Abstract
Objectives
Genome-wide association meta-analyses have recently identified multiple loci associated with blood pressure. We sought to validate previously identified blood pressure loci by replication in a single large homogenous population-based cohort, and to identify new genome-wide significant loci using both conventional and expression-guided approaches.
Methods
We examined the associations of 18 SNPs with genome-wide significance (P < 5.0 × 10−8, ‘primary’), and 13 suggestive SNPs (5.0 × 10−8 < P < 2.6 × 10−5, ‘secondary’), all from previously established genome-wide association studies, with self-reported blood pressure in 23,019 women from the Women’s Genome Health Study. We then targeted for replication 12 gene expression-associated SNPs (eSNPs) that were also previously associated with blood pressure phenotypes.
Results
Using these replication strategies, we found confirmatory evidence for 13/18 primary SNPs, 3/13 secondary SNPs, and 4/12 eSNPs in the Women’s Genome Health Study. Meta-analysis combining the Women’s Genome Health Study results with prior study results revealed one previously unrecognized blood pressure locus with genome-wide significance: a BLK-GATA4-adjacent region (P = 3.2 × 10−8).
Conclusion
In this analysis, conventional and eSNP-guided strategies were complementary and illustrate two ways for extending initial genome-wide association results for discovery of new genes involved in human disease. Using this strategy, we report a newly identified blood pressure locus, BLK-GATA4, that may further understanding of the complex genetic pathways regulating blood pressure.
Keywords: hypertension, genetics, blood pressure, women
Introduction
Hypertension (HTN) affects one in four adults worldwide, a proportion that is expected to increase to one in three by 2025 [1]. Due to the substantial heritability (30–60%) of blood pressure (BP) [2] and the dire clinical consequences of HTN, understanding the genetic basis of BP regulation is of great interest. Rare mutations in renal salt handling genes have been shown to be associated with substantial BP effects [3] but they are too rare to explain a substantial proportion of inter-individual BP variation within the population. Similarly, candidate gene studies to date have only explained a small fraction of the population-wide variation in BP, and data have been inconsistent [4, 5, 6].
Because BP is influenced by multiple common genetic variants, each with small independent effects, the identification of common polymorphisms associated with HTN susceptibility has been challenging. Recently, two large-scale genome-wide association studies (GWAS), both conducted by meta-analysis, identified common genetic variants associated with BP in individuals of European ancestry. The Cohorts for Heart and Aging Research in Genome Epidemiology (CHARGE) and Global BPgen consortia each identified 8 BP loci with P-values < 5.0 × 10−8 [7, 8]. We sought to validate previously identified BP loci by in silico replication, and to identify new genome-wide significant loci associated with BP in a large and homogeneous prospective cohort study, the Women’s Genome Health Study (WGHS) [9]. We used two complementary strategies to identify truly associated BP loci that might not satisfy conventional thresholds for genome-wide significance (P < 5 × 10−8), which is expected to minimize false positive results but may be too restrictive. First, SNPs were prioritized for replication solely based on association p-value in previous GWAS efforts. Second, the selection procedure was guided by an independent source of biological function through targeting SNPs that had not only prior evidence of some association with BP but also were associated with gene expression patterns in liver or lymphocyte derived cell-lines.
Methods
Participants
Analysis was conducted in the WGHS, a prospective cohort of participants in the Women’s Health Study (WHS) [10], which enrolled female health professionals from North America, ≥ 45 years of age without cardiovascular disease or other major chronic illnesses [11]. The WGHS is the subset of 28,345 (70.6%) of the original WHS participants, who provided a sample at baseline for blood-based analysis. The primary aim of the WGHS was to create a comprehensive genome-wide database of > 360 000 SNPs among initially healthy WHS participants, and to link genome-wide data to the existing epidemiologic databank of the parent WGHS.
Determination of BP phenotype
Baseline BP was self-reported in categories by the female health professionals, a group in which self-reported BP has been highly accurate when compared to chart review [12, 13, 14]. BP was broken into 9 categories for systolic BP (SBP) (< 110, 110–119, 120–129, 130–139, 140–149, 150–159, 160–169, 170–179, ≥ 180 mmHg), and 7 for diastolic BP (DBP) (<65, 65–74, 75–84, 85–89, 90–94, 95–104, ≥ 105 mmHg). The midpoint of each category was used for analysis. The use of self-reported BP in categories was acknowledged to be a limitation in our study from the inception, as it would predominantly limit our power to detect smaller associations, and underestimate the strength of any positive associations found. Prevalent HTN was defined as a history of physician-diagnosed HTN and ongoing HTN treatment, or SBP ≥ 140 or DBP ≥ 90 mmHg. To account for treatment effect, 10 and 5 mmHg were added to the measured SBP and DBP, respectively, if a participant was taking antihypertensive medication [15].
Genotyping
Detailed methods regarding genotyping have been previously described [9]. In brief, SNP genotyping was performed using the Illumina Infinium II assay [16] applied to the HumanHap300 Duo “+” platform, including a genome-wide set of haplotype-tagging SNP markers suitable for populations with European ancestry [17]. A focused panel of 45,882 missense and haplotype-tagging SNPs was included (the “+” content) to enhance coverage of genomic regions believed to be of significance in cardiovascular diseases. In the final experimental data, all samples had complete genotype information for 98% of the SNPs; all SNPs met Hardy-Weinberg equilibrium using an exact test (P > 1.0 × 10−6) and had successful genotyping for at least 90% of the samples. Among participants of European ancestry, imputation using Mach v. 1.0.16 (http://www.sph.umich.edu/csg/abecasis/mach/) and linkage disequilibrium relationships from the HapMap CEU population allowed estimation of dose of the coded allele at SNPs not determined experimentally or missing from samples which nonetheless met quality standards. The allele dose estimates were used in all regression analysis to evaluate SNP associations with BP phenotypes.
