms for P1 and 175 to 225 ms for N2) centered on the mean peak latencies were used to calculate the mean amplitudes of P1 and N2 for each individual. The same window was used to analyze the recordings from the Fz electrode. The Fz electrode site was chosen in addition to GFP because it had the best signal-to-noise ratio.
Statistical Analyses
A two-way mixed model multivariate ANCOVA was conducted using SPSS (Statistical Package for the Social Sciences; SPSS Inc., Chicago, IL) with group (top SIN vs. bottom SIN) serving as the between-group independent variable and condition (quiet vs. noise) as the within-group variable. The amplitudes for GFP and Fz served as dependent variables. Pearson’s correlations were calculated for the entire group (N=32) between HINT scores and GFP and Fz amplitudes, between HINT scores and age, and between HINT scores and reading. Because of its effect on cortical potentials as well as its relationship with SIN perception, age served as a covariate in all analyses. In SPSS, we used partial correlations and entered HINT scores and GFP and Fz amplitudes as variables and age as the covariate. For the repeated measures MANCOVA, we entered the amplitudes for GFP and Fz in both quiet and noise, the HINT group as the between-subjects factor, and age as the covariate. In a secondary analysis, we created a subset of 24 children ages 9 to 12 to minimize any effects of age. We repeated the correlations as well as the repeated measures ANCOVA with this subset.
Results
Effects of Noise
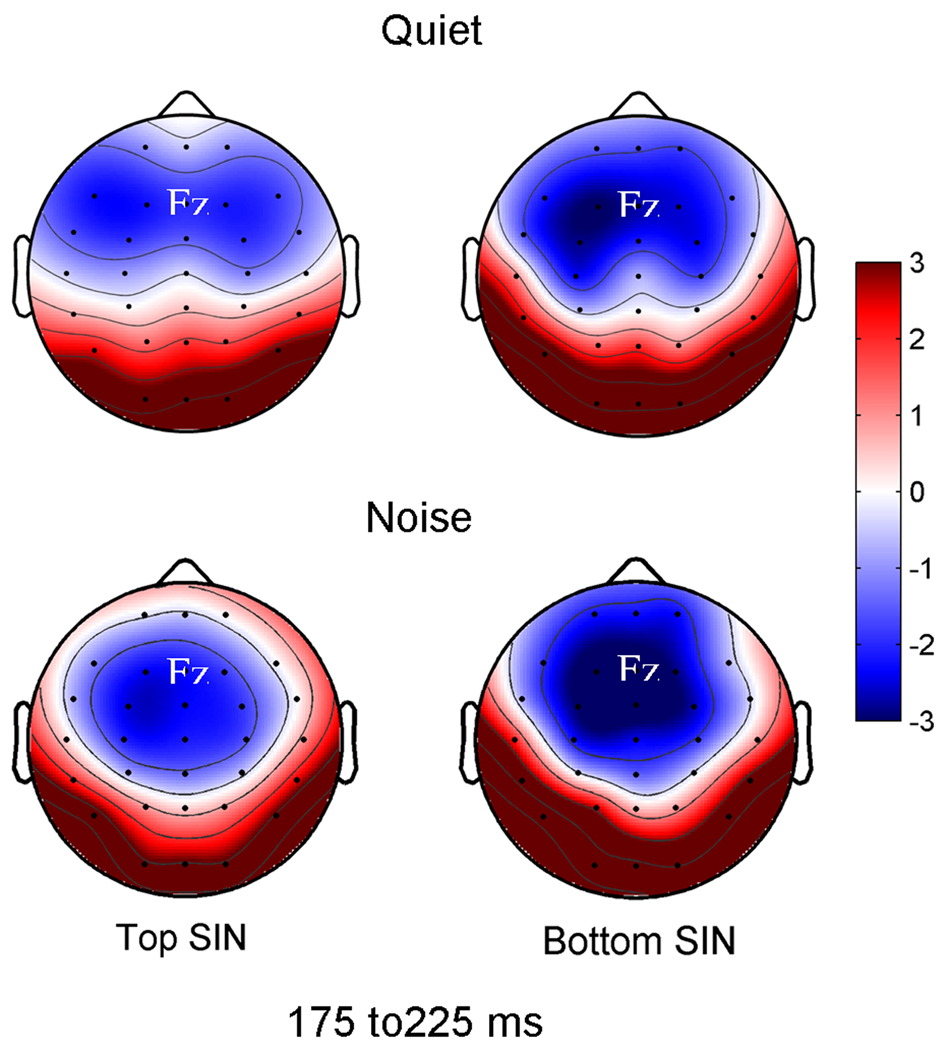
Background noise had a different effect on N2 amplitude in the top and bottom SIN groups. The addition of babble noise resulted in a significant increase in N2 amplitude in the bottom SIN group in the Fz channel (Quiet mean: −2.44; Noise mean: −3.20 µV; F1,14 = 4.861, p = 0.045) and a smaller N2 amplitude increase in GFP (Quiet mean: 2.91; Noise mean: 3.17 µV; F1,14 = 3.647, p = 0.077). In contrast, there were no significant noise-induced amplitude changes in the top group in either the Fz channel (Quiet mean: −2.09; Noise mean: −2.18 µV; F1,14 = 0.699, p = 0.471) or GFP (Quiet mean: 2.66; Noise mean: 2.41 µV; F1,14 = 0.029, p = 0.868) (Figure 2). A significant interaction was found between the quiet and noise conditions for the two groups for GFP (F1,29 = 4.1922, p = 0.048).
