Abstract
OBJECTIVE
The expression and effects of anti-inflammatory interleukins on endothelial cell (EC) activation and development of angiogenesis is uncharacterized. The purpose of this study is to characterize the expression and function of Interleukin-19 (IL-19), a recently described Th2 anti-inflammatory interleukin on EC pathophysiology.
METHODS and RESULTS
We demonstrate by immunohistochemistry and immunoblot that IL-19 is expressed in inflamed, but not normal human coronary endothelium, and can be induced in cultured human EC by serum and bFGF. IL-19 is mitogenic, chemotactic, and promotes cell EC spreading. IL-19 activates the signaling proteins STAT3, p44/42, and Rac1. In functional ex vivo studies, IL-19 promotes cord-like structure formation of cultured EC and also enhances microvessel sprouting in the mouse aortic ring assay. IL-19 induces tube formation in matrigel plugs in vivo.
CONCLUSIONS
These data are the first to report expression of the anti-inflammatory interleukin IL-19 in EC, and the first to indicate that IL-19 is mitogenic and chemotactic for EC, and can induce the angiogenic potential of EC.
INTRODUCTION
Endothelial cell (EC) paracrine and autocrine stimulation can result in migration and proliferation, and is an essential component of multiple normal and pathophysiological processes including atherosclerosis, permeability, wound healing, and angiogenesis (1–3). Angiogenesis is the growth of new blood vessels and normally occurs in the process of healing wounds and restoring blood flow after injury or insult. Both inflammatory and anti-inflammatory cytokine expression participate in wound healing and neo-vascularization, and many pro-inflammatory cytokines play an established role in angiogenesis. For example, pro-inflammatory cytokines such as IL-1β, IL-8, and IL-18 promote angiogenesis with direct, increased effects EC migration, proliferation, Matrix Metalloproteinase (MMP) production, tube formation; and increased vascularity in vivo (4–6). While it may be intuitive that the inflammatory state of ischemic tissue may dictate whether that tissue is neovascularized or becomes necrotic, the role of and direct effects of anti-inflammatory interleukins on EC in initiation and progression of neovascularization are less clear, and somewhat inconsistent. As examples, it is recognized that the M2, anti-inflammatory macrophage phenotype promotes tissue regeneration and angiogenesis by secretion of soluble factors. However, the prototypical anti-inflammatory cytokine, IL-10, has anti-angiogenic activity and is associated with VEGF down regulation, reduction of FGF and VEGF induced proliferation of microvascular EC (7). IL-10 also induced TIMP1 and reduced MMP2 production in EC (8). IL-4 can inhibit VEGF production and reduce vascularization, but can also induce migration and tube like structure formation in EC, activities consistent with angiogenesis (9–11). IL-20 also has reported pro and anti-angiogenic effects (12–14). Identification of cytokines and their receptors common to leukocytes and endothelial cells has the potential to link inflammatory and angiogenic processes. Characterization of these molecules and their function in these processes could lead to new therapies for tissue repair and neovascularization, and requires further investigation.
Interleukin-19 (IL-19) is an IL-10 family member expressed in monocytes, T, and B lymphocytes, and can be up regulated in these cells by LPS and G-CSF (15–18). Like IL-10, IL-19 has been ascribed to be a Th2, anti-inflammatory interleukin (18). Treatment of maturing antigen-presenting cells with IL-19 promotes of the Th2 (regulatory), rather than the Th1 (T helper) response, and induces IL-10, and decreases IFNγ expression in T cells (17,18). The expression and function of anti-inflammatory interleukins on EC pathophysiology remains uncharacterized, and the role of IL-19 in particular in wound healing or neovascularization has not been reported. To test the hypothesis that IL-19 has a non-Th2 regulatory function in EC, we designed experiments to determine a role for this interleukin in endothelial cell activation and angiogenesis. This is an inaugural study describing IL-19 effects in EC, and there are several unique findings in this report. We report that IL-19 expression can be induced in cultured EC, can activate multiple signal transduction proteins. The IL-20 receptor, the receptor for IL-19, is expressed on EC. IL-19 is mitogenic for EC, promotes tube-like structure formation on Matrigel by EC, microvessel formation in the mouse aortic ring assay, and in vivo in matrigel plugs. Because it is expressed by both leukocytes and EC, we propose that IL-19 be considered an inducible mediator of leukocyte/EC bi-directional communication in regulation of tissue repair and vessel regeneration. This has important implications for the role of anti-inflammatory factors in general in promotion of wound healing and vascular inflammation.
