Abstract
Ischemia impairs myocardial function and may contribute to the progression of heart failure. In this study, rats subjected to acute ischemia demonstrated reduced Ca2+ activated force as well as a decrease in myosin binding protein-C, titin and Ser23/24 phosphorylation of troponin I (TnI). All three proteins have been demonstrated to be downstream targets of β-adrenergic receptor activation (β-AR), leading to the hypothesis that decreased β-AR during ischemia leads to reduced protein phosphorylation and reduced rate constants of force relaxation. To test this hypothesis, force relaxation transients were recorded from permeabilized perfused and ischemic rat heart fibers following photolysis of the caged chelator diazo-2. Relaxation transients were best fit by double exponential functions whereby the majority (>70%) of the force decline was described by the fast rate constant, which was ~5 times faster than the slow rate constant. However, rate constants of relaxation between perfused and ischemic fibers were not different, despite significant decreases in sarcomeric protein phosphorylation in ischemic fibers. Treatment of perfused fibers with a cAMP analog increased Ser23/24 phosphorylation of TnI, yet the rate constants of relaxation remained unchanged. Interestingly, similar treatment of ischemic fibers did not impact TnI phosphorylation or force relaxation transients. Therefore, acute ischemia does not influence the rate constants of relaxation of permeabilized fibers. These results also suggest that the physiological level of sarcomeric protein phosphorylation is unlikely to be the primary driver of relaxation kinetics in permeabilized cardiac muscle fibers.
Keywords: troponin, heart, relaxation, ischemia, PKA
Introduction
Cardiac muscle relaxation is initiated by Ca2+ dissociation from Ca2+-troponin (Tn) C, leading to a series of conformational changes that restore the inhibitory effect of troponin I (TnI) and tropomyosin on the actomyosin crossbridge cycle (Gordon et al., 2000; Bers, 2002). The relaxation kinetics of cardiomyocytes can be significantly impacted by β-adrenergic receptor stimulation, with the resulting lusitropy modulated predominantly by downstream phosphorylation of phospholamban, myosin binding protein-C (MYBP-C) and TnI (Johns et al., 1997; Palmer and Kentish, 1998; Kentish et al., 2001; Bers, 2002). Phosphorylation of phospholamban following β-adrenergic receptor activation increases the kinetics of Ca2+ re-uptake into the sarcoplasmic reticulum, facilitating increased rates of relaxation (Bers, 2008). At the level of the contractile filaments, phosphorylation of MYBP-C may increase the kinetics of force development (Stelzer et al., 2007) whereas phosphorylation of TnI at Ser23/24 decreases myofilament Ca2+ sensitivity, may increase the rate of Ca2+ dissociation from TnC and accelerate the rate constant of force relaxation (Robertson et al., 1982; Zhang et al., 1995a; Wang and Kerrick, 2002; Stelzer et al., 2007). As well, novel phosphorylation targets such as titin may impact the passive force properties of the sarcomere (Fukuda et al., 2005). However, there remains some controversy over the relative contributions of the downstream sarcomeric targets to the observed lusitropic effect upon ®-adrenergic receptor stimulation (Zhang et al., 1995a; Zhang et al., 1995b; Johns et al., 1997; Li et al., 2000; Kentish et al., 2001). Experiments testing in vitro phosphorylated or pseudophosphorylated and nonphosphorylatable TnI expressed in the myocardium have both supported and refuted the role of Ser23/24 phosphorylation plays in increasing cardiac muscle relaxation rates (Zhang et al., 1995a; Johns et al., 1997; Fentzke et al., 1999; Kentish et al., 2001; Yasuda et al., 2007). MYBP-C knock-out models have suggested that TnI phosphorylation is primarily involved in the Ca2+ desensitization of force activation, whereas MYBP-C phosphorylation itself may increase the apparent rate constant of crossbridge detachment following stretch (Stelzer et al., 2007).
We have previously shown in both a rat ischemia-reperfusion model and a low myocardial blood flow model that transient changes in blood flow result in a decline in both maximal Ca2+-activated force (Fmax) and myofilament Ca2+ sensitivity (Chen and Ogut, 2006; Christopher et al., 2009; Han and Ogut, 2010). Interestingly, these states of altered blood flow are accompanied by an apparent decrease in phosphorylation of sarcomeric targets of β-adrenergic receptor activation, monitored as a decrease in Ser23/24 phosphorylation of TnI (Christopher et al., 2009; Han and Ogut, 2010). Therefore, we hypothesized that the reduced Ser23/24 TnI phosphorylation observed with ischemia would decrease the rate constant of force relaxation when compared to fibers from perfused hearts. To test this hypothesis, force relaxation transients of permeabilized fibers from perfused and ischemic rat hearts were recorded following photolysis of the caged chelator, diazo-2. Our results show that the biphasic relaxation transients from skinned perfused and ischemic cardiac muscle fibers were not different, suggesting that the impact of transient ischemia on the contractile filaments is predominantly responsible for systolic, rather than diastolic, dysfunction (Han and Ogut, 2010). Furthermore, we did not observe a strict relationship between the extent of phosphorylation of downstream sarcomeric targets of β-adrenergic receptor activation and the measured relaxation rate constants, suggesting that the phosphorylation of contractile proteins is not the driving force behind accelerated relaxation kinetics following β-adrenergic receptor activation.
Materials and Methods
In vivo rat ischemia model and muscle fiber preparation
The in vivo rat ischemia model has been described in greater detail previously (Bulteau et al., 2001; Chen and Ogut, 2006) and follows an experimental protocol approved by the Institutional Animal Care and Use Committee of the Mayo Clinic. In brief, adult male Sprague-Dawley rats (body wt. 250–350 g) were anesthetized using a mixture of ketamine and xylazine (10:2; 0.5–0.7 mL/kg) administered intramuscularly. An endotracheal tube was inserted for ventilation with O2-supplemented room air using a positive pressure respirator. Body temperature was maintained at 37°C during the procedure. The animal was allowed to stabilize for 15 min and then the heart was exposed by a midline thoracotomy and a ligature was placed near the bifurcation of the left coronary artery, restricting blood flow through the left anterior descending (LAD) and circumflex arteries for 30 min. Coronary occlusion was monitored by immediate pallor of the left ventricular free wall. For the time-matched perfused condition, the animal underwent surgery but without ligation. Following the procedure, the anterolateral papillary muscle, which derives blood supply from the LAD (Voci et al., 1995), was excised from the left ventricle and gently teased into strips (< ~250 ⎧m in width, ~1.2 mm in length) in relaxing solution (pCa8.0; 1×10−8 M free Ca2+) while on ice. For these studies, papillary muscles were used instead of the thinner trabeculae because the perfusion route of the latter could not be guaranteed due to the inherent variability of trabeculation in the ventricle. Prepared fiber strips were skinned for 2 h at 4°C with relaxing solution containing 1% Triton X-100. To test the effect of endogenous PKA activation, the permeabilization solution contained 1 mM 8-Br-cAMP, a cell permeable cyclic AMP analog (Calbiochem). For permeabilization and 8-Br-cAMP treatment at room temperature (22°C), the total time was reduced to 1 hour.
