Abstract
Objective
Toll like receptors (TLR) bridge innate immunity and host responses, including inflammation. Sterile inflammation such as a venous thrombus (VT) may involve TLR signaling, including TLR9.
Methods and Results
TLR9 signaling on thrombus resolution was investigated using a mouse model of stasis VT. VT were significantly larger in TLR9 −/− mice as compared with WT at 2 and 8 days, despite a 2 fold increase in thrombus PMN at 2d, and monocytes at 8d, while thrombus collagen and neovascularization was 55% and 37% less at 8d. Coincidently, decreased fibrinogen and increased thrombin-antithrombin complex were observed in TLR9 −/− mice thrombi. Vein wall IFNα, IL1α, and IL2 was significantly reduced in TLR9 −/− mice as compared to WT. Thrombus cell death pathway markers were not significantly altered at 2d, but caspase 1 was reduced in TLR9 −/− thrombi at 8d. MyD88 confers TLR9 intracellular signaling, but MyD88−/− mice had similar VT resolution as WT. However, inhibition of the NOTCH ligand delta-like 4 was associated with larger VT. Finally, stimulation with a TLR9 agonist was associated with smaller VT.
Conclusion
TLR9 signaling is integral for early and mid VT resolution through modulation of sterile inflammation, maintaining a TH1 milieu, and effects on the thrombosis pathway.
Introduction
Deep vein thrombosis (DVT) resolution is an inflammatory process.1 Well known factors that predispose to a DVT include systemic infectious processes such as pneumonia and urinary tract infection.2 Current therapy primarily involves anticoagulation with heparin, followed by a vitamin K antagonist.3 While effective, the risks of bleeding with anticoagulation are significant, and many patients have contraindications to anticoagulation.
Venous thrombosis (VT) pathophysiology is now better understood from experimental studies. Leukocytes, chemokines, and proinflammatory cytokines are all involved in the process of VT resolution and vein wall healing.1, 4–6 This process resembles sterile wound healing in the distinct phases of neutrophil (PMN) and monocyte influx, followed by fibrosis. Similarly, sterile inflammation results in apoptosis and necrosis, with matrix breakdown and release of cellular products, such as nucleotides and proteins, which must be cleared by the organism7–10 Although septic thrombophlebitis occurs, most DVT are sterile, and the pathways for clearance of the sterile thrombus have not been elucidated.
Recently, the role of toll like receptor (TLR) signaling in modulating sterile inflammation has become better defined in experimental injury models.7, 9–11 TLR signaling pathways bridge innate and adaptive immunity, are highly conserved, and most signal via the adaptor molecule MyD8811 TLR9 is located on endosomes, and binds viral DNA and as well as certain forms of self DNA.7, 11 In particular, the hypomethylated CpG motif binds to TLR9, conferring type I interferon expression and promotes a Th1 mileau.12 The CpG containing oligodeoxynucleotides (ODN) are immune adjuvants, and are beneficial for certain models of experimental disease.13, 14 Neutrophils, monocytes, lymphocytes and dendritic cells express TLR9,11 and TLR9 agonists can directly stimulate PMN function.15
In this study, the role of TLR9 signaling was examined in early and mid time VT resolution. We found that targeted TLR9 deletion or exogenous inhibition was associated with impaired VT resolution, and altered the local Th1 cytokine milieu, as well as several measures of clotting. Further, VT resolution was independent from MyD88, a primary intracellular signaling protein for TLR9, but is dependent, in part, on the NOTCH ligand, delta-like ligand 4 (DLL4). Finally, using a TLR9 stimulatory ODN, we were able to accelerate early VT resolution.
Methods
Animal Model
Male mice (BALB/c, C57/BL6, 20–30 gm, wild type [WT]) and BALB/c TLR9 −/− and C57/BL6 MyD88 −/− were used for all studies.16 For certain experiments, the TLR9 inhibitor, ODN 2088 or the TLR9 agonist, ODN 1826 and respective control ODNs (Invivogen, San Diego, CA), 200ug IP, 72 hrs preligation was administered to BALB/c mice, with harvest at day 2.13 In other experiments, anti-DLL4 anti-serum16 or rabbit serum control, 0.5 ml IP was given 1 day pre- and 1 day post-ligation to BALB/c mice, with harvest at day 2. For all surgical procedures, the mice underwent general anesthesia with isoflorane/O2. All animal studies were done with University of Michigan Animal Use Committee approval.
Stasis venous thrombosis (VT) was induced by IVC ligation.4, 6, 17 Briefly, the model consists of a laparotomy with 6 – 0 prolene ligation of the IVC below the renal veins and division of all visible side branches. At sacrifice, the thrombosed IVC was carefully dissected and removed for histological, or protein and molecular analysis after thrombus-vein wall separation. Mice were sacrificed on days 2 and 8, representing the early and mid time point resolution. The thrombus is easy to remove at, or before 8 days. Thrombus resolution is measured by true weight in gms (early) or by weight/length in mg/mm (late) time points. The remnant thrombus represents the balance of thrombosis and thrombolysis at the given time point of tissue harvest.
Thrombus bacterial culture
Swabs of thrombus and serum, under sterile conditions, were placed on blood and MacConkey agar plates (Remel, Lenexa, KS) using a sterile I-loop spreader. Positive control blood and MacConkey agar plates were swabbed as well. Plates were placed in a 37° incubator and were checked for bacterial growth at 9 and 16 hours.
Histological Analysis/ Immunohistochemical Staining/ Trichrome Staining
Immunohistochemical staining was performed on the paraffin embedded tissue sections (5 µm).4, 6 The following antigens were stained for: anti-PMN, (Accurate Chemical, Westbury, NY), anti-vWF (1:100, Serotec, Oxford, UK), anti-TLR9 (Imgenex, San Diego, CA), or anti-MAC 2 (1:200; Cedarlane, Hornby, ON). After processing, the slides were counterstained with hematoxylin and cover-slipped. In a blinded fashion, positive cells in 5 high power fields (hpf, 1000X) radially around the IVC wall were counted and totaled.4, 6
Masson's trichrome staining was done on each section taken from the same region of thrombosed IVC4 and was quantified by computerized analysis.