MATERIALS AND METHODS
Immunohistochemistry
Human coronary arteries were removed from non-failing hearts from organ donors which were deemed not appropriate for transplantation, and hearts which needed to be removed from patients with severe CAV at the time of re-transplantation and processed as we described (19).
Cells and Culture, Cell Proliferation, Migration assays, Rac1 activation, Western blotting, cord-like structure, flow cytometry, aortic ring assay, RT-PCR, mouse matrigel plug, and statistical analysis were all performed as we have described (19). All animal procedures were approved by the Institutional Animal Care and Use Committee of Temple University.
Cell Spreading
Spreading was performed as described and calculated by tracing the cell and measuring the area using Image-Pro Plus software (20).
For a detailed Materials and Methods section, please see the supplement material (available online at http://atvb.ahajournals.org).
RESULTS
Endothelial cells express Interleukin-19
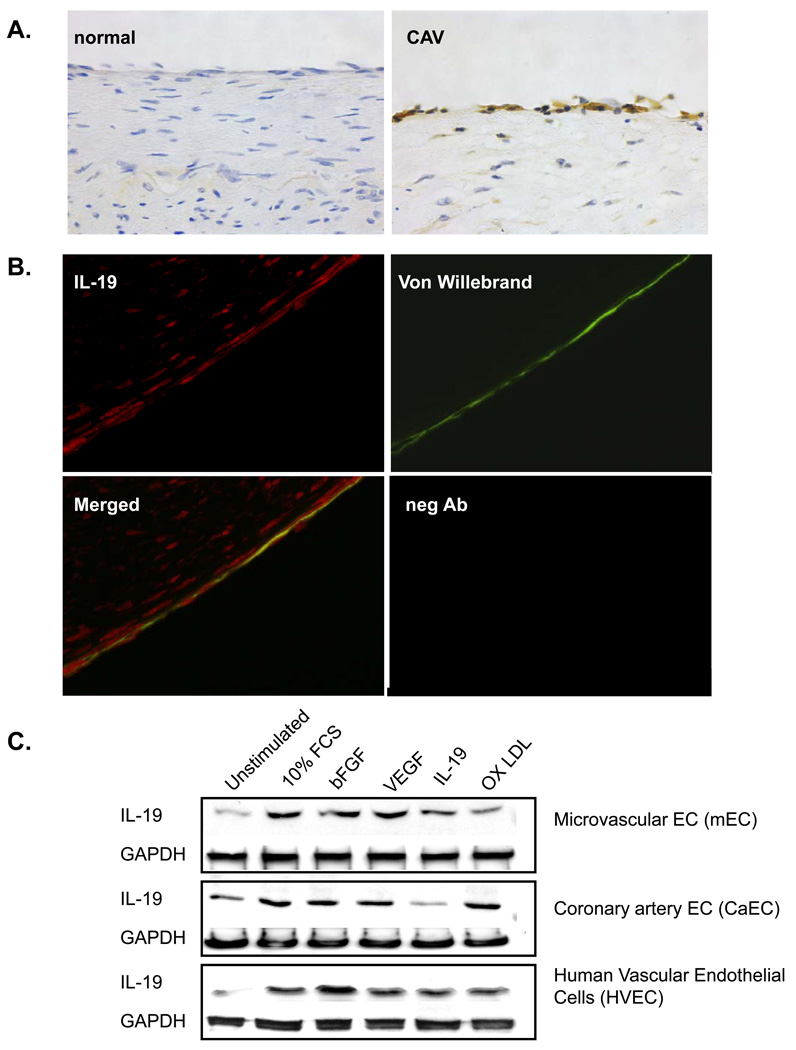
Immunohistochemical staining of normal and diseased human coronary arteries established IL-19 expression in human endothelium. Figure 1A demonstrates that IL-19 is expressed in EC in human coronary arteries with coronary artery transplant vasculopathy (CAV), a disease in which endothelium is chronically inflamed. We have previously shown that IL-19 is expressed in some SMC and CD45 positive cells in inflamed arteries (21). IL-19 immunoreactivity in endothelium in arteries with CAV was confirmed by co-localization with the EC marker von Willebrand factor (Figure 1B). In contrast, normal control coronary arteries did not show any expression of IL-19 in endothelium or other medial cells. Because CAV arteries exist in a milieu of cytokines and growth factors, we performed experiments to investigate differential expression of IL-19 by cytokines in cultured EC under more defined conditions. Human vascular endothelial cells (HVEC), coronary artery endothelial cells (CaEC), and coronary micro-endothelial cells (mEC) were cultured in serum-reduced media for 24 hours, then stimulated with a variety of soluble factors. Figure1C shows that all cultured EC express basal levels of IL-19 protein, but can be induced