Determination of Ser23/24 phosphorylation of TnI
To determine the extent of Ser23/24 phosphorylation of TnI, a multiplex Western blotting method was used (Han and Ogut, 2010). Fibers from the anterolateral papillary muscle were homogenized in SDS-PAGE sample buffer with protease and phosphatase inhibitors (Roche) and resolved by Bis-Tris SDS-PAGE. The homogenates were transferred to low fluorescence PVDF membrane (Hybond-LFP, GE Lifesciences), blocked, and incubated simultaneously with a mouse anti-cardiac muscle TnI monoclonal antibody (10T79E, Fitzgerald, Concord, MA) and a phospho-Ser23/24 TnI rabbit polyclonal antibody (Cell Signaling Technology, Danvers, MA). Following washing, the blots were incubated with Cy3 labeled anti-mouse IgG and Cy5 labeled anti-rabbit IgG (GE Lifesciences), washed, allowed to dry and scanned on a Typhoon 9410 imager at the appropriate channels. The scanned images were analyzed using ImageQuant TL software (GE Lifesciences). The relative extent of Ser23/24 phosphorylation of TnI was determined by dividing the value of the Ser23/24 phosphospecific antibody signal by that of the total TnI mAb signal within each sample. To compare the extent of Ser23/24 phosphorylation of TnI in perfused versus ischemic hearts, separate homogenates of the anterolateral papillary muscle from perfused and ischemic hearts were resolved, blotted and analyzed as described. The extent of Ser23/24 phosphorylation in the perfused samples was averaged and normalized to 100%, and the ratio in ischemic samples was reported relative to the perfused samples. To determine the effect of the PKA activator 8-Br-cAMP on Ser23/24 phosphorylation of TnI in perfused and ischemic rat hearts, fibers from the anterolateral papillary muscle were dispersed into eight groups, with four receiving 8-Br-cAMP treatment. All eight groups were subsequently resolved on a single gel and Western blotted to determine the extent of Ser23/24 phosphorylation of TnI.
MYBP-C and titin phosphorylation
To determine the relative change in phosphorylation of MYBP-C and titin, SDS-PAGE resolved samples were stained initially with Pro-Q Diamond phosphoprotein stain and then subsequently with Deep Purple total protein stain as previously described (Christopher et al., 2009; Han and Ogut, 2010). Following densitometry, the ratio of the phosphoprotein stain to the total protein stain was reported as the relative extent of phosphorylation. For MYBP-C, 29:1 8% SDS-PAGE was used to resolve homogenates. For titin, sample preparation and SDS-PAGE were done through slight modifications of published methods (Tatsumi and Hattori, 1995; Warren et al., 2003). Approximately 5 mg of tissue was ground over dry ice in grinding resin filled tubes (Bio-Rad), and then 500 µL of homogenization buffer (3% SDS, 75 mM DTT, 50 mM Bis-Tris, pH 6.4, protease and phosphatase inhibitors (Roche) and a trace of bromophenol blue for tracking) was added and the sample homogenized an additional 30 seconds at room temperature. The homogenate was then incubated at 60°C for 10 minutes, centrifuged at 10000 X g for 5 minutes to clear insoluble materials, and glycerol was added to 20% prior to aliquoting and storage at −80°C. Gels used to resolve titin isoforms were 37.5:1 3% acrylamide gels containing 0.25% agarose, 0.1% SDS and 350 mM Bis-Tris, pH 6.4, 2.6 mM ammonium persulfate and 6.6 mM N,N,N',N'-tetramethyl-ethane-1,2-diamine N,N,. The gels were run overnight at 5 mA / gel in a running buffer that contained 50 mM MOPS, 50 mM Tris, 0.1% SDS, 1 mM EDTA (final pH 7.6 – 7.7) with 5 mM sodium bisulfite added to the upper chamber.
Fiber mechanics setup
The workstation to test skinned fiber contraction has been previously described in detail (Chen and Ogut, 2006; Han and Ogut, 2010). The ends of the skinned fibers were fixed with glutaraldehyde, followed by attachment of aluminum T-clips. Each fiber was mounted on the stage of a computer-controlled workstation, with one end hooked to a length controller (model 312C, Aurora Scientific) and the other to a force transducer (Akers AE801). The fibers were stretched to an average sarcomere length of ~2.2 µm while they were in relaxing solution, and their length, width, and thickness were recorded. Unless indicated otherwise, the temperature of the solutions during experiments was maintained at 15 ± 0.1°C. Activating and relaxing solutions contained the following: 5 mM free MgATP, 1 mM free Mg2+, 10 mM total EGTA, 25 mM total BES, pH 7, 25 mM creatine phosphate and 15 units / mL creatine kinase. The ionic strength was maintained constant at 200 mM using potassium methanesulphonate and the concentration of free Ca2+ in the solutions was adjusted using an iterative program as previously described (Chen and Ogut, 2006). All chemicals were purchased from Sigma, except for ATP (Special Quality, < 0.5% ADP, Roche) and diazo-2 (Invitrogen).
Measurement of muscle relaxation using flash photolysis of diazo-2
Relaxation transients from permeabilized perfused and ischemic cardiac muscle fibers were recorded following flash photolysis of diazo-2. The maximum force per cross-sectional area (Fmax) of each fiber was measured using an initial control contraction by changing the bathing solution from pCa8.0 to pCa4.5 (Fig. 2A). After contraction, the fiber was returned to pCa8.0 relaxing solution for 5 min and then transferred to a pCa8 pre-activating solution that reduced the EGTA concentration to 0.1 mM and included 10 mM dithiothreitol. Subsequently, the fiber was allowed to develop force to a level approaching ~80% of Fmax by incubating in an EGTA-free pCa5.7 solution containing 2 mM diazo-2 and 10 mM dithiothreitol. The activated fiber was briefly suspended in air for flash photolysis using a Xe flash lamp system (Model JML-C2; Rapp OptoElectronic, Germany) and immediately returned to the relaxing solution. The relaxation transients were fit with a double exponential equation (y = yo + A1 exp (−kr1 x) + A2 exp (−kr2 x)) yielding fast (kr1) and slow (kr2) rate constants. The values A1 and A2 represent the percentage of the force decline described by the fast and slow rate constants, respectively. To examine the effect of exogenous phosphate (Pi) on muscle fiber relaxation, flash photolysis of diazo-2 was repeated on the same fiber activated in the presence of 0, 2.5 mM and 5 mM added Pi. In control experiments, there was no significant decline, defined as a greater than 10% decrease, in tension produced when fibers were tested with three consecutive activation – diazo-2 relaxation cycles (data not shown). Relaxation transients were collected at 15°C or 22°C, depending on the experimental conditions tested. Under certain conditions, the activation time course was noticeably prolonged, presumably due to the thickness of some fibers. Therefore, few relaxation transients may have been initiated when the fiber was close to, but not at, the steady state.
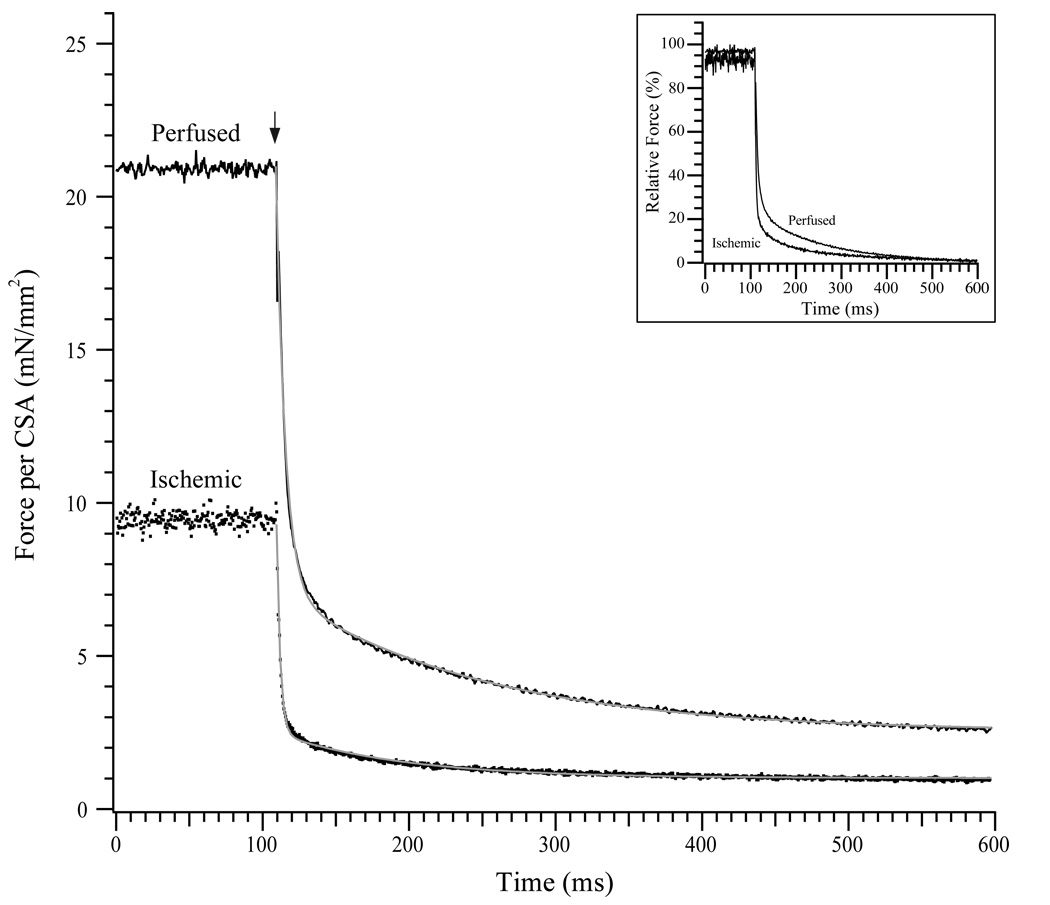
Fig. 2. Relaxation of perfused and ischemic fibers by Diazo-2.