Antigen Analysis by Bioplex and ELISA
Thrombus or vein wall homogenate underwent quantification of peptide mediators (normalized to total protein in the sample) for mouse TNFα, IL4, IL12, IL13, IL2, and IFNα, using the bio-plex multi-antigen technology (Biorad, Hercules, CA). 16, 18 IL1α was determined by a commercially available ELISA per manufacturer instructions (R and D). Serum and thrombus homogenate was assessed for urokinase plasminogen activator (uPA) and plasminogen activator inhibitor -1 (PAI-1) by ELISA (Innovative Research, Novi, MI).
Quantitative (Real-Time) Polymerase Chain Reaction
Expression of genes of interest were determined as follows:6, 17 messenger RNA was isolated by treatment of vein wall segments with TRIzol reagent and reverse transcribed by incubating with Oligo-(dT) primer and M-MLV Reverse Transcriptase (Life Technologies, Carlsbad, CA) at 94°C for 3 minutes followed by 40°C for 70 minutes. The resultant cDNA was amplified by Taq Polymerase (Promega, Madison, WI) in the SmartCycler quantitative polymerase chain reaction system (Cepheid, Sunnyvale, CA). SYBR Intercalating Dye (Roche, Indianapolis, IN) was used to monitor levels of cDNA amplification for each gene. The genes included: B-Actin, IL-1a; Jagged 1; Jagged 2; Notch1; Notch2; Notch3; Notch4; TLR9, TLR3, TLR4.
Analysis of Thrombosis Characteristics
Using the thrombus tissue homogenate preparation, assessment of plasmin, Factor X (FX), fibrinogen (all from Innovative Research, Novi, MI) and Thrombin- Antithrombin Complex (TAT, Enzyme Research Laboratories, South Bend, IN) were performed according to manufacturer’s instructions. Blood was also taken from the various genotypes of mice at baseline and subjected to thromboelastography.19
Analysis of Thrombus Cell Death / Caspase-1 / dsDNA Content
Thrombus homogenate was subjected to the cell death assay measuring histone associated DNA fragments (Roche, Mannheim, Germany), caspase-1 activity (R and D, Minneapolis, MN), and dsDNA (Invitrogen, Carlsbad, CA) according to manufacturers’ instructions.
Statistical Analysis
All data are represented as mean ±SE. Unpaired Student’s T-test and one-way ANOVA were used as appropriate for comparison between the groups at their individual time points and appropriate controls (Sigma Stat, SPSS, Inc. Chicago, IL). A P <.05 was assigned significance.
Results
TLR9 −/− is Associated with Impaired Thrombus Resolution and Increased Leukocyte Influx
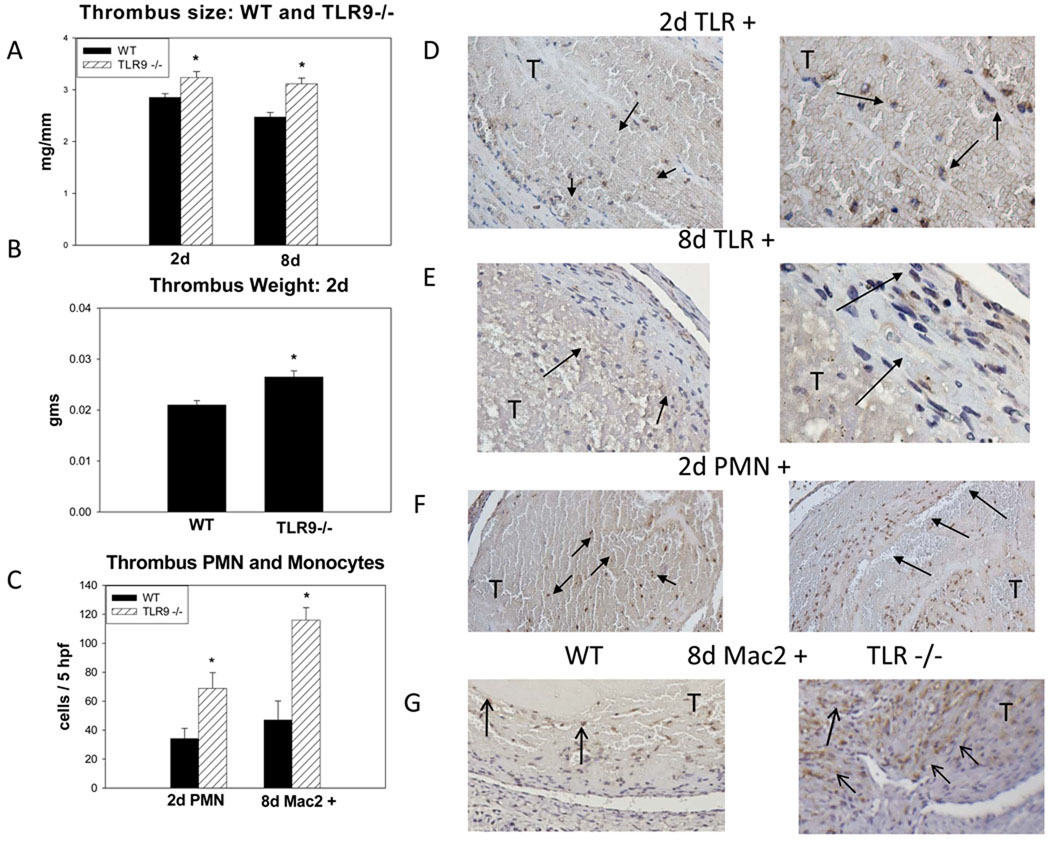
In our animal model, the thrombus tissue harvested represents the balance of thrombosis and thrombolysis. After IVC ligation to produce stasis, the overall thrombus size decreases over time.4, 6, 17 As compared with WT controls, the TLR9 −/− mice had approximately 20% larger thrombi, (p = .01; n = 9), at both 2d and 8d (Figure 1a,b). This was consistent whether measured as thrombus weight or as measured by weight/length ratio. Additional time points of 4 days showed 50% increase in size (p < .01, N = 6–8), while no significant difference was observed at 12d. To confirm TLR9’s role in VT resolution, a specific ODN 2088 inhibitor was administered to WT mice.13 As compared to control, the TLR9 inhibitor was associated with significantly larger thrombi at 2d (18 +/− 1 vs. 22 +/− 1; p = .02; n = 6 – 7), consistent with the TLR9−/− phenotype.