Selection of candidate SNPs for replication
We used two complementary methods to validate previous findings and identify novel associations with BP. First, on the basis of previously published p-value alone, candidate SNPs were selected from BP-related loci identified in previous studies, and classified as primary when the prior association was genome-wide significant (P < 5.0 × 10−8), or secondary when it was suggestive (5.0 × 10−8 < P < 5.6 × 10−5). For rs5761405, imputation was unavailable in Global BPgen, and a proxy rs2092201 within the same locus (r2 = 1.0) was selected for joint analysis with CHARGE and external validation in the WGHS. Similarly, rs880315 was used as a proxy for rs12046278 (r2 = 0.86). Two additional loci implicated in prior association studies (STK39 [18] and CDH13 [19]) were also included among the secondary SNPs for a total of 13.
Second, we selected an additional set of candidate SNPs for replication by targeting SNPs that were both associated with cis-gene expression levels in liver (n=3,322) [20] or immortalized lymphocyte (n=10,823) [21] derived cell lines and also associated with BP in previous analysis from CHARGE [7] The threshold for significance of association with BP in CHARGE was defined as P < 1/n, for n equal to the number of tissue specific SNPs interrogated, a threshold that was expected to generate one false-positive result per tissue examined. For liver expression, this threshold was P < 3.0 × 10−4; for lymphoblastoid cell lines the threshold was P < 9.2 × 10−5. A total of 24 eSNPs meeting these criteria from 12 loci were identified in CHARGE. Of these, one eSNP per locus was selected for a total of 12 eSNPs for analysis in the WGHS.
Statistical analysis
Association of SNP genotypes with BP in the WGHS was evaluated with cross-sectional analyses using an additive genetic model. Multiple linear regression was used for BP, and logistic regression for dichotomous HTN outcomes. We addressed the multiple testing as follows: a total of 43 SNPs were tested (18 primary SNPs, 13 secondary SNPs, and 12 eSNPs), thus significant replication for the primary trait was considered at P = 0.05/43 = 1.2 × 10−3. The primary trait was defined as either SBP or DBP, whichever association was more significant in prior studies. For non-primary traits, significant replication was considered at P = 0.05/(43×2) = 5.8 × 10−4. One-tailed P-values were used if the beta coefficients for the allele were in the same direction as in prior studies.
Using meta-analysis with inverse variance weighting and fixed effects, the WGHS results were combined with findings from prior studies. Of note, CDH13 was not included in the meta-analysis, as the previous report used a dominant, rather than additive genetic model [18]. Similarly, STK39 was not included, because the beta-coefficients and standard errors were not available from the previous study [19]. Neither locus met replication criteria within WGHS.
A trait-specific genetic BP risk score was developed using loci that had been identified as associated with BP at a P < 5.0 × 10−8 in prior or present analyses. For a locus with more than one candidate SNP, the SNP with the largest absolute beta-coefficient was selected. A risk score was constructed for each individual in the WGHS by summing the weighted dose of the risk alleles at the chosen SNPs, with weights equal in magnitude to the beta-coefficients in previous reports [7, 8] and risk allele chosen to be consistently associated with higher BP across all score SNPs. Both SBP and DBP risk scores were used to calculate odds ratios of HTN, with the reference group being those with median scores (SBP risk score = 8, DBP risk score = 4.5).
Results
The WGHS sample with BP data and genome-wide genotyping that was eligible for this investigation included 23,019 women of confirmed, self-reported European descent with mean age of 54 ± 7 years. The mean SBP was 125 ± 15 mmHg, DBP was 77 ± 10 mmHg, and 5,699 (25%) had a physician diagnosis of HTN at baseline. Thirteen percent were taking anti-hypertensive medications, 12% were smokers, and 44% were using hormone replacement therapy at baseline.
Replication of GWA-derived SNPs
Replication in the WGHS supported associations with 5 of 7 primary SNPs for SBP (5 of 6 SBP loci due to 2 SNPs within the same locus: SH2B3, ATP2B1, MTHFR, CYP17A1 [rs11191548 and rs1004467, pairwise r2 = 0.42], PLEKHA7) at the pre-specified P-value < 1.2 × 10−3. Similarly, 8 of 11 primary SNPs were associated with DBP in the WGHS (6 of 9 DBP loci, CACNB2, ATP2B1, CYP1A2-CSK-ULK3 [rs1378942 and rs6495122, r2 = 0.59], c10orf107, SH2B3 [rs653178 and rs3184504, r2 = 1.0], ZNF652) (Table 1). Among secondary SNPs, 2 of 8 SNPs were associated with SBP (CACNB2, CASZ1), and 1 of 5 with DBP (PLEKHA7) in the WGHS (Table 2). For beta coefficients, please see Supplemental Digital Content, Tables 1 and 2.
Table 1.