(Top) Representative tracings of force per cross-sectional area (CSA) for skinned perfused (solid line) and ischemic (dotted line) fibers. Each fiber is allowed to contract in pCa4.5 solution to determine Fmax, prior to returning to a pCa8 solution followed by a low EGTA pCa8 solution. Fibers were then allowed to contract to submaximal levels in a pCa5.7 solution containing diazo-2. Following each submaximal contraction, the force relaxation transient is recorded following photolysis of diazo-2 (denoted by *). (Bottom) Representative tracings for skinned perfused and ischemic fibers just prior to and following flash photolysis of diazo-2 (marked by ↓). Grey lines indicate biexponential fits to the force transient. The inset shows the transient relaxation curves when force is normalized to the maximum level prior to flash photolysis.
Measurement of muscle relaxation using flash photolysis of NPE-ATP
The rate constants of force relaxation from the Ca2+-free rigor state was measured using flash photolysis of NPE-ATP (Goldman et al., 1982). Muscle fibers in pCa8.0 relaxing solution were transferred to ATP-free pCa8.0 relaxing solution that included apyrase (Martin and Barsotti, 1994; Han and Ogut, 2010). The fibers were then changed to pCa8.0 relaxation solution containing NPE-ATP, incubated, and then briefly suspended in air for flash photolysis as described earlier. The ATP-induced relaxation transients from rigor were also fit to a double exponential equation.
Statistical analysis
All data are presented as average ± SEM. Statistical comparisons between fiber groups were performed by Student’s t-test using a Bonferroni correction when multiple comparisons were necessary.
Results
Troponin I, MYBP-C and titin phosphorylation in perfused and ischemic fibers
The phosphorylation of both TnI and MYBP-C represent well-established, downstream physiological targets following β-adrenergic receptor activation (Layland et al., 2005; Han et al., 2010). We examined the extent of Ser23/24 phosphorylation of TnI from the anterolateral papillary muscles of perfused and ischemic hearts to determine if ischemia altered the extent of phosphorylation (Fig. 1A). Relative to the extent of Ser23/24 phosphorylation in perfused hearts (100 ± 6.0%; n=8), ischemic hearts demonstrated a significant decrease in relative Ser23/24 phosphorylation to 69.0 ± 12.8% (n=8; P < 0.05). This reduction of TnI phosphorylation was mirrored by MYBP-C, with ischemic samples showing a relative ~50% decrease in phosphorylation (100 ± 16.1% vs 48.1 ± 24.0%, P < 0.05; Fig. 1B). Recent data have also suggested that titin is a downstream target of ®-adrenergic receptor activation (Fukuda et al., 2005, J Gen Physiol, 125, 257-71). Analysis of titin expression demonstrated that rat anterolateral papillary muscle expressed predominantly the N2B isoform (Fukuda et al., 2005, J Gen Physiol, 125, 257-71; Warren et al., 2004), and an ~60% decrease in phosphorylation was observed for ischemic samples (100 ± 25.4% vs 39.3 ± 9.4%, P < 0.05; Fig. 1C). Analysis of the samples did not demonstrate proteolytic products of TnI or MYBP-C. For titin, the T2 proteolytic fragment was present (Granzier and Irving, 1995), but there was not statistically significant difference in the extent of T2 fragment present in perfused versus ischemic fibers.
Figure 1. TnI, MYBP-C and titin phosphorylation in perfused and ischemic fibers.
(A, Top) Papillary muscle homogenates from four representative perfused and ischemic hearts were resolved and simultaneously blotted using a mouse α-TnI monoclonal antibody (Cy3-labeled secondary antibody detection) and a rabbit phospho-Ser23/24 TnI polyclonal antibody (Cy5-labeled secondary antibody detection). (Bottom) Densitometric analyses of the ratio of the Ser23/24 phosphorylated TnI signal (Cy5) divided by the total TnI signal (Cy3) for each lane. Homogenates were also resolved for sequential Pro-Q Diamond phosphoprotein and Deep Purple total protein staining to examine the relative phosphorylation levels of MYBP-C (B) and N2B titin (C) in perfused versus ischemic homogenates. Data are presented as average ± S.E.M, with the average ratio of the perfused fibers set to a relative level of 100%. * P < 0.05 versus the perfused condition.
Relaxation transients of perfused and ischemic fibers
The relaxation rate constants of perfused and ischemic fibers were measured by rapidly decreasing the free [Ca2+] within the skinned fibers by flash photolysis of diazo-2 (Fig. 2). The impact of the ischemia protocol was confirmed by a significant decrease in the Fmax of fibers from ischemic hearts (30.8 ± 0.9 mN/mm2, n=18) versus perfused hearts (43.4 ± 1.6 mN/mm2, n=20; P < 0.05). With exposure to the pCa5.7 / diazo-2 solution, the fibers produced 32.4 ± 1.2 mN/mm2 (perfused, n=20) and 23.9 ± 1.5 mN/mm2 (ischemic, n=18) of tension, which were 75% and 78% of the Fmax for perfused and ischemic fibers, respectively. Following flash photolysis of diazo-2, relaxation transients were collected and fit with a double exponential equation to provide fast (kr1) and slow (kr2) relaxation rate constants. In perfused fibers, the fast relaxation rate constant (24.4 ± 1.6 s−1, n=20) was not different than that for ischemic fibers (26.1 ± 1.6 s−1, n=18), nor was there a difference in the slow relaxation rate constants between perfused and ischemic fibers (4.4 ± 0.3 s−1 vs. 4.8 ± 0.4 s−1). The percentage of the relaxation transient described by the fast rate constant kr1 (A1; 80.3 ± 1.8% vs. 82.8 ± 1.6%) and the slow rate constant kr2 (A2; 19.7 ± 1.8% vs. 17.2 ± 1.6%) were also compared but were not different between perfused and ischemic groups (Table 1). To determine another index of relaxation, we also measured the half time (t½) for relaxation. Perfused fibers relaxed with a t½ of 43.2 ± 2.5 ms (n=20), which was not significantly different from that of ischemic fibers (39.9 ± 2.6 ms; n = 18).
Table 1.
Relaxation parameters of perfused and ischemic fibers
| Perfused | Ischemic | |||
|---|---|---|---|---|
| Diazo-2 | NPE-ATP | Diazo-2 | NPE-ATP | |
| kr1 (s−1) | 24.4 ± 1.6 | 262 ± 30 | 26.1 ± 1.6 | 247 ± 32 |
| kr2 (s−1) | 4.4 ± 0.3 | 13 ± 1 | 4.8 ± 0.4 | 14 ± 1 |
| n | 20 | 6 | 18 | 5 |
All data are presented as average ± SEM.