Figure 1.
a) Thrombus size was significantly larger at 2 + 8d in TLR9 −/− mice as measured by wt/L in mg/mm; b) A similar relationship was found measuring the VT weight directly, in gms; c) Both PMN and monocytes (Mac2+) cells were greater in the TLR9 −/− thrombi as compared with WTs; d) Intrathrombus TLR9 + cells at 2d (200X). The morphology is of primarily of PMN type (1000x), arrows; e) Intrathrombus TLR9+ cells at 8d (200X). The morphology is of mononuclear type (1000X); f) PMN counts were significantly increased at 2 d in TLR9 −/− mice as compared to WT (200X, 1000X), arrows; g) Mac2 + cells at 8d are increased in TLR9−/− thrombi. Most cells are in the thrombus periphery, arrows (200X). * = P < .05. T = thrombus.
TLR9 positive (+) cells in the WT thrombi remained relatively stable in number between 2 and 8 days (22 +/− 3, and 24 +/− 6, cells/5hpf), but the cell nuclear characteristics changed from predominately polymorphonuclear to mononuclear (Figure 1d,e). Of note, no TLR9+ cells were observed in the vein wall. Co-localization of PMN nuclear morphology with TLR9 staining was 93 +/− 3% at 2d and mononuclear morphology with TLR9 staining was 96 +/− 2% at 8d.
As compared with WT, TLR9 −/− mice had an ~2 fold increase in thrombus PMN at 2 days (Figure 1c,f). As few PMN are present in the thrombus at 8d,4 monocytes were evaluated. As compared with WT controls, TLR9 −/− had a 2 fold increased number of thrombus Mac-2+ monocytes (p < .01; n = 3 – 4) (Figure 1g). In both cases, these cells were primarily in the thrombus periphery.
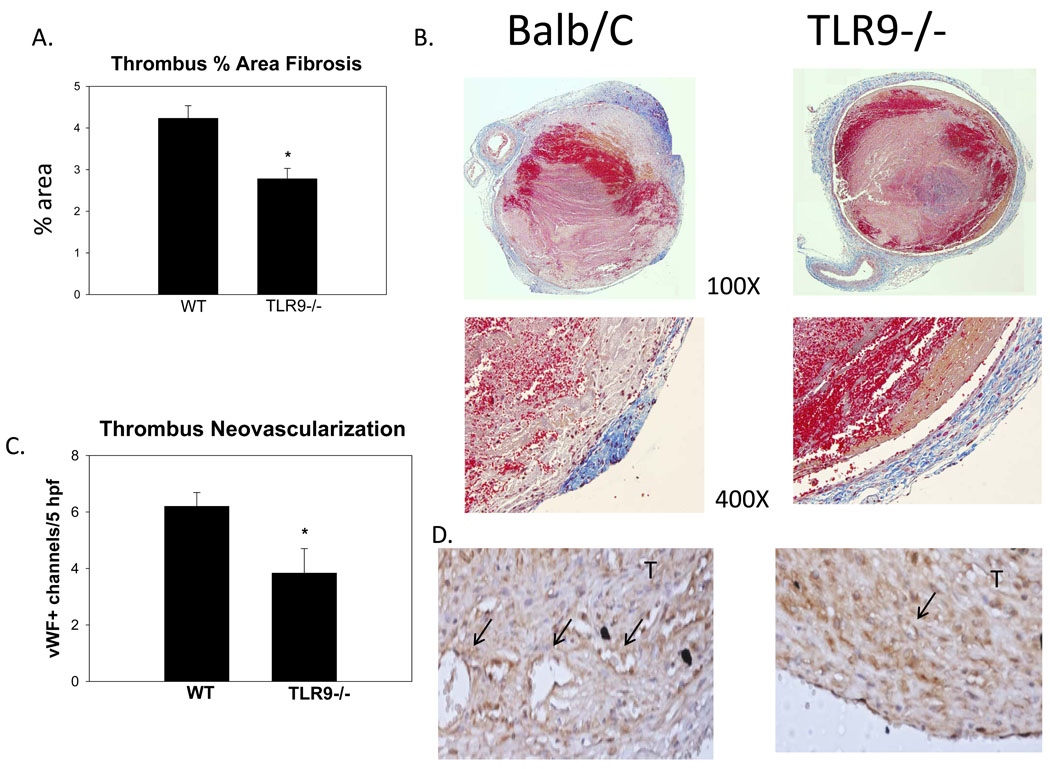
Thrombus fibrosis and neovascularization occurs as VT resolves and matures.1, 4, 6 By image assessment (trichrome percent positive staining), there was no significant difference between groups at 2d. At 8 days, the WT had approximately 45% greater fibrosis, as compared with TLR9 −/− thrombi (p < .001; n = 3 – 4)(Figure 2a–d), suggesting impaired thrombus maturation. Consistent with impaired maturation at 8d, TLR9−/− mice had 37% fewer vWF positive channels in the thrombus as compared with WT (p = .05, N = 5–6)(Figure 2e,f).