Replication of primary SNPs in WGHS by BP trait
| Trait | SNP | Chromosome (Position, NCBI 36.3) | Nearest Gene(s) | Allele (coded/other) | Freq. coded allele | CHARGE/Global BPgen | WGHS | WGHS (non-primary trait)† |
|---|---|---|---|---|---|---|---|---|
| p-value | p-value* | p-value* | ||||||
| SBP | rs3184504 | 12 (110,368,991) | SH2B3 | C/T | 0.51 | 5.7E-07 | 5.0E-06 | 2.3E-04 |
| rs2681492 | 12 (88,537,220) | ATP2B1 | C/T | 0.19 | 3.0E-11 | 6.0E-06 | 5.0E-06 | |
| rs17367504 | 1 (11,785,365) | MTHFR | A/G | 0.84 | 1.0E-05 | 4.0E-05 | 4.0E-05 | |
| rs11191548 | 10 (104,836,168) | NT5C2; CYP17A1 | C/T | 0.09 | 3.0E-07 | 2.1E-04 | 1.1E-03 | |
| rs381815 | 11 (16,858,844) | PLEKHA7 | C/T | 0.73 | 5.8E-07 | 1.1E-03 | 7.5E-05 | |
| rs1004467 | 10 (104,584,497) | CYP17A1 | A/G | 0.90 | 2.0E-06 | 2.8E-03 | 2.1E-03 | |
| rs12946454 | 17 (40,563,647) | ACBD4 | A/T | 0.74 | 4.0E-06 | 3.2E-01 | 2.8E-01 | |
| DBP | rs11014166 | 10 (18,748,804) | CACNB2 | A/T | 0.65 | 8.7E-07 | 2.2E-06 | 3.2E-05 |
| rs2681472 | 12 (88,533,090) | ATP2B1 | A/G | 0.83 | 3.7E-08 | 3.2E-06 | 1.6E-06 | |
| rs1378942 | 15 (72,864,420) | CSK | A/C | 0.66 | 6.0E-08 | 2.8E-05 | 4.6E-05 | |
| rs1530440 | 10 (63,194,597) | c10orf107 | C/T | 0.81 | 3.0E-06 | 3.1E-05 | 2.3E-06 | |
| rs653178 | 12 (110,492,139) | ATXN2; SH2B | C/T | 0.49 | 1.0E-07 | 2.1E-04 | 5.0E-06 | |
| rs3184504 | 12 (110,368,991) | SH2B3 | C/T | 0.51 | 1.7E-08 | 2.3E-04 | 5.0E-06 | |
| rs6495122 | 15 (72,912,698) | CSK-ULK3 | A/C | 0.43 | 8.1E-07 | 3.0E-04 | 9.5E-04 | |
| rs16948048 | 17 (44,795,465) | ZNF652 | A/G | 0.63 | 5.0E-06 | 4.9E-04 | 3.8E-03 | |
| rs2384550 | 12 (113,837,114) | TBX3-TBX5 | A/G | 0.35 | 1.3E-07 | 1.9E-02 | 3.6E-03 | |
| rs16998073 | 4 (81,403,365) | FGF5 | A/T | 0.76 | 7.0E-09 | 8.0E-02 | 5.5E-04 | |
| rs9815354 | 3 (41,887,655) | ULK4 | A/G | 0.17 | 7.8E-07 | 2.2E-01 | 9.5E-02 |
One-tailed p-value, a priori significance at P < 1.2 × 10−3 for primary trait, and P < 5.8 × 10−4 for the non-primary trait (met when indicated in bold face font)
Non-primary trait is DBP if the association was first reported with SBP in previous studies, and SBP if previous association was with DBP.
Table 2.
Replication of secondary SNPs in WGHS by BP trait
| Trait | SNP | Chromosome (Position, NCBI 36.3) | Nearest Gene | Allele (coded/other) | Freq. coded allele | Prior study* | WGHS | WGHS (non-primary trait)‡ |
|---|---|---|---|---|---|---|---|---|
| p-value | p-value† | p-value† | ||||||
| SBP | rs11014166 | 10 (18,748,804) | CACNB2 | A/T | 0.65 | 2.1E-06 | 3.2E-05 | 2.2E-06 |
| rs880315 | 1 (10,719,453) | CASZ1 | C/T | 0.35 | 2.1E-07 | 7.0E-04 | 7.0E-03 | |
| rs448378 | 3 (170,583,593) | MDS1 | A/G | 0.53 | 1.3E-06 | 3.4E-03 | 3.7E-02 | |
| rs1910252 | 8 (49,569,915) | EFCAB1 | C/T | 0.83 | 1.7E-06 | 3.0E-02 | 2.4E-01 | |
| rs2736376 | 8 (11,155,175) | MTMR9 | C/G | 0.13 | 1.9E-06 | 2.2E-01 | 3.3E-01 | |
| rs2092201 | 22 (24,910,475) | SEZ6L | C/T | 0.96 | 2.6E-05 | 2.3E-01 | 4.2E-01 | |
| rs7571613 | 2 (190,513,907) | PMS1 | A/G | 0.82 | 7.2E-07 | 3.4E-01 | 4.4E-01 | |
| rs6749447 | 2 (168,749,632) | STK39 | G/T | 0.27 | 1.6E-07 | 3.8E-01 | 1.5E-01 | |
| DBP | rs11024074 | 11 (16,873,795) | PLEKHA7 | C/T | 0.30 | 2.8E-07 | 3.4E-04 | 4.4E-03 |
| rs7016759 | 8 (49,574,969) | EFCAB1 | C/T | 0.17 | 1.9E-06 | 2.5E-01 | 3.9E-02 | |
| rs13423988 | 2 (68,764,770) | GPR73-ARGHGAP25 | C/T | 0.83 | 1.1E-06 | 3.9E-01 | 4.2E-01 | |
| rs13401889 | 2 (190,618,804) | MSTN | C/T | 0.22 | 9.7E-07 | 4.0E-01 | 4.1E-01 | |
| rs11646213 | 16 (66,940,701) | CDH13 | A/T | 0.41 | 5.6E-05 | 3.2E-01 | 2.8E-01 |
Prior study is CHARGE (Levy et al [7]), except for STK39 (Wang et al [18]) and CDH13 (Org et al [19])
One-tailed p-value, a priori significance at P < 1.2 × 10−3 for primary trait, and P < 5.8 × 10−4 for the non-primary trait (met when indicated in bold face font)
Non-primary trait is DBP if the association was first reported with SBP in previous studies, and SBP if previous association was with DBP.