Figure 2.
Effects of noise in top vs. bottom SIN groups. Headplots demonstrate greater neural activity in the noise condition in the bottom SIN group in the 175 to 225 ms. There were significant effects of noise for the Fz channel in the bottom SIN group range (F1,14 = 4.861, p = 0.045) but not in the top SIN group (F1,14 = 0.699, p = 0.471).
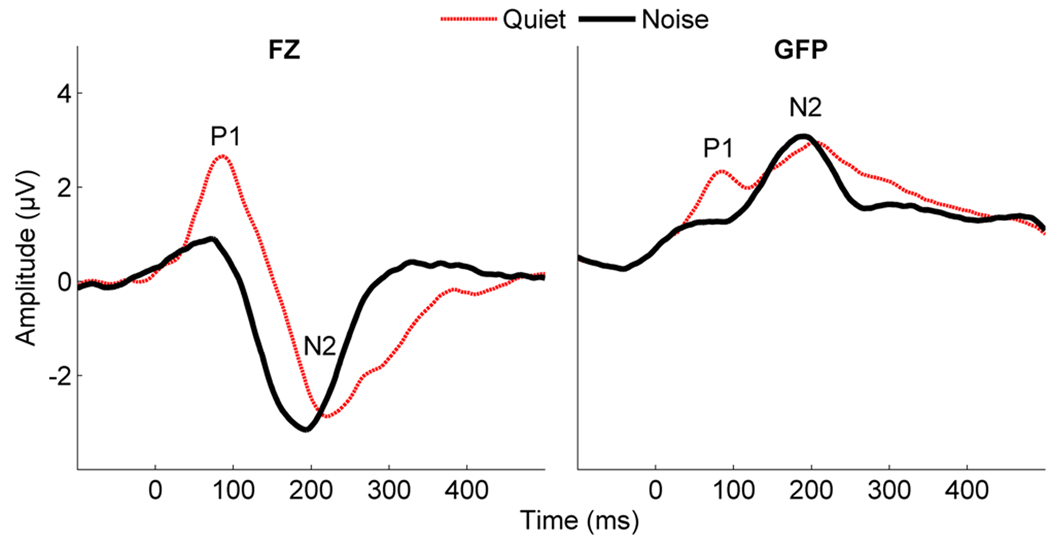
In both the top and bottom SIN groups, the addition of noise resulted in significantly decreased P1 amplitudes. Figure 3 demonstrates a main effect of noise on the P1 component in GFP (F1,31 = 5.644, p = .024) and an effect in the Fz channel that approaches significance (F1,31 = 3.725, p = .056) across all participants (N=32).
Figure 3.
Effects of noise on cortical-evoked responses to speech in grand averages across all subjects (N=32). The P1 component disappears in noise in both GFP and the Fz channel. In addition, the GFP waveform shows more focused activity in the noise condition.