Figure 2.
a, b) By trichrome thrombus analysis, thrombus maturation is impaired in TLR9 −/− mice as compared with WTs. Significantly less collagen was found in the TLR9 −/− thrombus sections as shown at 100X and 400X. c,d) Less thrombus neovascularization was found in the TLR9−/− mice at 8d, denoted by vWF+ channels, arrows (400X).* = P < .05. T = thrombus.
TLR9 −/− mice have altered in vivo clotting parameters
Ex vivo thrombogenesis was assessed in the two mouse genotypes by thromboelastography. Decay, alpha, and maximum amplitude parameters19 showed no significant differences between the WT and TLR9 −/− mice, suggesting the basic clotting mechanisms were not different between groups (data not shown).
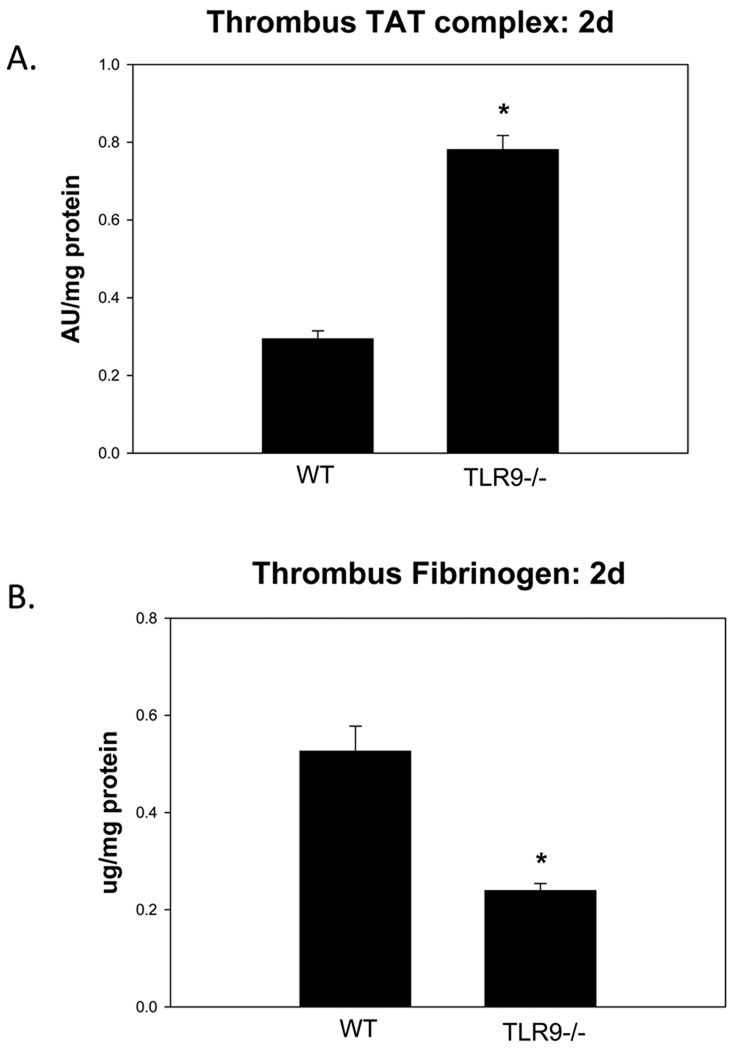
In vivo thrombus parameters were assessed to determine differences between groups in the early 2d thrombus milieu. As compared with WT controls, the TLR9 −/− thrombi had ~3 fold increased TAT complex (p < .001; n = 5 – 7) (Figure 3). Free fibrinogen was ~50% less in the TLR9 −/− thrombi as compared to WT controls (p < .001; n = 4 – 6). However, FX, plasmin, and both serum and thrombus uPA:PAI-1 balance17 was not significantly different between TLR9−/− and WT, at 2 or 8d (data not shown).
Figure 3.
Thrombus thrombin– anti thrombin Ag assessment. Increased TAT was observed in the TLR9 −/− mice as compared with WT; b) Free fibrinogen was reduced in TLR9 −/− mice as compared with WT. * = P < .05.
TLR9 −/− mice have early intact sterile cellular processes
As the TLR9 signaling pathway confers sterile inflammatory processing,10 several measures of this were ascertained. First, there was no significant difference between thrombus caspase-1 antigen at 2 days, in TLR9 −/− thrombi as compared with WT. At 8 days, caspase 1 was reduced ~ 3 fold in the TLR9 −/− thrombi as compared with WT (.07+/−.01 vs. .19 +/− .02, p < .001; n = 7 – 9). A cell death assay, reflecting histone associated DNA fragments, showed no significant difference between the WT or the TLR9 −/− groups at days 2 or 8. Assessment of dsDNA, a ligand for TLR9 that is present within the sterile inflammatory mileau,9 was higher in the VT than in the serum, by approximately 100 fold (.7+/− .13 vs. .03 +/− .09, ng/ml, p < .01; n = 3).
As TLR9 receptors are innate pattern recognition proteins for bacteria,12 we needed to confirm VT sterility. Importantly, there was no bacterial growth in either of the auger types from the VT sample (n = 4), confirming the sterile nature of the inflammation.