to express significantly increased IL-19 by 10% FCS and bFGF (P<0.05, n=3 experiments) (please see http://atvb.ahajournals.org, Supplemental Data Figure I). Other factors can also increase IL-19 expression, but not to a significant degree. Expression of IL-19 mRNA mirrors protein expression (data not shown). These data are the first to indicate that IL-19 expression is inducible in several EC types. Subsequent experiments were focused to determine a function and potential mechanism of IL-19 activity in EC.
Figure 1.
Endothelial cells express Interleukin-19. A. Representative immunohistochemical analysis of IL-19 expression in sections from human coronary arteries from a normal and a patient with cardiac allograft vasculopathy (CAV) stained with anti-IL-19 antibody. B. Fluorescence immunohistochemistry. All sections from human artery with CAV co-stained with IL-19 antibody (red), and Von Willebrand factor (green), merged, and negative control. 600X magnification for all. C. Representative immunoblot of differential induction of IL-19 protein by soluble factors. Primary HVEC, CaEC, and mEC were serum-starved for 24 hours, then stimulated with the indicated factors for 48 additional hours. Blots are representative of at least 3 independent experiments.
IL-19 activates EC signal transduction and enhances proliferation
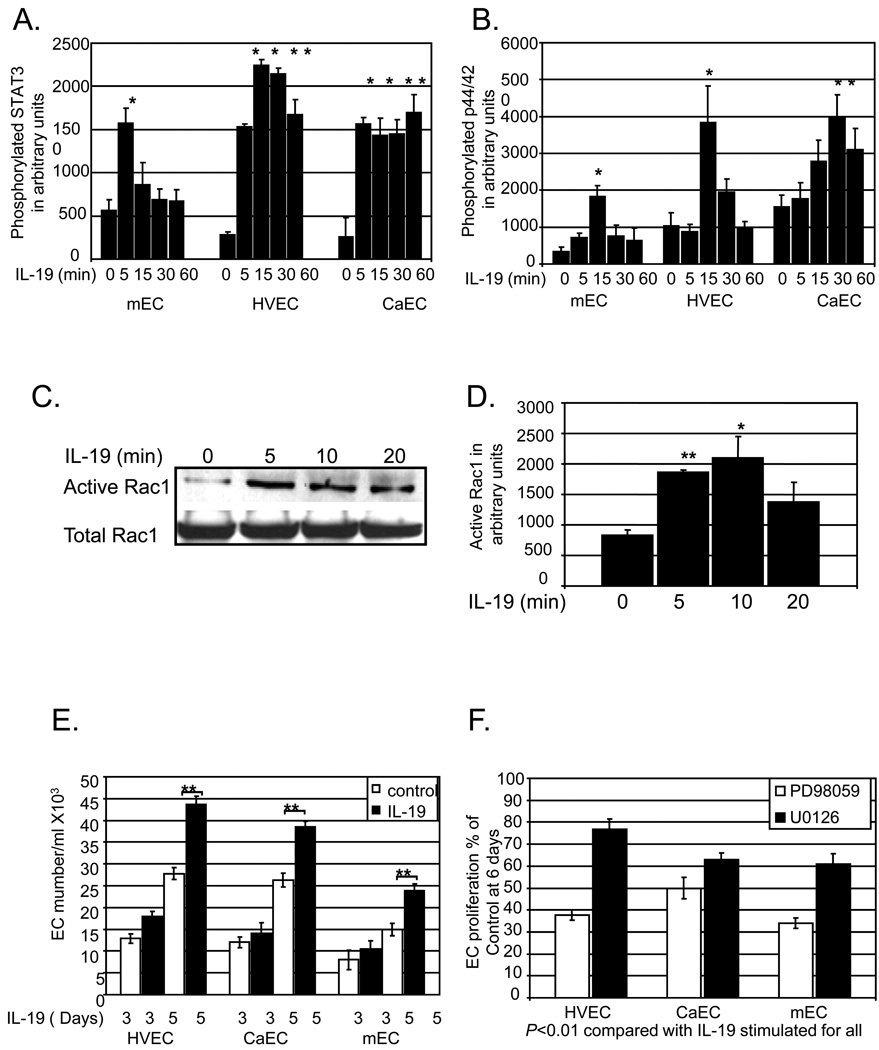
IL-19 can significantly induce rapid and transient activation of STAT3, Rac1, and MAPK p44/42 in all EC types examined (P< 0.05 or 0.01 compared with unstimulated for all) (Figure 2A–D) (for representative immunoblots, please see http://atvb.ahajournals.org, Supplemental Data Figure II). IL-19 signals through the IL-20 receptor, which is composed of α and β subunits, and is known to activate STAT proteins. Each EC type expressed detectible amounts of mRNA for each of the IL-20 receptor subunits (please see http://atvb.ahajournals.org, Supplemental Data Figure III).
Figure 2.