Between Group Differences
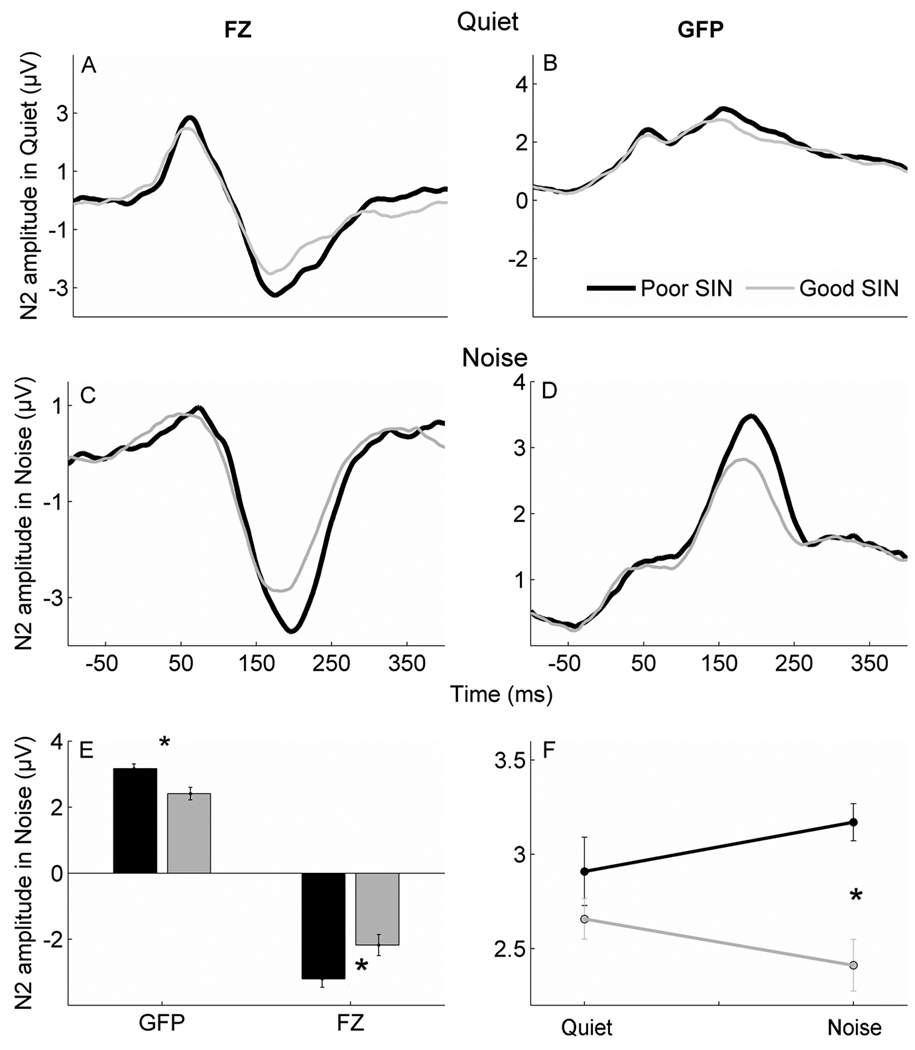
The bottom SIN group had greater N2 amplitudes than the top SIN group in the noise condition, but not in quiet. An effect of group was noted in the noise condition (F2,28 = 4.340, p = 0.049) with post-hoc independent t-tests indicating a significant p-value for GFP (F1,29 = 6.802, p = 0.014) and a p-value close to significant for the Fz channel (F1,29 = 4.1922, p = 0.050). In quiet, there were no significant differences between groups for either GFP (F1,29 = 0.009, p = 0.925) or the Fz channel (F1,29 = 0.082, p = 0.925) (Figure 4).
Figure 4.
Differences between top and bottom SIN groups in the quiet and noise conditions. Greater amplitudes are noted in the poor SIN group compared to the good group in both GFP and the Fz channel in noise but not in quiet. Top: Grand average waveforms in quiet show no significant differences in the Fz channel (A) or GFP (B) between the bottom SIN group (black) and the top SIN group (gray). Middle: Grand average waveforms in the noise condition demonstrate higher N2 amplitudes in the bottom SIN group (black) compared to the top SIN group (gray) for both the Fz channel (C) and GFP (D). Bottom right (E): Bar graphs demonstrated group differences in the noise condition bottom SIN > top SIN (p < 0.05). Bottom left (F): A significant interaction between the quiet and noise conditions for the two groups in GFP (p < 0.05) with bottom SIN > top SIN only in noise.