TLR9 −/− is associated with decreased Th1 cytokine levels
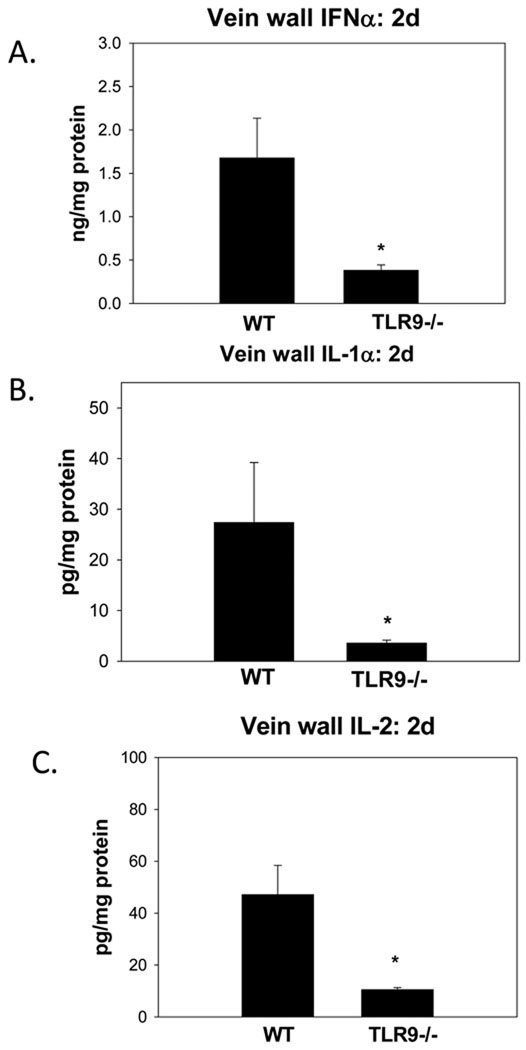
To characterize the thrombus cytokine milieu, ‘Th1’, and ‘Th2’ type cytokines were assessed. No significant differences in WT or TLR9 −/− thrombus or vein wall TNFα, IL- 4, IL-13, and IL-12 were found at 2d, and most of these had low, non detectable expression (data not shown). In contrast, vein wall IFNα was reduced 4 fold in TLR9 −/− as compared to WT (p < .01; n = 5 – 7) (Figure 4). Similarly, vein wall IL-1α was reduced 8 fold (p < .01; n = 4 – 5), and vein wall IL-2 was reduced 3.5 fold (p = .02; n = 5 – 8) in TLR9 −/−, as compared with WT controls.
Figure 4.
Profiles of vein wall cytokines in WT and TLR9 −/− mice. a) IFNα; b) IL1α; c) IL-2. All were reduced in TLR9 −/− compared with WT mice. * = P < .05.
Lack of MyD88 signaling does not affect VT resolution
To determine if TLR9 signaling effects on VT resolution were dependent on the MyD88 signaling pathway, we used genetically identical WT and MYD88 −/− mice, and subjected them to both stasis thrombosis. There was no significant difference in thrombus size of MyD88 −/− as compared with WT at 2 day (19 ± 7 vs. 23 +/− 1 mg; p = .18, N = 9 –10) or 8 day (24 ± 2 vs. 25 +/− 2 mg/mm, P = .70, N = 9 –11). Consistently, we found no significant difference in 2d PMN thrombus counts (data not shown).
TLR9 dependent VT resolution is dependent in part on the NOTCH ligand, delta-like 4
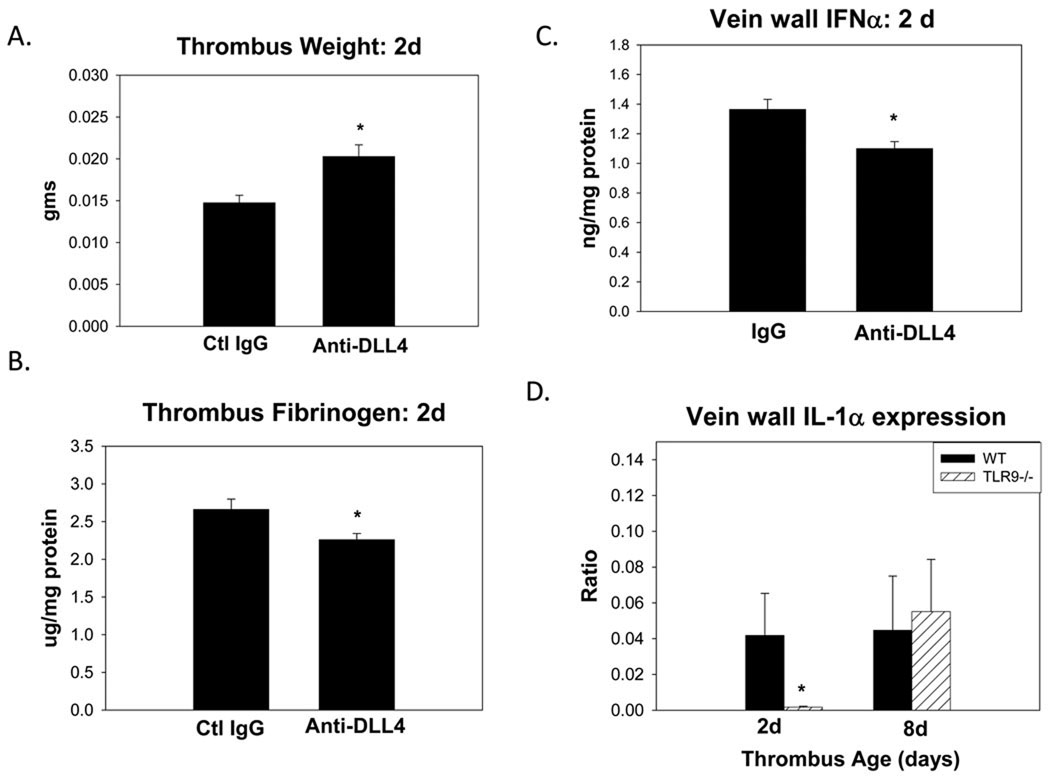
As MyD88 deletion did not impair VT resolution, recent studies have suggested that TLR9 signaling may require DLL4-Notch for activity.16, 18 Using neutralizing anti DLL4 antiserum, we found that the thrombus weight was increased by approximately 25% (p < .01; n = 8 – 9) (Figure 5). Thrombus PMN counts with DLL4 inhibition was increased by 15%, but this did not reach statistical significance (62 +/− 8 vs. 42 +/− 8 cells/5 hpf, p = .12; n = 5 – 8). There was no significant difference in thrombus TLR9+ cell staining in the treated or control mice at 2d (15 +/− 1 vs. 12 +/− 1 p=.10; N =4 – 5).