The association between candidate SNPs and BP was robust across different BP traits, as loci replicating with SBP in the WGHS also showed evidence of association with DBP, and vice versa. There was also significant association between 3 of 12 replicated primary, and 1 of 3 secondary SNPs with hypertension (Supplemental Digital Content, Table 3). Overall, the directional association (sign of beta) in the WGHS matched CHARGE or Global BPgen for 27 of 29 SNPs. Two secondary SNPs that were not associated with BP in WGHS had discordant beta coefficients compared with previous studies (rs13423988, P=0.39, and rs13401889, P=0.40).
Replication of expression-associated SNPs
Four of 12 loci selected for potential functional effects through their association with gene expression met replication thresholds for BP phenotypes within WGHS (Table 3). The validated eSNPs were rs7537765 (CLCN6 near MTHFR, NPPA; expressed gene CLCN6), rs6495126 (MPI, SCAMP2, ULK3; expressed genes ULK3, AK001918, RPP25), rs6601414 (MSRA; expressed gene C8orf5), and rs2898290 (BLK, GATA4; expressed genes C8orf5, FAM167A, BLK).
Table 3.
Association of CHARGE eSNPS with blood pressure phenotypes in the WGHS
| SNP | Chromosome (Position, NCBI 36.3) | Nearest Gene(s) | Expressed gene | Allele (coded/other) | Freq. coded allele | CHARGE p-values* | WGHS p-values† | ||
|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | ||||||
| Liver eSNPs | |||||||||
| rs7537765 | 1 (11,809,890) | CLCN6; MTHFR;NPPA | CLCN6 | A/G | 0.84 | 1.8E-05 | 9.2E-04 | 5.2E-05 | 1.1E-04 |
| rs249209 | 5 (79,902,965) | ANKRD34B | HSS00169533 | G/T | 0.41 | 8.5E-05 | 0.07 | 0.85 | 0.73 |
| rs525381 | 12 (255,053) | KDM5A; SLC6A13 | CCDC77; SLC6A12 | A/G | 0.28 | 9.3E-05 | 0.14 | 0.04 | 0.06 |
| rs739496 | 12 (110,372,042) | SH2B3; ATXN2 | HSS00340376 | A/G | 0.79 | 2.9E-04 | 1.3E-05 | 0.01 | 0.02 |
| rs6495126 | 15 (72,962,079) | MPI;SCAMP2; ULK3 | ULK3;RPP25; AK001918; | A/G | 0.30 | 3.0E-04 | 3.6E-05 | 1.1E-04 | 3.7E-05 |
| Lymphoblastoid cell line eSNPs | |||||||||
| rs1384883 | 1 (74,274,065) | LRRIQ3 | LRRC44;BC042056 | C/T | 0.46 | 9.9E-03 | 7.2E-05 | 0.51 | 0.56 |
| rs12466395 | 2 (190,488,943) | PMS1 | ORMDL1 | A/G | 0.22 | 8.8E-04 | 5.3E-05 | 0.37 | 0.28 |
| rs7571613 | 2 (190,513,907) | MSTN | ORMDL1;PMS1 | A/G | 0.82 | 7.3E-07 | 2.2E-06 | 0.67 | 0.88 |
| rs2272007 | 3 (41,971,140) | ULK4 | ULK4 | C/T | 0.83 | 0.87 | 1.5E-06 | 0.22 | 0.37 |
| rs4572871 | 4 (83,979,911) | SEC31A; SCD5 | SCD5 | A/G | 0.21 | 2.3E-05 | 9.7E-03 | 2.4E-03 | 3.1E-03 |
| rs6601414 | 8 (10,014,158) | MSRA | C8orf5 | A/G | 0.46 | 3.0E-04 | 3.4E-05 | 6.9E-04 | 0.01 |
| rs2898290 | 8 (11,471,318) | BLK;GATA4 | C8orf5;BLK; FAM167A; | C/T | 0.53 | 2.3E-05 | 7.0E-03 | 4.1E-04 | 0.02 |
Bold face font indicates statistical significance met in CHARGE at P = 1/n (n = number of eSNPs interrogated in CHARGE)
Bold face font indicates replication threshold met in WGHS at P < 1.2 × 10−3 for primary traits, and P < 5.8 × 10−4 for non-primary traits
Meta-analysis of WGHS results and prior studies
Table 4 provides meta-analysis results combining WGHS data with prior study results (for beta coefficients please see Supplemental Digital Content, Table 4). For GWA-derived candidate SNPs, one new genome-wide association emerged for SBP (rs880315; CASZ1), a locus that recently replicated with genome-wide significance in the Japanese population.[22] One additional association was suggestive with P = 9.1 × 10−8 (rs448378; MDS1). One locus previously associated with DBP emerged as newly genome-wide significant in association with SBP (CACNB2), and one locus previously associated with SBP emerged as newly significant in association with DBP (PLEKHA7). In meta-analysis of eSNP results, there were 3 loci that were associated with BP at P < 5.0 × 10−8: CLCN6-MTHFR-NPPA and ULK3 met genome-wide significance in prior studies, whereas the association at BLK-GATA4 was newly significant. Of note, the eSNP near ULK3 (rs6495126), and the GWA-derived SNP at ULK3 (rs6495122) are in modest linkage disequilibrium (r2 = 0.49), suggesting that SNP-SNP correlation may not fully explain this finding, and that rs6495126 may potentially represent a separate locus of interest. One additional locus was suggestive with P = 2.5 × 10−7 (SEC31A-SCD5).