Correlations
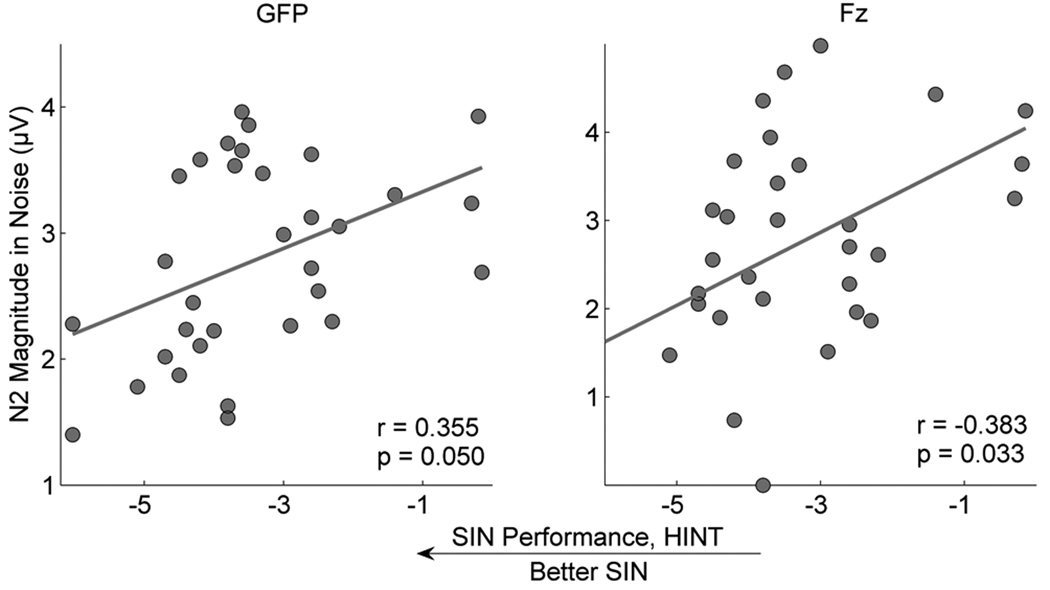
Correlations using the entire group (N=32) were significant between HINT scores and N2 amplitudes in the noise condition for the Fz electrode (r = −0.383, p = 0.033) and approaching significance for GFP (r = 0.355, p = 0.050) and (Figure 5) when partialling for age. SIN performance correlated with reading on WJIII Basic Reading (r = −0.392, p = 0.027) and TOWRE Total (r = −.383, p = 0.031) but did not correlate with phonological processing as assessed by CTOPP cluster scores (Rapid Naming: r = −0.130, p = 0.478; Phonological Awareness cluster score: r = −0.147, p = 0.422). As noted in Table 1, there were no significant differences for any of the reading or phonological processing measures between the top and bottom SIN groups.
Figure 5.
Relationships between N2 magnitudes and SIN perception. Scatterplots of the relationships between HINT-Front raw scores and N2 magnitudes in the noise condition in GFP and the Fz channel, demonstrating that better SIN perception is related to smaller N2 magnitudes in noise.
The differences in N2 magnitude between top and bottom SIN groups could not be attributed to differences in the overall noise levels of the participants. There were no group differences in SNR, computed as the root mean square amplitude (RMS) of the response portion (50 to 250 ms) of the waveform divided by the RMS of the pre-stimulus portion (−100 to 0 ms), in GFP (F1,29 = 0.266, p = 0.610) or the Fz (F1,29= 0.000, p = .983) electrode in the noise condition.
Effects of Age
There was no effect of age for either group (F2,28 = 1.559, p = 0.228) or condition (F2,28 = 1.422, p = 0.258), indicating that age was not a significant factor in our findings. Although age correlated with SIN performance (r = −0.420, p = 0.017), correlations between HINT and N2 amplitudes in noise remained significant when partialling for age, as noted above.
To demonstrate that SIN perception rather than age is the principal factor in our results, we restricted the age range to 9 to 12 year olds, eliminating 8 children from the dataset, thereby creating more closely age-matched top and bottom SIN groups. This analysis (N = 24), revealed that age did not correlate with HINT (r = 0.276, p = 0.193), and top and bottom SIN groups were not significantly different in age (p = 0.198). The correlations between HINT scores and N2 amplitudes were slightly stronger with this subset of children after partialling for age (Fz: r = −0.426, p = 0.043; GFP: r = 0.510, p = 0.013).
In this data subset, N2 amplitude significantly increases in noise in the bottom SIN group in GFP (Quiet mean: 2.60; Noise mean: 3.13 µV; F1,11 = 5.869, p = 0.034), and this increase in noise approaches significance in the Fz channel (Quiet mean: −1.81; Noise mean: −2.88 µV; F1,11 = 4.638, p = 0.054), but not in the top SIN group in either GFP (Quiet mean: 2.82; Noise mean: 2.52 µV; F1,11 = 1.102, p = 0.316) or the Fz channel (Quiet mean: −2.28; Noise mean: −2.16 µV; F1,11 = 0.078, p = 0.785). A significant interaction was found between the quiet and noise conditions for the two groups for GFP (F1,21 = 6.955, p = 0.015). The persistence of these results in a smaller age-restricted subset supports our hypothesis that N2 amplitude reflects differences in SIN perception.
Discussion
Our aim was to examine the relationship between SIN perception and cortical encoding of speech in noise in children. We found considerable variability in child SIN perception, as reflected by a standardized measure of hearing in noise. To examine the nature of this variability, we divided participants into top and bottom SIN groups. Overall we found that irrespective of group, P1 component was significantly reduced in the noise condition. Bottom SIN perceivers had greater N2 amplitudes in noise than top SIN perceivers.