Figure 5.
DLL4 antibody inhibition experiments. As compared with WT given IgG control: a) thrombus size was increased with DLL4 blockade at 2d; b) thrombus fibrinogen was reduced; c) vein wall IFNα was reduced. Gene expression of IL1α (d) was reduced in TLR9 −/− mice. * = p < .05.
Assessing thrombus characteristics, the TAT complex, plasmin and FX levels were not significantly altered with the anti DLL4, but free fibrinogen was reduced by 20%, as compared with Ig control (p = .03; n = 5).
Evaluating the cytokine milieu, vein wall IFNα was significantly reduced with anti DLL4 treatment as compared with IgG control (p = .01; n = 5) (Figure 5), but neither IL1α nor IL2 were significantly altered (data not shown).
To characterize the vein wall genetic expression of certain ligands related to TLR and NOTCH signaling, there was neither significant induction of TLR9 or DLL4 in the vein wall after VT nor a difference in the WT and the TLR9 −/− mice in either Jagged 1 – 3, or Notch 1 – 3 (data not shown). There was also no induction of vein wall TLR3 or TLR4 gene expression (data not shown). However, IL1α gene expression was reduced by ~3 fold (p = .03; n = 6 – 8) in TLR9 −/− as compared with WT at 2d.
TLR9 Agonist Accelerates VT Resolution
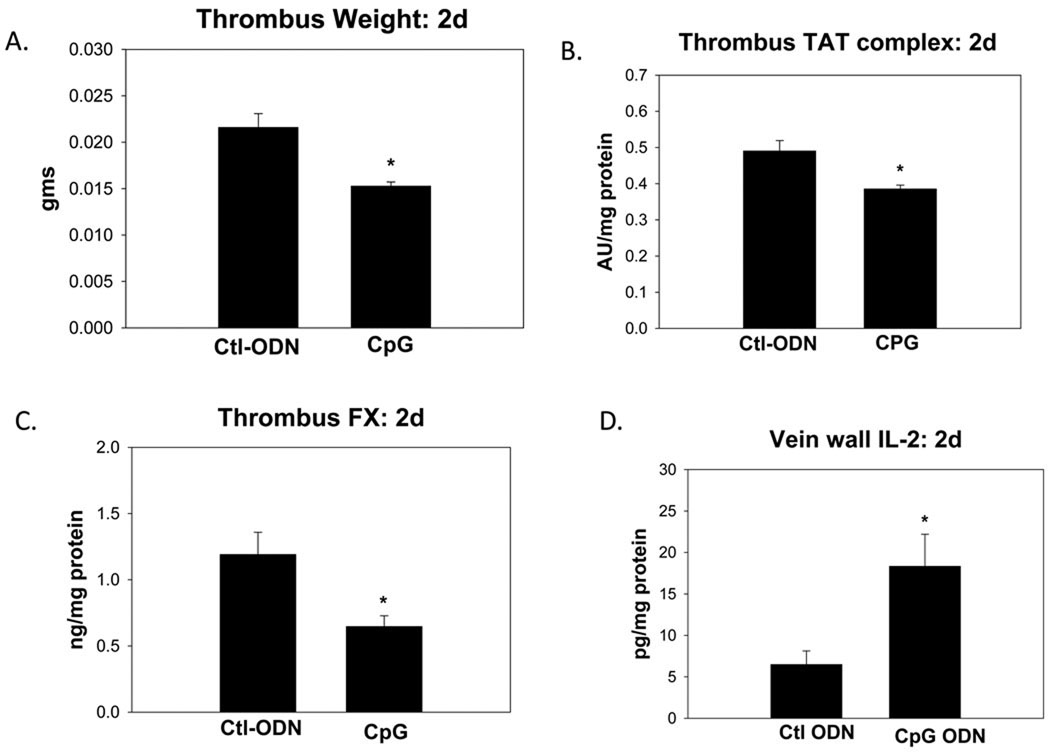
To determine if we could accelerate early thrombus resolution via stimulation of TLR9 signaling, we treated the WT mice with immunostimulatory ODN-1826,13 and a scrambled ODN as control. The ODN-1826 treated mice had 29% smaller thrombi, (p = .001; n = 12 – 13) (Figure 6), as compared to the ODN control. The thrombus PMN and TLR9 counts were not significantly altered by ODN-1826 administration as compared with ODN controls at 2d (data not shown).
Figure 6.
Stimulation with ODN-1826 (CpG) TLR9 agonist at 2d. As compared with control: a) Thrombus size was reduced; b) TAT was reduced; c) Factor X was reduced; d) Vein wall IL-2 was elevated. * P <.05.
Evaluating the thrombus parameters, TAT complex was reduced by ~10% in the ODN-1826 treated mice as compared with the ODN controls (p < .01; n = 6–7). Thrombus FX clot levels were also reduced with ODN-1826 treatment (p = .01, n = 7). Thrombus free fibrinogen and plasmin activity were not significantly different between groups.
Comparing the cytokine milieu, we found no significant difference in vein wall IFNα or IL1α levels. However, IL2 was elevated 2.5 fold with ODN-1826 as compared with the ODN control (p = .02; n = 7).
Discussion
Recent models have begun to illuminate the processes mediating sterile inflammation7, 8, 20 with the TLR9 pathway directly involved 9, 11 The data herein suggest a similarly important role, supported by these major findings : 1) thrombus resolution is impaired with genetic deletion and exogenous inhibition of TLR9 as well as DLL4, but not MYD88 pathways; 2) in vivo thrombus clotting mechanisms are altered and Th1 cytokines are reduced in TLR9−/− mice; and 3) CpG ODN stimulation of TLR9 accelerates early VT resolution.