IL-19 activates signal transduction and EC Proliferation. Human mEC, HVEC, or CaEC were cultured in serum -reduced media, then challenged with 100ng/ml IL-19 for the indicated times. Protein extracts were immunoblotted with anti-total or phospho-STAT3 (A.), or anti-total or phospho-p44/42 MAPK (B.). IL-19 activation of Rac1 was assayed by the GST-PAK sepharose pull-down assay (C.,D.). Densitometry for all experiments was performed and values normalized to total protein from at least 3 experiments. Asterisk indicates significant difference from unstimulated (* = P<0.05, ** = P<0.01 compared with unstimulated controls). E. IL-19 stimulates EC proliferation. Human mEC, HVEC, or CaEC in 1% FCS were treated with 100ng/ml IL-19and at 3, and 5 days they were trypsinized and counted in triplicate. (** = P<0.01 compared with unstimulated control), from three independent experiments. F. IL-19-induced EC proliferation is significantly reduced in EC treated with p44/42 inhibitors PD98059 and U0126 (5µM each) (** = P<0.01 compared with untreated control), from three independent experiments.
Because activation of p44/42 MAPK participates in EC mitogenesis we tested the hypothesis that IL-19 could promote proliferation of EC by itself, and/or enhance VEGF or bFGF mediated EC proliferation. HVEC, CaEC, mEC cultured in growth media were treated with IL-19 100ng/ml IL-19, and at 1, 3, and 5 days they were trypsinized and counted. In each EC type IL-19 significantly increased EC proliferation at 5 days (43.7+/−1.6 Vs 27.8+/−1,4, 38.4+/−1.3 Vs 26.3+/−1.5, 23.9+/−1.8 Vs 15+/−1.3 EC/ml, for HVEC, CaEC, and mEC, respectively, P<0.001 for all) (Figure 2E). This is not due to decreased apoptosis of these cells, as ascertained by Annexin V staining (please see http://atvb.ahajournals.org, Supplemental Data Figure IV). IL-19 did not induce VEGF or bFGF protein expression (please see http://atvb.ahajournals.org, Supplemental Data Figure V), nor enhance either VEGF or bFGF mediated HVEC proliferation (please see http://atvb.ahajournals.org, Supplemental Data Figure VI). To determine if IL-19 proliferative effects are contingent on p44/42 MAPK activity, each EC type was cultured in the presence of two different MAPK inhibitors; PD98059, or U0126. Each of these inhibitors at published EC50 concentrations (5µM each) significantly reduced IL-19-driven EC proliferation in the range of 23.3% – 66.0% (P<0.01 for each EC type) (Figure 2F). This indicates that IL-19 is mitogenic for multiple EC types, and utilizes the p44/42 MAPK pathway in this process.