The reduction of P1 amplitude across both groups indicates that sensory representation, as indexed by P1 (Sharma et al., 2002), is affected by the addition of noise. It has been suggested that the P1 component reflects a non-specific, pre-perceptual response to acoustic stimuli (Shtyrov et al., 1998; Ceponiene et al., 2005) and that the N2 component reflects a higher-order processing of sound content. We therefore expected to see differences in the N2 component, and not in P1. This selective enhancement of specific response components has also been found in musicians, in that musical experience does not result in an overall increase in gain but rather enhancement of salient aspects of the response (Lee et al., 2009; Strait et al., 2009). For example, musicians have stronger responses to the combination tones and higher harmonics of musical chords (important for melody recognition) but not for the fundamental frequency (Lee et al., 2009; reviewed in Kraus & Chandrasekaran, in press). Therefore, the selective enhancement of N2 co-occurring with a decrease in P1 amplitudes supports the notion that these cortical peaks reflect different aspects of auditory processing.
The finding of larger rather than smaller N2 amplitudes in the bottom SIN perceivers suggests that the top SIN perceivers may be recruiting fewer neural resources due to greater neural efficiency. Relationships between neural efficiency and cortical amplitudes have been previously offered as an explanation for the finding that cortical amplitudes are correlated with higher IQ scores (Robaey et al., 1995; Zhang et al., 2006), and our study implies a similar possible relationship between neural efficiency and SIN perception. The addition of babble noise results in an apparent increase in neural activity or effort, even when children are not attending to the stimulus. Decreased N2 amplitudes in the good SIN group may reflect greater inhibitory control (Ceponiene et al., 2002), a necessary function for suppressing unwanted background noise. Children with greater inhibitory ability, as determined by N2 amplitude, may be able to suppress the unwanted effects of background noise more effectively, thereby improving signal quality. The relationship between SIN perception and the inhibitory effects of the medial olivocochlear (MOC) auditory efferent system has been demonstrated (Kim et al., 2006; de Boer & Thornton, 2008). The high frequency range of MOC efferent function, as measured by contralateral suppression of distortion product otoacoustic emissions, correlates with speech perception ability in older adults (Kim et al., 2006). Furthermore, it has been noted that auditory training can result in improvements in speech perception that correlate with an increase in MOC activity (de Boer & Thornton, 2008). In addition to these known effects of peripheral inhibition on SIN ability, our results suggest a link between cortical inhibition and SIN perception.
An important aspect of our findings is that children in the bottom SIN group differed from those in the top SIN group only in the noise condition. This suggests that children with poor SIN do not suffer from a general sensory deficit. Rather, these results suggest a noise-specific difficulty that appears only when speech is processed under challenging listening conditions. Thus, the children with poor SIN performance may suffer from a noise-exclusion issue, similar to the deficits described by previous studies in the visual domain (Sperling et al. 2005). A noise-exclusion deficit may indicate a less efficient auditory system that is unable to dynamically adapt to challenging backgrounds (Chandrasekaran et al. 2009; Ahissar et al. 2006; Anderson et al., 2006). Noteworthy is that auditory expertise (musical training) is linked to enhanced perceptual and neural processing of speech in noise, suggesting that SIN ability is at least in part experience-dependent (Parbery-Clark et al., 2009b; Chandrasekaran & Kraus, 2010).
Noise-induced increases in evoked magnetic fields are thought to enhance concurrent sound segregation, ultimately leading to improved SIN perception (Alain et al., 2009). The greater amplitudes in children with poor SIN may be indicative of greater neural effort to facilitate the sound segregation process. Such results are consistent with imaging studies demonstrating that older adults with poor SIN perception use greater cognitive resources than younger adults with good SIN perception, who show sparser processing of speech in noise (Wong et al., 2009).
The finding of greater cortical-evoked deficits in children with poor SIN is a further demonstration that mechanisms central to the cochlea can contribute to deficits in SIN perception (Pienkowski and Eggermont, 2009; 2010). This aligns with studies showing a link between SIN perception and subcortical encoding of spectrotemporal features of speech in children (Cunningham et al., 2001; Hornickel et al., 2009; Anderson et al., 2010) and adults (Parbery-Clark et al., 2009b). Similarly, deficits in subcortical temporal processing have been linked to specific language impairment and dyslexia (Basu et al., 2010; Banai et al., 2005; 2009; Billiet & Bellis, in press). While peripheral hearing impairment is known to reduce spectrotemporal resolution contributing to impaired SIN ability, children and adults with audiometrically-normal hearing can have still experience SIN deficits indicating that hearing in noise depends on other factors besides audiometric thresholds (Billiet & Bellis, in press; Lewis et al., 2010). These factors likely reflect interplay of corticofugal and afferent processes. Although we employed a passive paradigm, it is likely that preconscious attentional processes affect the top-down modulation of responses (Tervaniemi et al., 2009; Hugdahl et al., 2003).