Deletion of TLR9 was associated with significantly increased thrombus size, with less neovascularization and fibrosis, despite increased thrombus PMN and monocyte influx. The increase in thrombus cellularity with deletion of TLR9 is consistent with other experimental models.20 Prior work has shown directly and indirectly that thrombus leukocyte influx is essential for VT resolution, but only with normal leukocyte functioning.4, 6 As TLR9 gene expression was neither induced nor were TLR9 protein or cells detected in the vein wall after the VT insult, this suggests that influxing cells with intact TLR9 signaling are important for VT resolution rather than the resident vein wall response. These data also suggest that TLR9 signaling affects downstream leukocyte functions for thrombus clearance, rather than being a function of the total number of leukocytes. For example, PMN’s are known to release plasminogen activators, and other proteases,21 but it is not known if the TLRs affect these processes. However, TLR9 stimulation may directly activate PMN’s, including IL8 release.15 Related work has suggested the importance of CXCR2-PMN role in experimental VT resolution, with a similar timeframe of impaired resolution (e.g. increased VT size at 2 and 8d).4 Lastly, while the TLR9−/− were less fibrotic, our model does not allow us to determine if this is more lysable or less likely to emobolize to the pulmonary circulation, which would be a critical outcome parameter in humans to evaluate.
While our model does not allow us to determine thrombotic propensity, it is likely that thrombolysis was not affected as both plasmin and the activating protease uPA and its inhibitor PAI-1, were not affected in the TLR9 −/− mice. Since our model reflects the relative balance of thrombosis and thrombolysis at any given time point, the data suggest that in vivo, but not ex vivo, thrombogenesis was affected with TLR9 deletion as decreased free fibrinogen and an increased TAT complexes in the TLR9 −/− mice thrombi were observed without a change in thromboelastographic parameters. Consistently, exogenous CpG stimulation of TLR9 was associated with less thrombus TAT. This is the first observation to suggest that TLR’s play a role in the hemostatic processes, and may modulate thrombogenesis. Alternatively, TLR9 deficiency may promote ongoing thrombogenesis by impairing the clearance of extracellular procoagulant RNA22 or by preventing the release of normal anti-inflammatory mediators.23
It was surprising that MyD88 did not confer the same phenotype as the TLR9 −/− mouse, given the central position of MyD88 in TLR9 signaling.11, 24 However, MyD88 is converged on by multiple TLRs and may have cancelled out their effects in VT resolution. For example, TLR9 −/− and MyD88 −/− have differing phenotypes in experimental lupus models.25 Consistently, no increase in proinflammatory cytokines was observed after VT (e.g. TNFαor IL-12), a known function of the MyD88 signaling pathway.24 In contrast, DLL4 inhibition produced a similar phenotype as TLR9 −/− with larger thrombi, and less IFNα, but less alteration in PMN influx and thrombotic parameters. Of note, NOTCH – DLL4 signaling has been shown to be important in arterial vascular homeostasis, although this is the first to assess its role in VT resolution.26 Taken together, this suggests TLR9 may signal via other pathways besides MyD88.16
Other pathways are also important for sterile inflammation resolution including IL1α,8 and was significantly reduced in the TLR9 −/− mice. IL1α may promote several processes to allow clearance of apoptotic cells, including induction of the caspase enzymes. Consistently, we observed decreased caspase 1 levels at 8d in TLR9 −/− mice. Caspases are important enzymes for activation of apoptotic pathways and modulating resolution of inflammation.8 Interestingly, while much of cellular breakdown products are assumed to be from leukocytes, platelets also have functional mRNA27 and make up a large portion of a VT. Exactly how the necrotic and apoptotic cellular DNA and RNA is processed in a VT has not been defined. While extracellular DNA may be primary, other cell peptides including High Mobility Group Box-1, Heat shock protein-1, as well as uric acid may also be important8, 10
The immune adjuvant, TLR9 agonist ODN-1826, has been tested in various disease models with strong Th1 effects.13, 14 The host recognizes self DNA for normal homeostasis, and is both a TLR9 dependent and independent process.28 The use of ODN-1826 was associated with a significant decrease in VT weight. This is striking, given that our model is full venous stasis, and LMWH only has delayed thrombus resolution effects.29 The Th1 cytokine IL-2 was elevated with ODN-1826 treatment, but not IL1α or IFNα. These data suggest that a pro Th1 thrombus milieu seems important for VT resolution, as previously observed.1, 6 Whether IL2 is directly responsible for accelerating VT resolution or is simply a surrogate will require further work. Unlike the TLR9 −/− mice which had less IFNα and ILα expression, these factors were not increased in the ODN-1826 treated mice. These data suggest both Th1 modulation as well as in vivo thrombogenesis may be conferred in part through TLR9 signaling. Our experiments do not show which of these factors is most important mechanistically, but future work will define the stimulatory factors for TLR9, as well as further VT modulation mechanisms associated with TLR9 signaling.
While new therapies are on the horizon for DVT treatment, these all primarily target the coagulation cascade and the bleeding risks remain.3 Indeed, a concern of those new agents is lack of antidotes.30 The next generation of DVT therapy should have rapid onset, require little monitoring, and have little bleeding risk. Stimulation of the sterile inflammatory processing pathway in the DVT may be ideal in this regard.