Table 4.
Meta-analysis of WGHS and CHARGE or Global BPgen results*
| Trait | SNP | Nearest gene(s) | Allele (coded/other) | Freq. coded allele | p-value* | |
|---|---|---|---|---|---|---|
| Primary SNPs | SBP | rs3184504 | SH2B3 | C/T | 0.51 | 1.9E-11 |
| rs2681492 | ATP2B1 | C/T | 0.19 | 4.9E-15 | ||
| rs17367504 | MTHFR | A/G | 0.84 | 1.2E-09† | ||
| rs11191548 | NT5C2;CYP17A1 | C/T | 0.09 | 1.1E-09† | ||
| rs381815 | PLEKHA7 | C/T | 0.73 | 1.1E-08 | ||
| rs1004467 | CYP17A1 | A/G | 0.90 | 1.1E-07 | ||
| rs12946454 | ACBD4 | A/T | 0.74 | 4.4E-04† | ||
| DBP | rs11014166 | CACNB2 | A/T | 0.65 | 9.7E-12 | |
| rs2681472 | ATP2B1 | A/G | 0.83 | 7.0E-13 | ||
| rs1378942 | CSK;CYP1A2 | A/C | 0.66 | 3.5E-11† | ||
| rs1530440 | c10orf107 | C/T | 0.81 | 9.6E-10† | ||
| rs653178 | ATXN2;SH2B3 | C/T | 0.49 | 1.2E-09† | ||
| rs3184504 | SH2B3 | C/T | 0.51 | 5.6E-11 | ||
| rs6495122 | CSK-ULK3 | A/C | 0.43 | 2.2E-09 | ||
| rs16948048 | ZNF652 | A/G | 0.63 | 4.5E-08† | ||
| rs2384550 | TBX3-TBX5 | A/G | 0.35 | 1.3E-07 | ||
| rs16998073 | FGF5 | A/T | 0.76 | 2.1E-07† | ||
| rs9815354 | ULK4 | A/G | 0.17 | 3.7E-05 | ||
| Secondary SNPs | SBP | rs11014166 | CACNB2 | A/T | 0.65 | 4.6E-10 |
| rs880315 | CASZ1 | C/T | 0.35 | 5.2E-09 | ||
| rs448378 | MDS1 | A/G | 0.53 | 9.1E-08 | ||
| rs1910252 | EFCAB1 | C/T | 0.83 | 2.6E-06 | ||
| rs2736376 | MTMR9 | C/G | 0.13 | 1.2E-04 | ||
| rs2092201 | SEZ6L | C/T | 0.96 | 6.7E-04 | ||
| rs7571613 | PMS1 | A/G | 0.82 | 2.3E-04 | ||
| DBP | rs11024074 | PLEKHA7 | C/T | 0.30 | 1.1E-09 | |
| rs7016759 | EFCAB1 | C/T | 0.17 | 8.9E-05 | ||
| rs13423988 | GPR73-ARGHGAP25 | C/T | 0.83 | 8.1E-04 | ||
| rs13401889 | MSTN | C/T | 0.22 | 8.7E-04 | ||
| eSNPs | SBP | rs7537765 | CLCN6;MTHFR | A/G | 0.84 | 2.6E-09 |
| rs249209 | ANKRD34B | G/T | 0.41 | 5.0E-03 | ||
| rs525381 | KDM5A;SLC6A13 | A/G | 0.28 | 2.5E-05 | ||
| rs7571613 | MSTN | A/G | 0.82 | 2.3E-04 | ||
| rs4572871 | SEC31A;near SCD5 | A/G | 0.21 | 2.5E-07 | ||
| rs2898290 | BLK;GATA4 | C/T | 0.53 | 3.2E-08 | ||
| DBP | rs739496 | SH2B3;ATXN2 | A/G | 0.79 | 1.2E-06 | |
| rs6495126 | MPI;SCAMP2;ULK3 | A/G | 0.30 | 3.4E-09 | ||
| rs1384883 | LRRIQ3 | C/T | 0.46 | 1.2E-03 | ||
| rs12466395 | PMS1 | A/G | 0.22 | 2.0E-04 | ||
| rs2272007 | ULK4 | C/T | 0.83 | 3.7E-05 | ||
| rs6601414 | MSRA | A/G | 0.46 | 2.0E-06 |
Bold face font indicates genome-wide significance met at P < 5.0 × 10−8
Of note, CDH13 was not included in the meta-analysis, as the previous report used a dominant, rather than additive genetic model [18]. Similarly, STK39 was not included, because the beta-coefficients and standard errors were not available from the previous study [19]
Meta-analysis combines CHARGE and WGHS, unless indicated by footnote, in which case the analysis combines Global BPgen and WGHS results
Blood pressure risk score
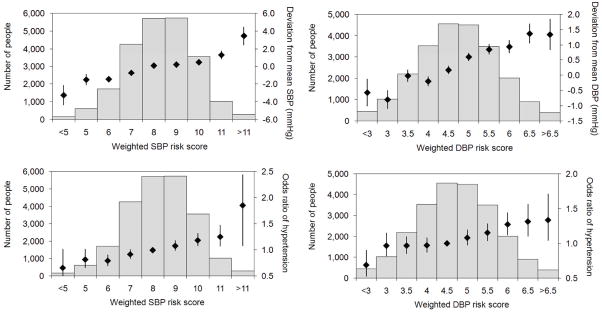
Weighted genetic risk scores were evaluated for the association with the deviation of SBP and DBP from the mean, and the odds ratio for prevalent HTN (Figure 1). As expected, there was a dose-response relationship between increasing risk score and higher BP: the range of the SBP risk score was associated with a 6 mmHg spread in SBP, and the odds ratio of HTN ranged from 0.7 for individuals with a risk score < 5 (95% CI 0.4–1.0, P=0.03), to an odds ratio of 1.9 for a risk score > 11 (95% CI 1.4–2.4, P<0.0001) when compared to individuals with a median risk score of 8. The proportion of total variance in BP (increment in r2) explained in the WGHS by the SBP risk score was 0.3%, and that of the DBP risk score was 0.4%, after accounting for age, age2, and BMI. The relatively minor contribution of aggregate genetic loci to overall variation in BP is consistent with prior studies [7, 8].