IL-19 activates EC spreading and migration
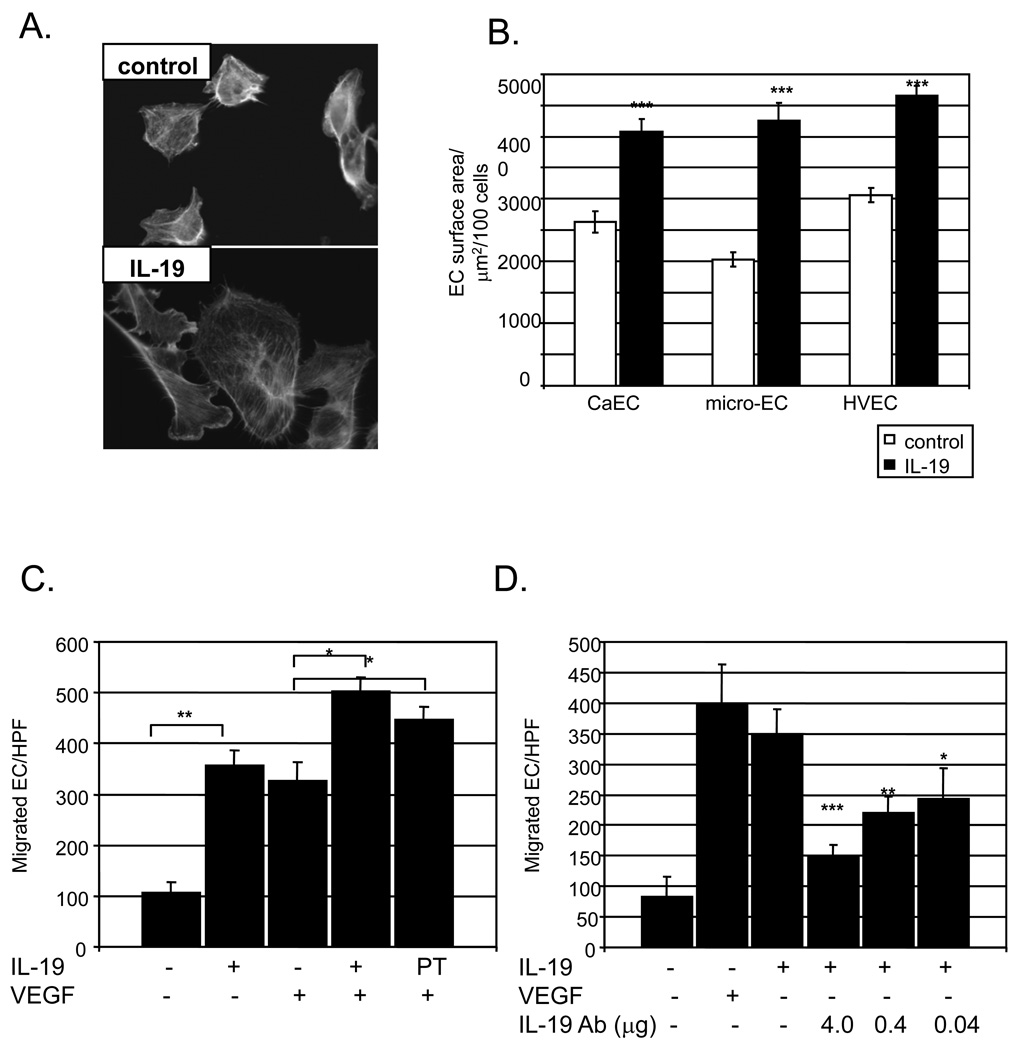
p44/42 MAPK, Rac1, and STAT3 regulate cytoskeletal organization and actin dynamics, and we hypothesized that IL-19 would influence EC spreading and/or migration. In all three EC types, IL-19 significantly enhanced cell spreading as compared with unstimulated cells (3060+/−110 Vs 4650+/−110, 2620+/−170 Vs 4087+/−190, 20.3+/−120 Vs 4260+/−290 µm2, P<0.001 for HVEC, CaEC, mEC, respectively) (Figure 3B).