Measurement of the relaxation transient from the rigor state (0 Ca2+, 0 ATP) was triggered by photolytic release of ATP from NPE-ATP (Goldman et al., 1982; Martin and Barsotti, 1994). The Ca2+-free rigor force in fibers from perfused hearts was 18.7 ± 1.3 mN/mm2 (n=6), significantly higher than in fibers from ischemic hearts (14.1 ± 1.4 mN/mm2, n=5; P < 0.05). Relaxation from Ca2+-free rigor by ATP demonstrated significantly faster rate constants as compared to the relaxation transient from activated fibers relaxed by flash photolysis of diazo-2. Fibers from perfused and ischemic hearts had relaxation transients that were fell fit by a double exponential equation (Fig. 3). Nonetheless, the fast and slow rate constants for perfused fibers (262 ± 30 s−1 and 13 ± 1 s−1; n=6) were not different than for ischemic fibers (247 ± 32 s−1 and 14 ± 1 s−1; n=5).
Fig. 3. Relaxation of perfused and ischemic fibers from rigor using NPE-ATP.
Representative tracings of force per cross-sectional area (CSA) versus time for skinned perfused and ischemic fibers from rigor by flash photolysis of NPE-ATP. Each fiber is allowed to reach rigor force in a Ca2+ free, 0 ATP rigor solution containing NPE-ATP, and the relaxation transient is initiated by flash photolysis (marked by ↓). Grey lines indicate biexponential fits to the force transients. The inset shows the transient relaxation curves when force is normalized to the maximum level prior to flash photolysis.
Effects of 8-Br-cAMP on TnI phosphorylation and relaxation transients
The effect of increasing Ser23/24 phosphorylation of TnI on the relaxation rate was determined by 8-Br-cAMP treatment. Anterolateral papillary muscle fibers from perfused and ischemic rat hearts were teased apart and separated into groups that were left untreated or treated with 8-Br-cAMP prior to examining the extent of Ser23/24 phosphorylation of TnI. When compared to untreated perfused fibers, the Ser23/24 phosphorylation level increased from a relative level of 100 ± 1.8% to 119.2 ± 1.8% (n = 4; P < 0.05) (Fig. 4). However, 8-Br-cAMP treatment of anterolateral papillary muscle fibers from ischemic rat hearts did not result in an increase in Ser23/24 phosphorylation of TnI (Fig. 5). When normalized to untreated ischemic fibers (100 ± 5%), the relative level of Ser23/24 phosphorylation of TnI following treatment with 8-Br-cAMP was 97.8 ± 3.1% (n = 4).
Fig. 4. Effect of 8-Br-cAMP treatment on Ser23/24 phosphorylation of TnI and relaxation transients in perfused fibers.
(Left) Fibers from perfused rat hearts were left untreated (control) or treated with 8-Br-cAMP, followed by multiplex Western blotting to determine the extent of Ser23/24 phosphorylation of TnI. Relative to the untreated, control perfused fibers, there was a significant increase in the Ser23/24 phosphorylation of TnI following 8-Br-cAMP treatment. (Right) Typical relaxation transients of fibers from a perfused rat heart left untreated (−cAMP) or treated with 8-Br-cAMP (+cAMP). Grey lines indicate biexponential fits to the force transients. The inset shows the transient relaxation curves when force is normalized to the maximum level prior to flash photolysis. Data are presented as average ± S.E.M. * P < 0.05 versus the untreated, control perfused condition.
Fig. 5. Ser23/24 phosphorylation of TnI and relaxation transients following 8-Br-cAMP treatment of ischemic fibers.
(Left) Fibers from ischemic rat hearts were left untreated (control) or treated with 8-Br-cAMP, followed by multiplex Western blotting to determine the extent Ser23/24 phosphorylation of TnI. Relative to the untreated control ischemic fibers, there was no significant change in the Ser23/24 phosphorylation of TnI following 8-Br-cAMP treatment. (Right) Two representative relaxation transients recorded from untreated control (−cAMP) and treated (+cAMP) ischemic heart fibers. Grey lines indicate biexponential fits to the force transients. The inset shows the transient relaxation curves when force is normalized to the maximum level prior to flash photolysis. Data are presented as average ± S.E.M.
We also determined the effect of 8-Br-cAMP treatment on the relaxation transients in a new cohort of perfused and ischemic rat hearts. Treatment of perfused fibers with 8-Br-cAMP did not significantly influence kr1 or kr2 (23.6 ± 2.3 s−1 and 4.6 ± 0.6 s−1) versus untreated fibers (22.8 ± 2.7 s−1 and 3.7 ± 0.4 s−1; n = 8). Treatment of ischemic fibers with 8-Br-cAMP also had no significant effect on kr1 or kr2 (21.1± 1.8 s−1 and 4.0 ± 0.3 s−1) when compared to untreated ischemic fibers (19.8 ± 2.7 s−1 and 3.7 ± 0.7 s−1; n = 6). A small group of perfused fibers were treated with 8-Br-cAMP at room temperature (22°C) to increase the extent of TnI phosphorylation. The recorded relaxation transients at 22°C demonstrated no significant change in either kr1 (30.3 ± 1.9 s−1 vs. 28.8 ± 2.1 s−1; n=2) or kr2 (6.2 ± 0.2 s−1 vs. 5.9 ± 1.2 s−1; n=2), although treatment of perfused fibers at room temperature with 8-Br-cAMP increased the relative level of Ser23/24 phosphorylation of TnI by 32.4 ± 5.2% (n=2) over the baseline untreated level. Initial observations in rabbit hearts have demonstrated that β-adrenergic receptor activation at 37°C resulted in an ~50% increase in overall TnI phosphorylation (Solaro et al., 1976). This magnitude change is in line with our observations of a ~20 – 30% increase in phosphorylation at temperatures ranging from 4°C to 22°C. Therefore, the changes in TnI phosphorylation achieved by 8-Br-cAMP activation are significant and in line with prior whole heart data (Solaro et al., 1976).
Effects of added Pi on relaxation transients
The direct effect of increasing exogenous [Pi] on relaxation rate constants was measured in order to determine if the response of perfused and ischemic fibers was unique (Han and Ogut, 2010). A set of 12 perfused and 11 ischemic fibers were successively tested in the presence of 0, 2.5 and 5 mM added Pi (Fig. 6). When added Pi was increased from 0 to 5 mM, kr1 in perfused fibers increased by 29% from 25.5 ± 1.9 s−1 to 32.9 ± 1.8 s−1 (n=12; P < 0.05) whereas ischemic fibers had a 16% increase from 29.5 ± 1.5 s−1 to 34.2 ± 1.6 s−1 (n=11; P < 0.05). This trend was reversed for kr2, which decreased 31% from 4.8 ± 0.3 s−1 to 3.3 ± 0.2 s−1 (P < 0.05) in perfused fibers and 31% from 5.2 ± 0.3 s−1 to 3.6 ± 0.2 s−1 (P < 0.05) in ischemic fibers after addition of 5 mM Pi (Table 2). In this set of experiments, the percentage of the relaxation transients described by the fast and slow rate constants were not different between groups and were not significantly impacted by added Pi (data not shown).
Fig. 6. Relaxation transients of perfused and ischemic fibers with increasing [Pi].
Representative tracings of force for skinned perfused (A) and ischemic (B) fibers. On the slow time scale (left), each fiber is allowed to contract in pCa4.5 solution to gauge the maximum force per fiber, prior to returning to a pCa8 solution followed by a low EGTA pCa8 solution. Fibers were then allowed to contract to submaximal levels in a pCa5.7 solution containing diazo-2 and different concentrations of added Pi. Following each submaximal contraction, the force relaxation transient was recorded following photolysis of diazo-2 and presented on a fast time scale (right). The three relaxation transients initiated by flash photolysis of diazo-2 (↓) in the presence of 0, 2.5 and 5 mM added Pi are shown together, with insets presenting the transients after normalization of force levels. Grey lines indicate biexponential fits to the force transients.
Table 2.