Figure 1. SBP and DBP risk scores.
Aggregate effects of risk alleles on blood pressure phenotypes are summarized in a weighted risk score for SBP and DBP, respectively. The relationship between risk score and deviation from mean SBP or DBP are shown in the top panels. Black diamonds indicate the mean BP deviation for each risk score grouping, with black whiskers indicating the standard errors. The bottom panels show black diamonds to indicate the relationship between SBP (left) and DBP (right) risk score and odds ratios of HTN, with black whiskers representing 95% CIs. The p-values for slopes across risk score groups were all highly significant (P < 0.0001 for all 4 comparisons)
Discussion
While demonstrating the utility of a strategy for functional enrichment based on gene expression, the major new finding from this work is the successful identification of novel genome-wide significant BP loci. Our eSNP analysis identified rs2898290 as a novel locus associated with BP on a genome-wide level, an eSNP that is in linkage disequilibrium with a SNP located within GATA4 (rs13259242, r2 = 0.56, distance 159,124 bp, based on HapMap CEU population, release 22). GATA4 is known to be involved in the regulation of brain natriuretic peptide [23], and has been linked to congenital heart disease and the development of cardiac hypertrophy in response to pressure overload [24]. The latter pathway may be mediated via atrial natriuretic peptide, which recently has been shown to reduce GATA4 phosphorylation and GATA4-dependent transcriptional activity, resulting in inhibition of endothelin-1 gene expression in cardiac fibroblasts [25]. While GATA4 has not previously been linked to hypertension, its regulation of endothelin-1 gene expression, a potent vasoconstrictor, is a biologically plausible mechanism by which common variants at the GATA4 locus may influence BP phenotypes.
Furthermore, rs880315 located in an intronic region of CASZ1, a zinc finger transcription factor, was associated with SBP after meta-analysis of WGHS and CHARGE results. While this association is newly genome-wide significant in a population of European descent, it appeared to have a stronger association with BP in the Japanese population, where the association was genome-wide significant for both SBP and DBP.[22] The gene encodes castor homolog-1, a zinc finger transcription factor that is thought to be involved in cell cycle signaling and the regulation of apoptosis. It resides on chromosome 1p36.22, and is expressed in a variety of tissues, including cardiac myocytes. Loss of the chromosomal region around CASZ1 is frequently seen in neuroectodermal tumors [26, 27].
These findings support the concept that targeted searches for variants associated with gene expression may be useful in the identification of significant loci associated with clinical phenotypes in the absence of genome-wide significance. Previous studies have provided a basis for this concept by linking expression-associated SNPs to BP [7] and other phenotypes, such as dyslipidemia [28]. To our knowledge, this is the first study to replicate successfully and by design both conventional and gene expression-associated loci. We provide initial evidence that our eSNP-based approach may be a rapid and cost-effective strategy for identifying relevant loci now that databases of eSNPs are increasingly available. Nevertheless, broader replication efforts are needed to corroborate this approach.
Two recent studies have replicated results from CHARGE and Global BPgen in Asian populations: the Korean Association Resource (KARE) study showed replication for 4 BP loci (ATP2B1, CSK, CYP17A1, and PLEKHA7) [29], and 7 additional loci (CASZ1, MTHFR, ITGA9, FGF5, CYP17A1-CNNM2, ATP2B1, and CSK-ULK3) were replicated in a Japanese study combining 3 separate cohorts [22]. The WGHS is unique in exceeding the sample size of any other single cohort study used in genome-wide association analysis examining BP, and it alone approaches the size of prior consortia. Our study results extend previous findings of CHARGE and Global BPgen by providing external validation of a number of loci including CYP17A1 (a gene associated with a rare Mendelian form of HTN [30]) and ATP2B1 (encoding a plasma membrane calcium/calmodulin-dependent ATPase known to regulate BP in animal models [31]). For functions of other BP-related loci, please see Supplemental Digital Content, Table 5. Unlike prior GWAS, ours was conducted exclusively in women. Despite known sex differences in the natural history of BP [32], it is notable that previous BP genetic loci from cohorts including both men and women could be confirmed in this large cohort of women. Further studies are needed to explore the potential for gene-sex interactions. At the same time, the lack of heterogeneity with respect to sex in the WGHS might have improved our ability to validate loci.