Figure 3.
IL-19 promotes EC spreading and migration. A.,B. Human EC grown on glass coverslips were treated with IL-19 for 3 hours or left untreated. Rhodamine phalloidin was added to stain filamentous actin. Images were captured and calculated by tracing the cell and measuring the area using Image-Pro Plus software. Data shown are mean ± s.e.m. of 100 cells from each population from three separate experiments. IL-19 induces EC migration. C. HVEC were seeded in medium containing 0.2% BSA, with or without IL-19, or VEGF as a positive control. Some EC were pre-treated with IL-19 16 hours prior to seeding in migration chambers. (** = P<0.01 compared with unstimulated controls). D. IL-19 neutralizing antibody significantly reduced IL-19 induced HVEC migration at all concentrations of neutralizing antibody used. *** = P<0.0001, compared with no antibody control).
To determine if IL-19 induced EC migration, HVEC were seeded onto the top chamber in medium containing 0.2% BSA, with or without 100ng/ml IL-19, or VEGF as a positive control, in the lower chamber. Some EC were pre-treated with IL-19 for 16 hours prior to trypsinization and seeding in migration chambers. Figure 3C shows that addition of IL-19 significantly increases HVEC migration (107.5+/−19.3 compared with 357.6+/− 29.9 EC/HPF for unstimulated Vs. IL-19 treated, P<0.01 n = 3), which is comparable to values obtained for VEGF (317.0+/-36.9 EC/HPF), and there was no significant difference between IL-19 and VEGF-treated HVEC. Interestingly, co-incubation of VEGF and IL-19 resulted in significantly more migration than VEGF alone (503.1+/−26.4 Vs 317.0+/−36.9 EC/HPF for combined and VEGF, respectively, P<0.05). Also interesting was that EC pre-incubated with IL-19, prior to seeding in the chamber, but did not have IL-19 in the lower chamber also migrated significantly more rapidly than VEGF alone (447.3+/−24.3 Vs 317.0+/−36.9 EC/HPF, P<0.5, n=3, for pre-treated Vs unstimulated EC, respectively) (Figure 3D). There was no statistical difference in migration between HVEC which had VEGF and IL-19 in the lower chamber and those which were pre-treated with IL-19, but only had VEGF in the lower chamber. Experiments were also performed in which IL-19 neutralizing antibody was added to the lower chamber. Figure 3B shows that IL-19 neutralizing antibody significantly reduced IL-19 induced HVEC migration at all concentrations of neutralizing antibody used. VEGF does not induce IL-19 expression (please see http://atvb.ahajournals.org, Supplemental Data Figure VII), nor does IL-19 neutralizing antibody reduce VEGF-induced migration (please see http://atvb.ahajournals.org, Supplemental Data Figure VIII). Together, these data are the first to show that IL-19 stimulates endothelial cell signaling, spreading, and migration.