Relaxation parameters of perfused and ischemic fibers in the presence of Pi and Diazo-2
| Perfused | Ischemic | |||||
|---|---|---|---|---|---|---|
| 0 mM Pi | 2.5 mM Pi | 5 mM Pi | 0 mM Pi | 2.5 mM Pi | 5 mM Pi | |
| kr1 (s−1) | 25.5 ± 1.9 | 28.4 ± 1.4 | 32.9 ± 1.8† | 29.5 ± 1.5 | 29.8 ± 1.4 | 34.2 ± 1.6† |
| kr2 (s−1) | 4.8 ± 0.3 | 3.6 ± 0.2* | 3.3 ± 0.2* | 5.2 ± 0.3 | 4.1 ± 0.2* | 3.6 ± 0.2† |
| n | 12 | 12 | 12 | 11 | 11 | 11 |
All data are presented as average ± SEM.
P < 0.05 versus respective 0 mM Pi
P < 0.05 versus respective 0 and 2.5 mM Pi
Discussion
Reduced phosphorylation of sarcomeric PKA targets during ischemia
Characterizing the molecular mechanisms underlying cardiac muscle force activation and relaxation during ischemia is important in clarifying the etiology of heart failure (Bolli and Marban, 1999). Although brief ischemic episodes are known to depress myocardial contractility, the individual contributions of contractile proteins to the measured fiber mechanics remain under investigation (Hofmann et al., 1993; Gao et al., 1995; Decker et al., 2005; Biesiadecki et al., 2007; Bito et al., 2007). To this end, it has previously been demonstrated that TnI phosphorylation is reduced during ischemia and in heart failure (Wolff et al., 1996; Chen and Ogut, 2006; El-Armouche et al., 2007; Hamdani et al., 2008; Christopher et al., 2009). As TnI is a proven downstream target of PKA during ®-adrenergic receptor-induced lusitropy (Solaro et al., 1976; Garvey et al., 1988), we hypothesized that reduced Ser23/24 phosphorylation of TnI in ischemic fibers was indicative of altered sarcomeric protein phosphorylation and would signal a reduction in the rate constants of force relaxation. As expected, the reduction in Ser23/24 TnI phosphorylation was accompanied by reductions in other sarcomeric proteins. Examination of MYBP-C phosphorylation demonstrated a 50% reduction in phosphorylation in ischemic fibers. For titin, rat anterolateral papillary muscles predominantly expressed the N2B isoform(Warren et al., 2004) and showed a 60% decrease in phosphorylation in ischemic fibers. The concomitant reduction in phosphorylation of these three proteins implicated a common reduction in ®-adrenergic receptor mediated signaling to the sarcomere in ischemic myocardium, as all three have been shown to be PKA targets (Solaro et al., 1976; Garvey et al., 1988; Fukuda et al., 2005).
Prior studies have demonstrated that heart failure leads to reduced β-adrenergic receptor activity through a change in receptor density, decline in substrate sensitivity to agonist stimulation, and changes in the activity of PKA through altered regulatory subunit expression (Bristow et al., 1982; Zakhary et al., 1999). Our data complement these findings and demonstrate that brief ischemia may also impair the intrinsic mechanism of PKA activation in the myocardium as evidenced by reduced phosphorylation of downstream sarcomeric targets (Fig. 1; Han and Ogut, 2010). Previous studies in ischemic fibers have demonstrated a reproducible Ca2+ desensitization (Kusuoka et al., 1990; Hofmann et al., 1993; Gao et al., 1995; Chen and Ogut, 2006; Han and Ogut, 2010) in the face of declining Ser23/24 phosphorylation of TnI (Chen and Ogut, 2006; Christopher et al., 2009). These data signify a point of uncoupling: whereas a decline in TnI phosphorylation would normally produce an increase in Ca2+ sensitivity (Garvey et al., 1988; Zhang et al., 1995b) ischemic fibers do not recapitulate this relationship. Therefore, the direct relationship between endogenous PKA activation, TnI phosphorylation and Ca2+ desensitization of the myocardium is not well conserved as a result of ischemia. We hypothesize that this is due to the multimodal impact of altered blood flow on the myocardium, as evidenced by significant changes in the post-translational state of TnI, MYBP-C and titin in addition to other myofibrillar proteins including troponin T and nebulette (McDonald et al., 1998; Chen and Ogut, 2006; Yuan et al., 2006; Christopher et al., 2009; Han and Ogut, 2010).
Troponin I phosphorylation and relaxation transients in permeabilized ischemic fibers
To determine the impact of ischemia on the relaxation transients, we used permeabilized anterolateral papillary muscle fibers from the in vivo rat model of ischemic myocardium. Consistent with previous reports, ischemia resulted in an ~30% decrease in Fmax (Chen and Ogut, 2006; Han and Ogut, 2010). Given the significant difference in sarcomeric protein phosphorylation (Fig. 1), this presented an appropriate model to examine how protein post-translational state contributed to the mechanics of permeabilized muscle fiber relaxation during ischemia. Upon photolysis of diazo-2, the relaxation transients of cardiac muscle fibers demonstrated a biphasic profile, as expected (Fig. 2; (Palmer et al., 1991; Simnett et al., 1998; Mulligan et al., 1999; Saeki et al., 2004)). The majority of the force decline in both perfused and ischemic fibers was described by the fast rate constant, kr1, which was not different between perfused and ischemic fibers (Table 1). Therefore, the significant decline in phosphorylation of downstream ®-adrenergic activation targets, monitored through Ser23/24 phosphorylation of TnI, did not correlate with slowed relaxation transients in ischemic fibers, which may be contrary to the expected relationship between TnI phosphorylation and relaxation in normal hearts (Zhang et al., 1995a; Kentish et al., 2001; Yasuda et al., 2007). To address this discrepancy, we attempted to increase the level of Ser23/24 phosphorylation to a higher point in the physiological range in order to determine if the 30% difference between perfused and ischemic fibers did not exceed a necessary threshold to see an effect on relaxation kinetics. The cell permeable cAMP analog 8-Br-cAMP was used to activate the endogenous PKA, with Ser23/24 phosphorylation of TnI being both the intended downstream effect while serving as a proxy for relative PKA activity (Garvey et al., 1988; Han and Ogut, 2010). We observed that 8-Br-cAMP treatment of perfused fibers at 4°C significantly increased Ser23/24 phosphorylation of TnI by ~20% over baseline, but there was no significant increase in TnI phosphorylation for ischemic fibers (Figs. 4,5). As expected, the relaxation transients in ischemic fibers were unchanged by the treatment. However, despite the increase in TnI phosphorylation in perfused fibers, there were no changes in the relaxation transients. This finding was further strengthened in experiments wherein the relative increase in Ser23/24 phosphorylation of TnI was ~30% over baseline by treatment with 8-Br-cAMP at 22°C. Although the changes in TnI phosphorylation at 4 and 22°C were significant and comparable to the ~50% increase in TnI phosphorylation following β-adrenergic activation of the heart at 37°C (Solaro et al., 1976), there was no significant acceleration of the relaxation transients. These results suggest that TnI phosphorylation at Ser23/24 was not the primary driver for increased relaxation kinetics in skinned, perfused fibres loaded with diazo-2. More importantly, changes to the post-translational states of contractile proteins during myocardial ischemia do not markedly impair relaxation transients in permeabilized fibers.