Acknowledgments
Supported in part by NIH HL083918 and HL092129 (PKH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. 2008;28:387–391. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- 2.Gangireddy C, Rectenwald JR, Upchurch GR, Wakefield TW, Khuri S, Henderson WG, Henke PK. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:335–341. doi: 10.1016/j.jvs.2006.10.034. discussion 341-332. [DOI] [PubMed] [Google Scholar]
- 3.Geerts W. Antithrombotic and Thrombolytic Therapy. Chest. 2008;133:381s–451s. [Google Scholar]
- 4.Henke PK, Varga A, De S, Deatrick CB, Eliason J, Arenberg DA, Sukheepod P, Thanaporn P, Kunkel SL, Upchurch GR, Jr, Wakefield TW. Deep vein thrombosis resolution is modulated by monocyte CXCR2-mediated activity in a mouse model. Arterioscler Thromb Vasc Biol. 2004;24:1130–1137. doi: 10.1161/01.ATV.0000129537.72553.73. [DOI] [PubMed] [Google Scholar]
- 5.Henke PK, Varma MR, Moaveni DK, Dewyer NA, Moore AJ, Lynch EM, Longo C, Deatrick CB, Kunkel SL, Upchurch GR, Jr, Wakefield TW. Fibrotic injury after experimental deep vein thrombosis is determined by the mechanism of thrombogenesis. Thromb Haemost. 2007;98:1045–1055. [PubMed] [Google Scholar]
- 6.Henke PK, Pearce CG, Moaveni DM, Moore AJ, Lynch EM, Longo C, Varma M, Dewyer NA, Deatrick KB, Upchurch GR, Jr, Wakefield TW, Hogaboam C, Kunkel SL. Targeted Deletion of CCR2 Impairs Deep Vein Thombosis Resolution in a Mouse Model. J Immunol. 2006;177:3388–3397. doi: 10.4049/jimmunol.177.5.3388. [DOI] [PubMed] [Google Scholar]
- 7.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 9.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 10.Barton GM. A calculated response: control of inflammation by the innate immune system. J Clin Invest. 2008;118:413–420. doi: 10.1172/JCI34431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol. 2004;25:381–386. doi: 10.1016/j.it.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nat Med. 2003;9:831–835. doi: 10.1038/nm0703-831. [DOI] [PubMed] [Google Scholar]
- 13.Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, Lessov NS, Simon RP, Stenzel-Poore MP. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis HL, Weeratna R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 15.Jozsef L, Khreiss T, El Kebir D, Filep JG. Activation of TLR-9 induces IL-8 secretion through peroxynitrite signaling in human neutrophils. J Immunol. 2006;176:1195–1202. doi: 10.4049/jimmunol.176.2.1195. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Schaller M, Hogaboam CM, Standiford TJ, Sandor M, Lukacs NW, Chensue SW, Kunkel SL. TLR9 regulates the mycobacteria-elicited pulmonary granulomatous immune response in mice through DC-derived Notch ligand delta-like 4. J Clin Invest. 2009;119:33–46. doi: 10.1172/JCI35647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deatrick KB, Eliason JL, Lynch EM, Moore AJ, Dewyer NA, Varma MR, Pearce CG, Upchurch GR, Wakefield TW, Henke PK. Vein wall remodeling after deep vein thrombosis involves matrix metalloproteinases and late fibrosis in a mouse model. J Vasc Surg. 2005;42:140–148. doi: 10.1016/j.jvs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Schaller MA, Neupane R, Rudd BD, Kunkel SL, Kallal LE, Lincoln P, Lowe JB, Man Y, Lukacs NW. Notch ligand Delta-like 4 regulates disease pathogenesis during respiratory viral infections by modulating Th2 cytokines. J Exp Med. 2007;204:2925–2934. doi: 10.1084/jem.20070661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallimore MJ, Harris SL, Tappenden KA, Winter M, Jones DW. Urokinase induced fibrinolysis in thromboelastography: a model for studying fibrinolysis and coagulation in whole blood. J Thromb Haemost. 2005;3:2506–2513. doi: 10.1111/j.1538-7836.2005.01615.x. [DOI] [PubMed] [Google Scholar]
- 20.Raymond T, Schaller M, Hogaboam CM, Lukacs NW, Rochford R, Kunkel SL. Toll-like receptors, Notch ligands, and cytokines drive the chronicity of lung inflammation. Proc Am Thorac Soc. 2007;4:635–641. doi: 10.1513/pats.200706-067TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henke PK, Varma MR, Deatrick KB, Drewyer NA, Lynch EM, Moore AJ, Dubay DA, Sukheepod P, Pearce CG, Upchurch GR, Jr, Kunkel SL, Franz MG, Wakefield TW. Neutrophils modulate post-thrombotic vein wall remodeling but not thrombus neovascularization. Thromb Haemost. 2006;95:272–281. doi: 10.1160/TH05-02-0099. [DOI] [PubMed] [Google Scholar]
- 22.Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, Song Y, Tzima E, Kennerknecht E, Niepmann M, von Bruehl ML, Sedding D, Massberg S, Gunther A, Engelmann B, Preissner KT. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miles K, Clarke DJ, Lu W, Sibinska Z, Beaumont PE, Davidson DJ, Barr TA, Campopiano DJ, Gray M. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. J Immunol. 2009;183:2122–2132. doi: 10.4049/jimmunol.0804187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 25.Baccala R, Hoebe K, Kono DH, Beutler B. Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 26.Fung E, Tang SM, Canner JP, Morishige K, Arboleda-Velasquez JF, Cardoso AA, Carlesso N, Aster JC, Aikawa M. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation. 2007;115:2948–2956. doi: 10.1161/CIRCULATIONAHA.106.675462. [DOI] [PubMed] [Google Scholar]
- 27.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishii KJ, Akira S. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 2006;27:525–532. doi: 10.1016/j.it.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Moaveni DK, Lynch EM, Luke C, Sood V, Upchurch GR, Wakefield TW, Henke PK. Vein wall re-endothelialization after deep vein thrombosis is improved with low-molecular-weight heparin. J Vasc Surg. 2008;47:616–624. doi: 10.1016/j.jvs.2007.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merli G, Spyropoulos AC, Caprini JA. Use of emerging oral anticoagulants in clinical practice: translating results from clinical trials to orthopedic and general surgical patient populations. Ann Surg. 2009;250:219–228. doi: 10.1097/SLA.0b013e3181ae6dbe. [DOI] [PubMed] [Google Scholar]