IL-19 promotes endothelial cell cord-like structure formation
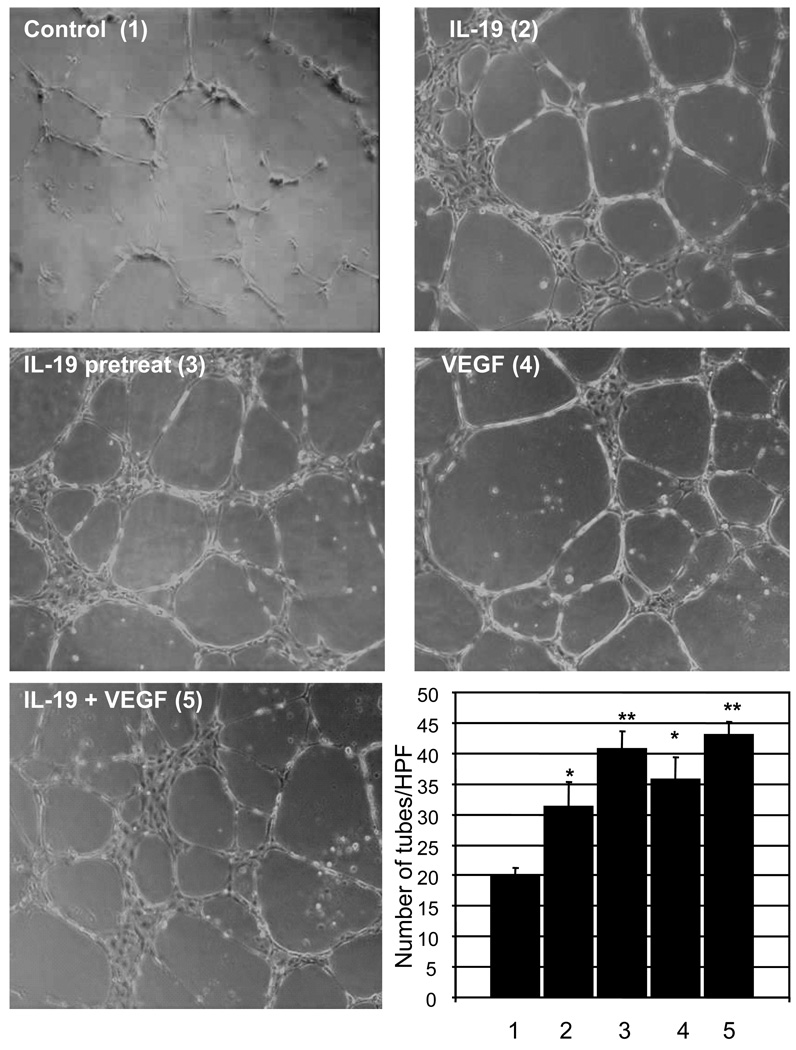
We hypothesized that IL-19 might play a role in endothelial migration and organization leading to tube or cord-like structure formation. HVEC were seeded onto growth factor reduced matrigel, in the presence of 10% FCS. Some HVEC were treated with IL-19, some co-treated with IL-19 and VEGF, and some pretreated with IL-19, and then treated with VEGF, or with VEGF alone as a positive control. The number of cord-like structures were counted manually per multiple representative images and an average was calculated for each condition. Data presented in Figure 4 show that IL-19 can induce significantly more cord-like structures than control samples (20.0+/−1.2 compared with 31.3+/−3.9 cords for control and IL-19 treated, respectively, (P<0.05, n=3). These results were similar to migration experiments in that EC pre-treated with IL-19, but not incubated with IL-19 in the assay, continued to form cord-like structures to a similar degree compared with IL-19, VEGF, or co-treated HVEC (40.6+/−2.9 Vs 31.3+/−3.9, 35.6+/−3.6, 43.0+/−2.1, for pre-treated, IL-19, VEGF, and co-treated HVEC, respectively). Together, this suggests that IL-19 plays a role in directed EC migration and organization.
Figure 4.
IL-19 promotes EC cord structure formation. HVEC were seeded into growth factor reduced matrigel, in the presence or absence of IL-19, VEGF, or both, and after 16 hours, the number of cord structures were counted and an average was calculated for each condition. Three images were taken per well from random fields. Each condition was performed in triplicate. (* = P<0.05, ** = P<0.01 compared with unstimulated control).