Of the primary BP loci we studied, 4 were not supported by highly significant association within WGHS (PLCD3, TBX3-TBX5, FGF5, and ULK4), but all had the same direction of effects as prior studies (sign of beta). The non-replicating SNPs may reflect associations that are intrinsically weaker in women than men, or may be due to variable SNP imputation quality, complex underlying haplotype structures not accounted for in the analysis, or limitations in power for the given sample size in WGHS.
While our results indicate a strong correlation between a BP genetic risk score and BP traits in WGHS, the aggregate of the effect of the validated loci in the genetic risk score did not explain a large proportion of the inter-individual variability in BP, a finding that is consistent with prior studies [7, 8]. Many BP-related loci, including loci harboring rare variants, remain unidentified, and complex gene-gene and gene-environment interactions are unaccounted for, rendering our risk score incomplete. The role, if any, for the genetic risk score in the prediction of incident HTN and long-term BP-related outcomes is unknown.
Aside from the restriction of the WGHS to the socio-economic group defined by white, female, health care professionals, the major potential limitation of our study is the use of self-reported estimates of BP. However, this limitation would be expected to bias our findings toward the null, limiting the ability to detect associations, and thus is in sharp contrast with the high rate of validation of candidate associations in the current analysis using the WGHS. Prior evidence suggests that within a population of health professionals, self-reported BP appears extremely accurate when validated against directly measured blood pressure [12]. Furthermore, the validity of this approach has been examined in the Nurses’ Health Study, in which 99% of women who reported high blood pressure had their diagnosis confirmed by chart review [13]. Previous analyses from the WHS have shown self-reported BP to be a strong predictor of cardiovascular risk, with relative risks similar in magnitude to other major studies, and SBP is in fact the strongest cardiovascular risk factor after age in WHS, which strengthens the validity of self-reported BP [33, 34]. In a prior report from the WGHS, self-reported BP progression was also associated with NPPA gene polymorphisms, and provides evidence that despite these limitations in outcome assessment, a significant genetic association could be detected [35]. We relied on assigning midpoints from each BP category (spanning up to 10 mmHg for SBP), which may have limited precision of the primary outcome measurement, given that the effect sizes of each locus we detected were < 1 mmHg/allele. Furthermore, we adjusted for antihypertensive treatment effect by adding 10 and 5 mmHg to SBP and DBP, as was done in CHARGE[7]. However, adjustment in Global BPgen was done using 15 and 10 mmHg, respectively, and thus may have decreased our power to validate results specifically driven by Global BPgen[8]. Again, these limitations in assessment of BP would be expected to bias our results toward the null. However, given the limited precision of self-reported BP assessment in categories, we cannot exclude potential small effects and false negative findings of genetic loci evaluated in our study. Finally, validation of our findings in an additional independent sample would be an important consideration in the future.
Conclusion
Replication of several previously identified genetic loci and putative functional variants associated with BP was successful in an independent large cohort of women health professionals of European ancestry. Using a novel approach to select candidate SNPs by incorporating both GWAS-derived loci, as well as a strategy for functional enrichment based on a gene expression, we report a new genetic locus associated with SBP at a genome-wide significance level: GATA4, a gene thought to regulate brain natriuretic peptide and myocyte hypertrophy. Functional characterization of this locus may further understanding of BP regulation and HTN pathophysiology, improve early risk detection, and spawn new therapeutic interventions. In addition, the bioinformatics analysis strategy implemented here to identify new loci is broadly applicable to areas beyond HTN and may hasten a better understanding of the genetic underpinnings of other complex human diseases.
Supplementary Material
Acknowledgments
The authors acknowledge the essential role of the CHARGE Consortium in providing supplemental results to allow application of the genetic risk score to WGHS, as well as meta-analysis of WGHS and CHARGE data.
Funding Sources
The WGHS is supported by HL-043851 and HL-69757 from the National Heart, Lung, and Blood Institute and CA-047988 from the National Cancer Institute, the Donald W. Reynolds Foundation and the Fondation Leducq, with collaborative scientific support and funding for genotyping provided by Amgen. This work was supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (J.E.H., D.L, contract No. N01-HC-25195).