In conclusion, our findings demonstrate a relationship between higher-level perception and obligatory cortical activity and, specifically, that greater N2 response magnitude in noise is associated with poorer SIN perception. These differences in cortical processing emerge only in challenging listening conditions. Cortical processing in children is malleable in response to short-term training (Warrier et al., 2004; Hayes et al., 2003); therefore, the evidence of deficits in central auditory processing suggests the viability of auditory training targeting these deficits.
Acknowledgements
This work was funded by the National Institutes of Health grant RO1 DC01510 to N.K. and the Hugh Knowles Center of Northwestern University. We wish to thank Trent Nicol, Erika Skoe, Dana Strait, and Jane Hornickel for their helpful comments and suggestions regarding the manuscript and for their help with data analysis. We would especially like to thank the children and their families who participated in the study.
References
- Ahissar M, Nahum M, Nelken I, Hochstein S. Reverse hierarchies and sensory learning. Philos. T. R. Soc. B. 2009;364:285–299. doi: 10.1098/rstb.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain C, Quan J, McDonald K, Roon PV. Noise-induced increase in human auditory evoked neuromagnetic fields. Eur. J. Neurosci. 2009;30:132–142. doi: 10.1111/j.1460-9568.2009.06792.x. [DOI] [PubMed] [Google Scholar]
- Banai K, Nicol T, Zecker S, Kraus N. Brainstem timing: Implications for cortical processing and literacy. J. Neurosci. 2005;25:9850–9857. doi: 10.1523/JNEUROSCI.2373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai K, Hornickel JM, Skoe E, Nicol T, Zecker S, Kraus N. Reading and subcortical auditory function. Cereb. Cort. 2009;19:2699–2707. doi: 10.1093/cercor/bhp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu M, Krishnan A, Weber-Fox C. Brainstem correlates of temporal auditory processing in children with specific language impairment. Dev. Sci. 2010;13:77–91. doi: 10.1111/j.1467-7687.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- Bench J, Kowal Ã, Bamford J. The BKB (Bamford-Kowal-Bench) Sentence Lists for Partially-Hearing Children. Brit. J. Audiol. 1979;13:108–112. doi: 10.3109/03005367909078884. [DOI] [PubMed] [Google Scholar]
- Billiet C, Bellis TJ. The relationship between brainstem temporal processing and performance on tests of central auditory function in children with reading disorders. J. Speech Lang. Hear. Res. doi: 10.1044/1092-4388(2010/09-0239). (in press) [DOI] [PubMed] [Google Scholar]
- Bradlow AR, Kraus N, Hayes E. Speaking Clearly for Children With Learning Disabilities: Sentence Perception in Noise. J. Speech Lang. Hear. Res. 2003;46:80–97. doi: 10.1044/1092-4388(2003/007). [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Shestakova A, Balan P, Alku P, Yiaguchi K, Naatanen R. Children's auditory event-related potentials index sound complexity and "Speechness". Intl. J. Neurosci. 2001;109:245. doi: 10.3109/00207450108986536. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Rinne T, Näätänen R. Maturation of cortical sound processing as indexed by event-related potentials. Clin Neurophysiol. 2002;113:870–882. doi: 10.1016/s1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Alku P, Westerfield M, Torki M, Townsend J. ERPs differentiate syllable and nonphonetic sound processing in children and adults. Psychophysiology. 2005;42:391–406. doi: 10.1111/j.1469-8986.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran B, Hornickel J, Skoe E, Nicol TG, Kraus N. Context-dependent encoding in the human auditory brainstem relates to hearing speech in noise: Implications for developmental dyslexia. Neuron. 2009;64:311–319. doi: 10.1016/j.neuron.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Kraus N. Music, Noise-Exclusion, and Learning. Music Perception. 2010;27:297–306. [Google Scholar]
- Cunningham J, Nicol T, Zecker S, Kraus N. Speech-Evoked Neurophysiologic Responses in Children with Learning Problems: Development and Behavioral Correlates of Perception. Ear Hear. 2000;21:554–568. doi: 10.1097/00003446-200012000-00003. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Nicol TG, Zecker SG, Bradlow AR, Kraus N. Neurobiologic responses to speech in noise in children with learning problems: deficits and strategies for improvement. Clin. Neurophsyiol. 2001;112:758–767. doi: 10.1016/s1388-2457(01)00465-5. [DOI] [PubMed] [Google Scholar]