Our data are consistent with a prior study reporting that TnI phosphorylation does not increase the rate constants of relaxation in guinea pig skinned trabeculae (Johns et al., 1997). In the cited study, myofibrils were treated with PKA, resulting in a relative desensitization to Ca2+ but no change in the relaxation transients. This is generally supported by additional studies in transgenic mice expressing a Ser23/24Ala mutant of TnI that demonstrate the importance of these sites primarily for the Ca2+ sensitivity of force production (Stelzer et al., 2007). However, the relative insensitivity of the force relaxation rates of perfused myocardium to 8-Br-cAMP treatment is seemingly at odds with prior work suggesting a direct relationship between force relaxation transients and Ser23/24 phosphorylation of TnI. In transfected cardiomyocytes expressing a pseudophosphorylated Ser23/24Asp mutant of TnI, relaxation transients were accelerated regardless of isoproterenol treatment (Yasuda et al., 2007). Notably, Kentish et al. (Kentish et al., 2001) have demonstrated that in slow skeletal TnI expressing myocardium, PKA treatment is unable to accelerate relaxation kinetics as this isoform lacks the identified PKA phosphorylation sites present in cardiac muscle TnI. Conversely, PKA treatment of wild type mouse cardiac myofibrils resulted in a relative increase in TnI phosphorylation and a significant decrease in the half-time of relaxation due primarily to an increase in the slow rate constant. These results support a role for TnI phosphorylation in accelerating the relaxation transient, while seemingly assigning a smaller role to MYBP-C phosphorylation. It is noteworthy that the ~40 ms half-time for relaxation in the rat cardiac muscles fibers measured here is significantly shorter than the ~150 ms as reported by Kentish et al. (Kentish et al., 2001) in mouse cardiac muscle fibers. This appears to be attributable to a significant difference in the magnitude and contribution of the fast and slow phases to the overall relaxation transient. Notably, whereas the fast rate constant described ~80% of the force decline in our measurements, this number appears significantly smaller in the Kentish study and results in a large increase in the contribution of the phase of relaxation described by the slow rate constant. Our data support previous reports suggesting that the second phase of the relaxation transient may represent the slower, forward progress of the crossbridge cycle through the ADP release step, leading to eventual detachment (Palmer et al., 1991; Johns et al., 1997; Palmer and Kentish, 1998; Simnett et al., 1998; Kentish et al., 2001). Therefore, if cardiac TnI phosphorylation significantly increases the rate of ADP release from AM·ADP, then relaxation transients initiated in fibers containing a higher proportion of AM·ADP crossbridges may readily show a decrease in the half-time of relaxation. Furthermore, it remains unclear if there is a necessary threshold of Ser23/24 phosphorylation of TnI that must be surpassed in order to observe a significant acceleration of the relaxation transient. Experimental systems testing the impact of phosphorylation following treatment with purified PKA catalytic subunits likely reach higher levels of TnI phosphorylation (Zhang et al., 1995a; Kentish et al., 2001) as compared to the strategy of activating the endogenous PKA present in the heart (Solaro et al., 1976). Accordingly, treatment with PKA catalytic subunits may exceed physiological levels of phosphorylation to reveal functional impacts not readily observed when phosphorylation fluctuates within physiological ranges.
Rates of Ca2+ and ATP-dependent relaxation in permeabilized ischemic fibers
The absence of a relationship between the extent of Ser23/24 phosphorylation of TnI and relaxation rates required additional experiments to ensure that increases in overall crossbridge cycling could indeed be measured in this experimental system. For this purpose exogenous phosphate was used as it increases the crossbridge cycling rate and accelerates the time course of relaxation (Hibberd et al., 1985; Araujo and Walker, 1994; Simnett et al., 1998; Gordon et al., 2000; Saeki et al., 2004). Relaxation transients were recorded from perfused or ischemic fibers tested sequentially in the presence of increasing added [Pi] (Fig. 6). These data demonstrated that the addition of 5 mM Pi accelerated the fast rate constant of relaxation while reducing the slow rate constant as compared to the baseline (Table 2). However, the divergent response of the two rate constants (kr1, kr2) to added Pi suggests that relaxation transients following flash photolysis of diazo-2 describe two distinct pathways leading to detachment (Palmer et al., 1991; Simnett et al., 1998; Mulligan et al., 1999; Saeki et al., 2004). The percentage of the force decline attributable to kr1 (A1) is significantly larger than that for kr2 (A2), implicating the process involving the rapid rate constant as the predominant one (Mulligan et al., 1999; Saeki et al., 2004). Since the fast rate constant increased with added Pi, it implicates kr1 as the reversal of the power stroke under physiological conditions. The slow rate constant then likely represents a forward transition whereby ADP bound crossbridges must release ADP prior to proceeding to detachment and force relaxation (Palmer et al., 1991; Johns et al., 1997; Simnett et al., 1998). For relaxation transients to accelerate significantly in this system, TnI phosphorylation would be required to have a significant impact on the reversal of the power stroke. However, the relaxation transients recorded from perfused and ischemic fibers suggest that TnI phosphorylation does not have a large effect on this process, especially at low Ca2+ concentrations. It is further noted that although relaxation transients from striated muscle fibers are biphasic, the rate constants of the two processes are different depending on the intracellular milieu. Specifically, if the endogenous Pi in the fiber is scavenged to well below physiological levels (> 10 µM; (Millar and Homsher, 1990; Pate et al., 1998)), the first rate constant, kr1, becomes significantly slower than the second rate constant, kr2 (Luo et al., 2002; Tesi et al., 2002; Belus et al., 2008). These data suggest that very low levels of Pi presumably limit detachment through backward progression of the crossbridge cycle and favour detachment through the forward transition that requires initial ADP release. It is currently difficult to apply these data to physiological and pathophysiological scenarios wherein Pi concentrations are in the low millimolar range (Itoya et al., 1996; Wu et al., 2008) wherein the first phase of the relaxation transient is rapid. Therefore, our experiments focused on concentrations of exogenous Pi within the ranges projected during altered blood flow to the myocardium rather than sub-physiological levels achieved by efficient phosphate scavenger systems. These data were complemented by an examination of the relaxation transients from Ca2+ and ADP-free rigor by flash photolysis of NPE-ATP (Fig. 3). Although these transients were significantly faster than for diazo-2, as expected (Martin and Barsotti, 1994; Palmer and Kentish, 1998), there was no observed difference in the rate constants of relaxation between perfused and ischemic fibers. The relatively rapid rate constants are consistent with this force relaxation process requiring only dissociation of ADP-free rigor crossbridges by ATP rather than progression through a slower crossbridge transition (e.g. ADP release from AM·ADP) prior to detachment.
In agreement with prior work, the relaxation rate constants reported in this study were not sensitive to the steady state force level (Tesi et al., 2002; Saeki et al., 2004), but rather reflected the crossbridge states within the myofibril prior to photolysis. This is particularly important as the Fmax in ischemic fibers is significantly reduced as compared to perfused fibers (Chen and Ogut, 2006; Han and Ogut, 2010), and could have confounded the results. Our previous data have suggested that the impaired rate constant of force production in ischemic fibers is observed only when they are activated from the relaxed state by Ca2+ using photolysis of NP-EGTA, but not from the Ca2+-rigor state following activation by photolysis of NPE-ATP (Han and Ogut, 2010). These data suggest that ischemia results in a select number of thin filament regulatory units that are no longer efficiently activated by Ca2+ and are therefore unable to support crossbridge attachment and subsequent force production. However, impaired activation of ischemic fibers by Ca2+ did not correlate with impaired relaxation induced by withdrawal of Ca2+. This asymmetry merits additional study.