Footnotes
Disclaimers: NONE
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, DeStefano AL, Larson MG, O’Donnell CJ, Lifton RP, Gavras H, et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension. 2000;36:477–483. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koivukoski L, Fisher SA, Kanninen T, Lewis CM, von Wowern F, Hunt S, et al. Meta-analysis of genome-wide scans for hypertension and blood pressure in Caucasians shows evidence of susceptibility regions on chromosomes 2 and 3. Hum Mol Genet. 2004;13:2325–2332. doi: 10.1093/hmg/ddh237. [DOI] [PubMed] [Google Scholar]
- 5.Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4:45–61. doi: 10.1097/00125817-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Chang YP, Liu X, Kim JD, Ikeda MA, Layton MR, Weder AB, et al. Multiple genes for essential-hypertension susceptibility on chromosome 1q. Am J Hum Genet. 2007;80:253–264. doi: 10.1086/510918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridker PM, Chasman DI, Zee RY, Parker A, Rose L, Cook NR, Buring JE. Rationale, design, and methodology of the Women’s Genome Health Study: a genome-wide association study of more than 25,000 initially healthy american women. Clin Chem. 2008;54:249–255. doi: 10.1373/clinchem.2007.099366. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Pare G, Parker A, Zee RY, Danik JS, Buring JE, et al. Loci related to metabolic-syndrome pathways including LEPR, HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women’s Genome Health Study. Am J Hum Genet. 2008;82:1185–1192. doi: 10.1016/j.ajhg.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 12.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 13.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 14.Conen D, Ridker PM, Buring JE, Glynn RJ. Risk of cardiovascular events among women with high normal blood pressure or blood pressure progression: prospective cohort study. BMJ. 2007;335:432. doi: 10.1136/bmj.39269.672188.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension. 2003;41:207–210. doi: 10.1161/01.hyp.0000044938.94050.e3. [DOI] [PubMed] [Google Scholar]
- 16.Gunderson KL, Steemers FJ, Ren H, Ng P, Zhou L, Tsan C, et al. Whole-genome genotyping. Methods Enzymol. 2006;410:359–376. doi: 10.1016/S0076-6879(06)10017-8. [DOI] [PubMed] [Google Scholar]
- 17.Gunderson KL, Kuhn KM, Steemers FJ, Ng P, Murray SS, Shen R. Whole-genome genotyping of haplotype tag single nucleotide polymorphisms. Pharmacogenomics. 2006;7:641–648. doi: 10.2217/14622416.7.4.641. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, O’Connell JR, McArdle PF, Wade JB, Dorff SE, Shah SJ, et al. From the Cover: Whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci. 2009;106:226–231. doi: 10.1073/pnas.0808358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Org E, Eyheramendy S, Juhanson P, Gieger C, Lichtner P, Klopp N, et al. Genome-wide scan identifies CDH13 as a novel susceptibility locus contributing to blood pressure determination in two European populations. Hum Mol Genet. 2009;18:2288–2296. doi: 10.1093/hmg/ddp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schadt EE, Molony C, Chudin E, Hao K, Yang X, Lum PY, et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 2008;6:e107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, Wong KC, et al. A genome-wide association study of global gene expression. Nat Genet. 2007;39:1202–1207. doi: 10.1038/ng2109. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi F, Isono M, Katsuya T, Yamamoto K, Yokota M, Sugiyama T, et al. Blood pressure and hypertension are associated with 7 Loci in the Japanese population. Circulation. 121:2302–2309. doi: 10.1161/CIRCULATIONAHA.109.904664. [DOI] [PubMed] [Google Scholar]
- 23.He Q, Mendez M, LaPointe MC. Regulation of the human brain natriuretic peptide gene by GATA-4. Am J Physiol Endocrinol Metab. 2002;283:E50–57. doi: 10.1152/ajpendo.00274.2001. [DOI] [PubMed] [Google Scholar]
- 24.Pikkarainen S, Tokola H, Kerkela R, Ruskoaho H. GATA transcription factors in the developing and adult heart. Cardiovasc Res. 2004;63:196–207. doi: 10.1016/j.cardiores.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Glenn DJ, Rahmutula D, Nishimoto M, Liang F, Gardner DG. Atrial natriuretic peptide suppresses endothelin gene expression and proliferation in cardiac fibroblasts through a GATA4-dependent mechanism. Cardiovasc Res. 2009;84:209–217. doi: 10.1093/cvr/cvp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan ZR, Wang R, Solomon J, Luo X, Sun H, Zhang L, Shi Y. Identification and characterization of survival-related gene, a novel cell survival gene controlling apoptosis and tumorigenesis. Cancer Res. 2005;65:10716–10724. doi: 10.1158/0008-5472.CAN-05-2176. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Yang X, Tan F, Cullion K, Thiele CJ. Molecular cloning and characterization of human Castor, a novel human gene upregulated during cell differentiation. Biochem Biophys Res Commun. 2006;344:834–844. doi: 10.1016/j.bbrc.2006.03.207. [DOI] [PubMed] [Google Scholar]
- 28.Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong KW, Jin HS, Lim JE, Kim S, Go MJ, Oh B. Recapitulation of two genomewide association studies on blood pressure and essential hypertension in the Korean population. J Hum Genet. 2010;55:336–341. doi: 10.1038/jhg.2010.31. [DOI] [PubMed] [Google Scholar]
- 30.Costa-Santos M, Kater CE, Auchus RJ. Two prevalent CYP17 mutations and genotype-phenotype correlations in 24 Brazilian patients with 17-hydroxylase deficiency. J Clin Endocrinol Metab. 2004;89:49–60. doi: 10.1210/jc.2003-031021. [DOI] [PubMed] [Google Scholar]
- 31.Monteith GR, Kable EP, Kuo TH, Roufogalis BD. Elevated plasma membrane and sarcoplasmic reticulum Ca2+ pump mRNA levels in cultured aortic smooth muscle cells from spontaneously hypertensive rats. Biochem Biophys Res Commun. 1997;230:344–346. doi: 10.1006/bbrc.1996.5956. [DOI] [PubMed] [Google Scholar]
- 32.Khoury S, Yarows SA, O’Brien TK, Sowers JR. Ambulatory blood pressure monitoring in a nonacademic setting. Effects of age and sex. Am J Hypertens. 1992;5:616–623. doi: 10.1093/ajh/5.9.616. [DOI] [PubMed] [Google Scholar]
- 33.Glynn RJ, L’Italien GJ, Sesso HD, Jackson EA, Buring JE. Development of predictive models for long-term cardiovascular risk associated with systolic and diastolic blood pressure. Hypertension. 2002;39:105–110. doi: 10.1161/hy1201.097199. [DOI] [PubMed] [Google Scholar]
- 34.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145:21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 35.Conen D, Cheng S, Steiner LL, Buring JE, Ridker PM, Zee RY. Association of 77 polymorphisms in 52 candidate genes with blood pressure progression and incident hypertension: the Women’s Genome Health Study. J Hypertens. 2009;27:476–483. doi: 10.1097/hjh.0b013e32832104c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.