- de Boer J, Thornton ARD. Neural Correlates of Perceptual Learning in the Auditory Brainstem: Efferent Activity Predicts and Reflects Improvement at a Speech-in-Noise Discrimination Task. J. Neurosci. 2008;28:4929–4937. doi: 10.1523/JNEUROSCI.0902-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EA, Warrier CM, Nicol TG, Zecker SG, Kraus N. Neural plasticity following auditory training in children with learning problems. Clin Neurophysiol. 2003;114:673–684. doi: 10.1016/s1388-2457(02)00414-5. [DOI] [PubMed] [Google Scholar]
- Heinrich A, Schneider BA, Craik FI. Investigating the influence of continuous babble on auditory short-term memory performance. Quarterly J. of Exp. Psych. 2007;61:735–751. doi: 10.1080/17470210701402372. [DOI] [PubMed] [Google Scholar]
- Hornickel J, Skoe E, Nicol T, Zecker S, Kraus N. Subcortical differentiation of stop consonants relates to reading and speech-in-noise perception. Proc. Natl. Acad. Sci. 2009;106:13022–13027. doi: 10.1073/pnas.0901123106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K, Bodner T, Weiss E, Benke T. Dichotic listening performance and frontal lobe function. Cog. Brain Res. 2003;16:58–65. doi: 10.1016/s0926-6410(02)00210-0. [DOI] [PubMed] [Google Scholar]
- Jasper H. The 10–20 electrode system of the International Federation. Electroencephal. and Clin. Neurophysiol. 1958;10:370–375. [Google Scholar]
- Jodo E, Kayama Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephal. and Clin. Neurophysiol. 1992;82:477–482. doi: 10.1016/0013-4694(92)90054-l. [DOI] [PubMed] [Google Scholar]
- Kim S, Frisina RD, Frisina DR. Effects of age on speech understanding in normal hearing listeners: Relationship between the auditory efferent system and speech intelligibility in noise. Speech Comm. 2006;48:855–862. [Google Scholar]
- Kraus N, Chandrasekeran B. Music training for the development of auditory skills. Nat. Rev. Neurosci. doi: 10.1038/nrn2882. (in press) [DOI] [PubMed] [Google Scholar]
- Lee KM, Skoe E, Kraus N, Ashley R. Selective subcortical enhancement of musical intervals in musicians. J. Neurosci. 2009;29:5832–5840. doi: 10.1523/JNEUROSCI.6133-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D, Hoover B, Choi S, Stelmachowicz P. Relationship between speech perception in noise and phonological awareness skills for children with normal hearing. Ear Hear. 2010 doi: 10.1097/AUD.0b013e3181e5d188. vol. Publish Ahead of Print, p10.1097/AUD.1090b1013e3181e1095d1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather N, Wendling BJ, Woodcock RW. Essentials of WJ III Tests of Achievement Assessment. New York: John Wiley & Sons, Inc.; 2001. [Google Scholar]
- Parbery-Clark A, Skoe E, Lam C, Kraus N. Musician Enhancement for Speech-In-Noise. Ear Hear. 2009a;30:653–661. doi: 10.1097/AUD.0b013e3181b412e9. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A, Skoe E, Kraus N. Musical Experience Limits the Degradative Effects of Background Noise on the Neural Processing of Sound. J. Neurosci. 2009b;29:14100–14107. doi: 10.1523/JNEUROSCI.3256-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Souza PE. Effects of aging on auditory processing of speech. Inter. J. Audio.l. 2003;42:S11–S16. [PubMed] [Google Scholar]
- Pienkowski M, Eggermont JJ. Intermittent exposure with moderate-level sound impairs central auditory function of mature animals without concomitant hearing loss. Hear. Res. 2010;261:30–35. doi: 10.1016/j.heares.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Eggermont JJ. Long-term, partially-reversible reorganization of frequency tuning in mature cat primary auditory cortex can be induced by passive exposure to moderate-level sounds. Hear. Res. 2009;257:24–40. doi: 10.1016/j.heares.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin. Neurophysiol. 2000;111:220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Robaey P, Cansino S, Dugas M, Renault B. A comparative study of ERP correlates of psychometric and Piagetian intelligence measures in normal and hyperactive children. Electroencephal. and Clin. Neurophysiol. /Evoked Potentials Section. 1995;96:56–75. doi: 10.1016/0013-4694(94)00174-j. [DOI] [PubMed] [Google Scholar]