The contribution of these various downstream effectors of β-adrenergic receptor stimulation to the function of the heart during normal and altered blood flow are essential questions given the significant change in β-adrenergic receptor activity in heart failure (Bristow et al., 1982; Rockman et al., 2002) and the efficacy of ®-adrenergic receptor antagonists in the clinical setting (Hjalmarson et al., 2000). Our data suggest that the integrated effects of altered β-adrenergic receptor mediated signaling is likely to be more pronounced in the intact fiber. For example, previous studies have assigned a significant role for titin in determining passive stiffness properties that may in turn relate to diastolic function (Fukuda et al., 2005; Kruger et al., 2009). In that respect, the changes in titin phosphorylation observed in this model may be more relevant for the contractility of the intact heart than for the active force relaxation transient demonstrated by permeabilized fibers following photolysis of diazo-2. Therefore, the integrated effect of altered β-adrenergic receptor mediated signaling on the active force relaxation properties of permeabilized fibers appears to be modest. In effect, these results may suggest that the altered Ca2+ transient in intact fibers following β-adrenergic receptor stimulation may be important in unmasking the impact of altered sarcomeric protein phosphorylation on relaxation transients. Aside from its effects on the sarcomeric proteins TnI, MYBP-C and titin, stimulation of the receptor also accelerates Ca2+ cycling through the phosphorylation of phospholamban, which is believed to play a major role in the faster relaxation of the myocardium (Luo et al., 1994; Hoit et al., 1995; Johns et al., 1997; Li et al., 2000; MacLennan and Kranias, 2003). It is proposed that >70% of the cytoplasmic Ca2+ is removed by the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase, which is tonically inhibited by dephosphorylated phospholamban in the absence of ®-adrenergic receptor stimulation (Bers, 2002). Our results showed that altering the Ser23/24 phosphorylation of TnI within physiological levels was not sufficient to increase the relaxation rate constants in permeabilized perfused or ischemic muscle fibers following rapid Ca2+ chelation. Even though TnI phosphorylation may alter the apparent Ca2+ off rate constant from TnC, this rate constant remains significantly faster than the rate constant of force relaxation (Ashley et al., 1991; Johns et al., 1997; Palmer and Kentish, 1998; Gordon et al., 2000; Luo et al., 2002). Furthermore, the rate constants of Ca2+ dissociation from purified TnC have not had a strict or direct relationship to the measured relaxation rate constant in fibers (Palmer and Kentish, 1998; de Tombe et al., 2007; Kreutziger et al., 2008). Therefore, we hypothesize that in the integrated thin filament, the impact of phospholamban phosphorylation on the Ca2+ transient is the primary driver for the positive lusitropy following β-adrenergic receptor stimulation (Li et al., 2000). In an intact, integrated system, the decrease in intracellular Ca2+ is much slower than that produced during the photolysis of diazo-2. Therefore, we can not rule out that changes in the phosphorylation of TnI could modulate or influence the rate of crossbridge detachment from the thin filament during cardiac relaxation where the load is dynamic. However, in the isolated, permeabilized muscle fiber setting, our data demonstrate that TnI phosphorylation did not meaningfully regulate the rate constants of force relaxation following photolysis of diazo-2.
In conclusion, our present study demonstrates that compared to control perfused fibers, acute ischemia does not influence the rate constants of fiber relaxation upon rapid chelation of Ca2+. Therefore, these results broadly suggest that systolic function in the ischemic heart, rather than diastolic function, may be more vulnerable to changes in the post-translational state of the contractile proteins in the sarcomere.
Acknowledgments
We thank Dr. Frank Brozovich for discussions during the preparation of the manuscript.
Financial Disclosure: This work was supported by NIH grant HL078845 and HL078845-S1 to O.O. Y.S.H. was supported in part by T32HL007111.
References
- Araujo A, Walker JW. Kinetics of tension development in skinned cardiac myocytes measured by photorelease of Ca2+ Am J Physiol. 1994;267:H1643–H1653. doi: 10.1152/ajpheart.1994.267.5.H1643. [DOI] [PubMed] [Google Scholar]
- Ashley CC, Mulligan IP, Lea TJ. Ca2+ and activation mechanisms in skeletal muscle. Q Rev Biophys. 1991;24:1–73. doi: 10.1017/s0033583500003267. [DOI] [PubMed] [Google Scholar]
- Belus A, Piroddi N, Scellini B, Tesi C, Amati GD, Girolami F, Yacoub M, Cecchi F, Olivotto I, Poggesi C. The familial hypertrophic cardiomyopathy-associated myosin mutation R403Q accelerates tension generation and relaxation of human cardiac myofibrils. J Physiol. 2008;586:3639–3644. doi: 10.1113/jphysiol.2008.155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- Biesiadecki BJ, Kobayashi T, Walker JS, John Solaro R, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res. 2007;100:1486–1493. doi: 10.1161/01.RES.0000267744.92677.7f. [DOI] [PubMed] [Google Scholar]
- Bito V, van der Velden J, Claus P, Dommke C, Van Lommel A, Mortelmans L, Verbeken E, Bijnens B, Stienen G, Sipido KR. Reduced force generating capacity in myocytes from chronically ischemic, hibernating myocardium. Circ Res. 2007;100:229–237. doi: 10.1161/01.RES.0000257829.07721.57. [DOI] [PubMed] [Google Scholar]
- Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, Szweda LI. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem. 2001;276:30057–30063. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- Chen FC, Ogut O. Decline of contractility during ischemia-reperfusion injury: actin glutathionylation and its effect on allosteric interaction with tropomyosin. Am J Physiol Cell Physiol. 2006;290:C719–C727. doi: 10.1152/ajpcell.00419.2005. [DOI] [PubMed] [Google Scholar]
- Christopher B, Pizarro GO, Nicholson B, Yuen S, Hoit BD, Ogut O. Reduced force production during low blood flow to the heart correlates with altered troponin I phosphorylation. J Muscle Res Cell Motil. 2009;30:111–123. doi: 10.1007/s10974-009-9180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tombe PP, Belus A, Piroddi N, Scellini B, Walker JS, Martin AF, Tesi C, Poggesi C. Myofilament calcium sensitivity does not affect cross-bridge activation-relaxation kinetics. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1129–R1136. doi: 10.1152/ajpregu.00630.2006. [DOI] [PubMed] [Google Scholar]
- Decker RS, Decker ML, Kulikovskaya I, Nakamura S, Lee DC, Harris K, Klocke FJ, Winegrad S. Myosin-binding protein C phosphorylation, myofibril structure, and contractile function during low-flow ischemia. Circulation. 2005;111:906–912. doi: 10.1161/01.CIR.0000155609.95618.75. [DOI] [PubMed] [Google Scholar]
- El-Armouche A, Pohlmann L, Schlossarek S, Starbatty J, Yeh YH, Nattel S, Dobrev D, Eschenhagen T, Carrier L. Decreased phosphorylation levels of cardiac myosin-binding protein-C in human and experimental heart failure. J Mol Cell Cardiol. 2007;43:223–229. doi: 10.1016/j.yjmcc.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol. 1999;517:143–157. doi: 10.1111/j.1469-7793.1999.0143z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N, Wu Y, Nair P, Granzier HL. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol. 2005;125:257–271. doi: 10.1085/jgp.200409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WD, Atar D, Backx PH, Marban E. Relationship between intracellular calcium and contractile force in stunned myocardium. Direct evidence for decreased myofilament Ca2+ responsiveness and altered diastolic function in intact ventricular muscle. Circ Res. 1995;76:1036–1048. doi: 10.1161/01.res.76.6.1036. [DOI] [PubMed] [Google Scholar]
- Garvey JL, Kranias EG, Solaro RJ. Phosphorylation of C-protein, troponin I and phospholamban in isolated rabbit hearts. Biochem J. 1988;249:709–714. doi: 10.1042/bj2490709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman YE, Hibberd MG, McCray JA, Trentham DR. Relaxation of muscle fibres by photolysis of caged ATP. Nature. 1982;300:701–705. doi: 10.1038/300701a0. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68:1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdani N, de Waard M, Messer AE, Boontje NM, Kooij V, van Dijk S, Versteilen A, Lamberts R, Merkus D, Dos Remedios C, Duncker DJ, Borbely A, Papp Z, Paulus W, Stienen GJ, Marston SB, van der Velden J. Myofilament dysfunction in cardiac disease from mice to men. J Muscle Res Cell Motil. 2008;29:189–201. doi: 10.1007/s10974-008-9160-y. [DOI] [PubMed] [Google Scholar]