- Scott SK, Stuart R, Lindsay W, Richard JSW. A positron emission tomography study of the neural basis of informational and energetic masking effects in speech perception. J. Acoust. Soc. Am. 2004;115:813–821. doi: 10.1121/1.1639336. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman M, Spahr A. A Sensitive Period for the Development of the Central Auditory System in Children with Cochlear Implants: Implications for Age of Implantation. Ear Hear. 2002;23:532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kraus N, J. McGee T, Nicol TG. Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel syllables. Electroencephal .and Clin. Neurophysiol. /Evoked Potentials Section. 1997;104:540–545. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Shield BM, Dockrell JE. The effects of noise on children at school: a review. Building Acoustics. 2003;10:97–116. [Google Scholar]
- Shield BM, Dockrell JE. The effects of environmental and classroom noise on the academic attainments of primary school children. J. Acoust. Soc. Am. 2008;123:133–144. doi: 10.1121/1.2812596. [DOI] [PubMed] [Google Scholar]
- Shtyrov Y, Kujala T, Ahveninen J, Tervaniemi M, Alku P, Ilmoniemi RJ, Näätänen R. Background acoustic noise and the hemispheric lateralization of speech processing in the human brain: Magnetic mismatch negativity study. Neuroscience Letters. 1998;251:141–144. doi: 10.1016/s0304-3940(98)00529-1. [DOI] [PubMed] [Google Scholar]
- Skrandies W. Brain mapping of visual evoked activity - topographical and functional components. Acta Neurologica Taiwanica. 2005;14:164–178. [PubMed] [Google Scholar]
- Sperling AJ, Zhong-Lin L, Manis FR, Seidenberg MS. Deficits in perceptual noise exclusion in developmental dyslexia. Nat. Neurosci. 2005;8:862–863. doi: 10.1038/nn1474. [DOI] [PubMed] [Google Scholar]
- Strait DL, Skoe E, Kraus N, Ashley R. Musical experience and neural efficiency: effects of training on subcortical processing of vocal expressions of emotion. Eur. J. Neurosci. 2009;29:661–668. doi: 10.1111/j.1460-9568.2009.06617.x. [DOI] [PubMed] [Google Scholar]
- Sussman E, Steinschneider M, Gumenyuk V, Grushko J, Lawson K. The maturation of human evoked brain potentials to sounds presented at different stimulus rates. Hear. Res. 2008;236:61–79. doi: 10.1016/j.heares.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaniemi M, Kruck S, Baene WD, Schröger E, Alter K, Friederici AD. Top-down modulation of auditory processing: effects of sound context, musical expertise and attentional focus. Eur. J. Neurosci. 2009;30:1636–1642. doi: 10.1111/j.1460-9568.2009.06955.x. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of word reading efficiency (TOWRE) Austin, Tx: Pro-Ed; 1999. (ed. Vol.) [Google Scholar]
- Wagner RK, Torgesen JK, Flowers DL. Comprehensive test of phonological processing (CTOPP) Austin, TX: Pro-Ed; 1999. (ed. Vol.) [Google Scholar]
- Warrier CM, Johnson KL, Hayes EA, Nicol T, Kraus N. Learning impaired children exhibit timing deficits and training-related improvements in auditory cortical responses to speech in noise. Exp. Brain Re.s. 2004;157:431–441. doi: 10.1007/s00221-004-1857-6. [DOI] [PubMed] [Google Scholar]
- Whiting KA, Martin BA, Stapells DR. The Effects of Broadband Noise Masking on Cortical Event-Related Potentials to Speech Sounds /ba/ and /da. Ear Hear. 1998;19:218–231. doi: 10.1097/00003446-199806000-00005. [DOI] [PubMed] [Google Scholar]
- Wible B, Nicol T, Kraus N. Correlation between brainstem and cortical auditory processes in normal and language-impaired children. Brain. 2005;128:417–423. doi: 10.1093/brain/awh367. [DOI] [PubMed] [Google Scholar]
- Wong PCM, Jin JX, Gunasekera GM, Abel R, Lee ER, Dhar S. Aging and cortical mechanisms of speech perception in noise. Neuropsychologia. 2009;47:693–703. doi: 10.1016/j.neuropsychologia.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Allen (TX): Riverside Publishing; 1989–1990. [Google Scholar]
- Zhang QA, Shi JA, Luo YA, Zhao DB, Yang JC. Intelligence and information processing during a visual search task in children: an event-related potential study. Neuroreport. 2006;17:747–752. doi: 10.1097/01.wnr.0000215774.46108.60. [DOI] [PubMed] [Google Scholar]
- Zhu J, Garcia E. The Weschler Abbreviated Scale of Intelligence (WASI) New York: Psychological Corporation; 1999. [Google Scholar]
- Ziegler JC, Pech-Georgel C, George F, Alario F-X, Lorenzi C. Deficits in speech perception predict language learning impairment. Proc. Natl. Acad. Sci. 2005;102:14110–14115. doi: 10.1073/pnas.0504446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JC, Pech-Georgel C, George F, Lorenzi C. Speech-perception-in-noise deficits in dyslexia. Dev. Sci. 2009;12:732–745. doi: 10.1111/j.1467-7687.2009.00817.x. [DOI] [PubMed] [Google Scholar]