- Han YS, Ogut O. Regulation of fibre contraction in a rat model of myocardial ischemia. PLoS One. 2010;5:e9528. doi: 10.1371/journal.pone.0009528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YS, Tveita T, Prakash YS, Sieck GC. Mechanisms underlying hypothermia-induced cardiac contractile dysfunction. Am J Physiol Heart Circ Physiol. 2010;298:H890–H897. doi: 10.1152/ajpheart.00805.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd MG, Dantzig JA, Trentham DR, Goldman YE. Phosphate release and force generation in skeletal muscle fibers. Science. 1985;228:1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vitovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Janosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF) MERIT-HF Study Group. JAMA. 2000;283:1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- Hofmann PA, Miller WP, Moss RL. Altered calcium sensitivity of isometric tension in myocyte-sized preparations of porcine postischemic stunned myocardium. Circ Res. 1993;72:50–56. doi: 10.1161/01.res.72.1.50. [DOI] [PubMed] [Google Scholar]
- Hoit BD, Khoury SF, Kranias EG, Ball N, Walsh RA. In vivo echocardiographic detection of enhanced left ventricular function in gene-targeted mice with phospholamban deficiency. Circ Res. 1995;77:632–637. doi: 10.1161/01.res.77.3.632. [DOI] [PubMed] [Google Scholar]
- Itoya M, Mallet RT, Gao ZP, Williams AGJ, Downey HF. Stability of high-energy phosphates in right ventricle: myocardial energetics during right coronary hypotension. Am J Physiol. 1996;271:H320–H328. doi: 10.1152/ajpheart.1996.271.1.H320. [DOI] [PubMed] [Google Scholar]
- Johns EC, Simnett SJ, Mulligan IP, Ashley CC. Troponin I phosphorylation does not increase the rate of relaxation following laser flash photolysis of diazo-2 in guinea-pig skinned trabeculae. Pflugers Arch. 1997;433:842–844. doi: 10.1007/s004240050353. [DOI] [PubMed] [Google Scholar]
- Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- Kreutziger KL, Piroddi N, Scellini B, Tesi C, Poggesi C, Regnier M. Thin filament Ca2+ binding properties and regulatory unit interactions alter kinetics of tension development and relaxation in rabbit skeletal muscle. J Physiol. 2008;586:3683–3700. doi: 10.1113/jphysiol.2008.152181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- Kusuoka H, Koretsune Y, Chacko VP, Weisfeldt ML, Marban E. Excitation-contraction coupling in postischemic myocardium. Does failure of activator Ca2+ transients underlie stunning? Circ Res. 1990;66:1268–1276. doi: 10.1161/01.res.66.5.1268. [DOI] [PubMed] [Google Scholar]
- Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Li L, Desantiago J, Chu G, Kranias EG, Bers DM. Phosphorylation of phospholamban and troponin I in beta-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol. 2000;278:H769–H779. doi: 10.1152/ajpheart.2000.278.3.H769. [DOI] [PubMed] [Google Scholar]
- Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- Luo Y, Davis JP, Smillie LB, Rall JA. Determinants of relaxation rate in rabbit skinned skeletal muscle fibres. J Physiol. 2002;545:887–901. doi: 10.1113/jphysiol.2002.031757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- Martin H, Barsotti RJ. Relaxation from rigor of skinned trabeculae of the guinea pig induced by laser photolysis of caged ATP. Biophys J. 1994;66:1115–1128. doi: 10.1016/S0006-3495(94)80892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KS, Moss RL, Miller WP. Incorporation of the troponin regulatory complex of post-ischemic stunned porcine myocardium reduces myofilament calcium sensitivity in rabbit psoas skeletal muscle fibers. J Mol Cell Cardiol. 1998;30:285–296. doi: 10.1006/jmcc.1997.0603. [DOI] [PubMed] [Google Scholar]
- Millar NC, Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J Biol Chem. 1990;265:20234–20240. [PubMed] [Google Scholar]
- Mulligan IP, Palmer RE, Lipscomb S, Hoskins B, Ashley CC. The effect of phosphate on the relaxation of frog skeletal muscle. Pflugers Arch. 1999;437:393–399. doi: 10.1007/s004240050793. [DOI] [PubMed] [Google Scholar]
- Palmer RE, Simnett SJ, Mulligan IP, Ashley CC. Skeletal muscle relaxation with diazo-2: the effect of altered pH. Biochem Biophys Res Commun. 1991;181:1337–1342. doi: 10.1016/0006-291x(91)92085-x. [DOI] [PubMed] [Google Scholar]
- Palmer S, Kentish JC. Roles of Ca2+ and crossbridge kinetics in determining the maximum rates of Ca2+ activation and relaxation in rat and guinea pig skinned trabeculae. Circ Res. 1998;83:179–186. doi: 10.1161/01.res.83.2.179. [DOI] [PubMed] [Google Scholar]
- Pate E, Franks-Skiba K, Cooke R. Depletion of phosphate in active muscle fibers probes actomyosin states within the powerstroke. Biophys J. 1998;74:369–380. doi: 10.1016/S0006-3495(98)77794-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem. 1982;257:260–263. [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- Saeki Y, Kobayashi T, Yasuda S, Nishimura S, Sugiura S, Yamashita H, Sugi H. Role of Ca2+ in determining the rate of tension development and relaxation in rat skinned myocardium. J Mol Cell Cardiol. 2004;36:371–380. doi: 10.1016/j.yjmcc.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Simnett SJ, Johns EC, Lipscomb S, Mulligan IP, Ashley CC. Effect of pH, phosphate, and ADP on relaxation of myocardium after photolysis of diazo 2. Am J Physiol. 1998;275:H951–H960. doi: 10.1152/ajpheart.1998.275.3.H951. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Moir AJ, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976;262:615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- Stelzer JE, Patel JR, Walker JW, Moss RL. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res. 2007;101:503–511. doi: 10.1161/CIRCRESAHA.107.153650. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Hattori A. Detection of giant myofibrillar proteins connectin and nebulin by electrophoresis in 2% polyacrylamide slab gels strengthened with agarose. Anal Biochem. 1995;224:28–31. doi: 10.1006/abio.1995.1004. [DOI] [PubMed] [Google Scholar]
- Tesi C, Piroddi N, Colomo F, Poggesi C. Relaxation kinetics following sudden Ca(2+) reduction in single myofibrils from skeletal muscle. Biophys J. 2002;83:2142–2151. doi: 10.1016/S0006-3495(02)73974-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voci P, Bilotta F, Caretta Q, Mercanti C, Marino B. Papillary muscle perfusion pattern. A hypothesis for ischemic papillary muscle dysfunction. Circulation. 1995;91:1714–1718. doi: 10.1161/01.cir.91.6.1714. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kerrick WG. The off rate of Ca(2+) from troponin C is regulated by force-generating cross bridges in skeletal muscle. J Appl Physiol. 2002;92:2409–2418. doi: 10.1152/japplphysiol.00376.2001. [DOI] [PubMed] [Google Scholar]
- Warren CM, Krzesinski PR, Campbell KS, Moss RL, Greaser ML. Titin isoform changes in rat myocardium during development. Mech Dev. 2004;121:1301–1312. doi: 10.1016/j.mod.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis. 2003;24:1695–1702. doi: 10.1002/elps.200305392. [DOI] [PubMed] [Google Scholar]
- Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered beta-adrenergically mediated protein phosphorylation. J Clin Invest. 1996;98:167–176. doi: 10.1172/JCI118762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Zhang EY, Zhang J, Bache RJ, Beard DA. Phosphate metabolite concentrations and ATP hydrolysis potential in normal and ischaemic hearts. J Physiol. 2008;586:4193–4208. doi: 10.1113/jphysiol.2008.154732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Coutu P, Sadayappan S, Robbins J, Metzger JM. Cardiac transgenic and gene transfer strategies converge to support an important role for troponin I in regulating relaxation in cardiac myocytes. Circ Res. 2007;101:377–386. doi: 10.1161/CIRCRESAHA.106.145557. [DOI] [PubMed] [Google Scholar]
- Yuan C, Guo Y, Ravi R, Przyklenk K, Shilkofski N, Diez R, Cole RN, Murphy AM. Myosin binding protein C is differentially phosphorylated upon myocardial stunning in canine and rat hearts-- evidence for novel phosphorylation sites. Proteomics. 2006;6:4176–4186. doi: 10.1002/pmic.200500894. [DOI] [PubMed] [Google Scholar]
- Zakhary DR, Moravec CS, Stewart RW, Bond M. Protein kinase A (PKA)-dependent troponin-I phosphorylation and PKA regulatory subunits are decreased in human dilated cardiomyopathy. Circulation. 1999;99:505–510. doi: 10.1161/01.cir.99.4.505. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ Res. 1995a;76:1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhao J, Potter JD. Phosphorylation of both serine residues in cardiac troponin I is required to decrease the Ca2+ affinity of cardiac troponin C. J Biol Chem. 1995b;270:30773–30780. doi: 10.1074/jbc.270.51.30773. [DOI] [PubMed] [Google Scholar]