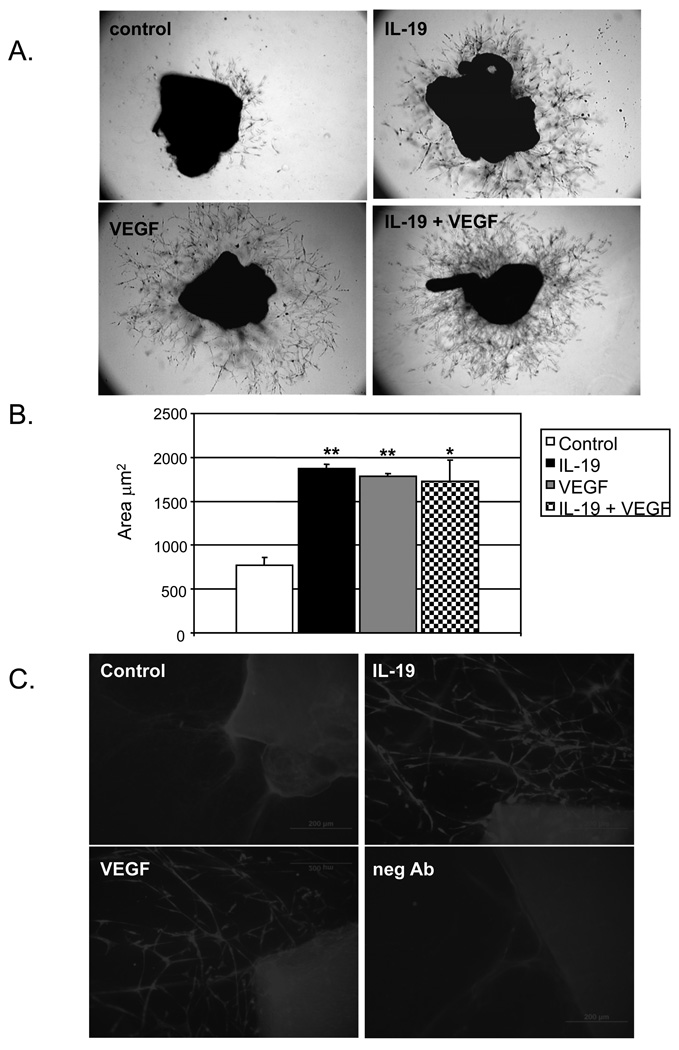
IL-19 promotes microvessel formation
Because IL-19 effected cord-like structure formation, we hypothesized that IL-19 could also regulate endothelial cell sprouting using two independent assays; ex vivo from aortic rings, and in vivo utilizing the matrigel plug angiogenesis assay (22,23). Mouse thoracic aorta was sectioned, placed onto growth factor reduced matrigel, and incubated in media containing IL-19, PBS, or VEGF as a positive control. Sprouting from rings were analyzed daily, and on the sixth day photographed and outgrowth area quantitated by image analysis. Figure 5 shows that aortic rings incubated with IL-19 showed significantly greater sprouting area than matrigel alone (770.0+/−85.1 Vs 1877.3+/−48.1 µm2 for unstimulated and IL-19 treated, respectively (P<0.01, n=6). This was similar to values for the VEGF positive control, and rings incubated with both IL-19 and VEGF (1784.8+/−28.8, and 1730.0+/−478.0µm2 for VEGF and IL-19 and VEGF co-treated, respectively). Immunostaining was performed on whole mounts of aortic rings. Sprouting microvessels were immunoreactive with PECAM1 antibody, demonstrating the presence of EC in these structures.
Figure 5.
IL-19 promotes EC microvessel formation in mouse aortic rings. Sectioned mouse thoracic aorta was cultured in triplicate in growth factor reduced matrigel in the presence or absence of IL-19, VEGF, or both. B. Densiometric quantitation of area of microvessel sprouting (* = P<0.05, ** = P<0.01 compared with unstimulated control) from three independent experiments. C. Rings that were cultured with media only (control), IL-19, or VEGF for 6 days were immuno-stained with PECAM1 antibody, or secondary antibody only (neg Ab).
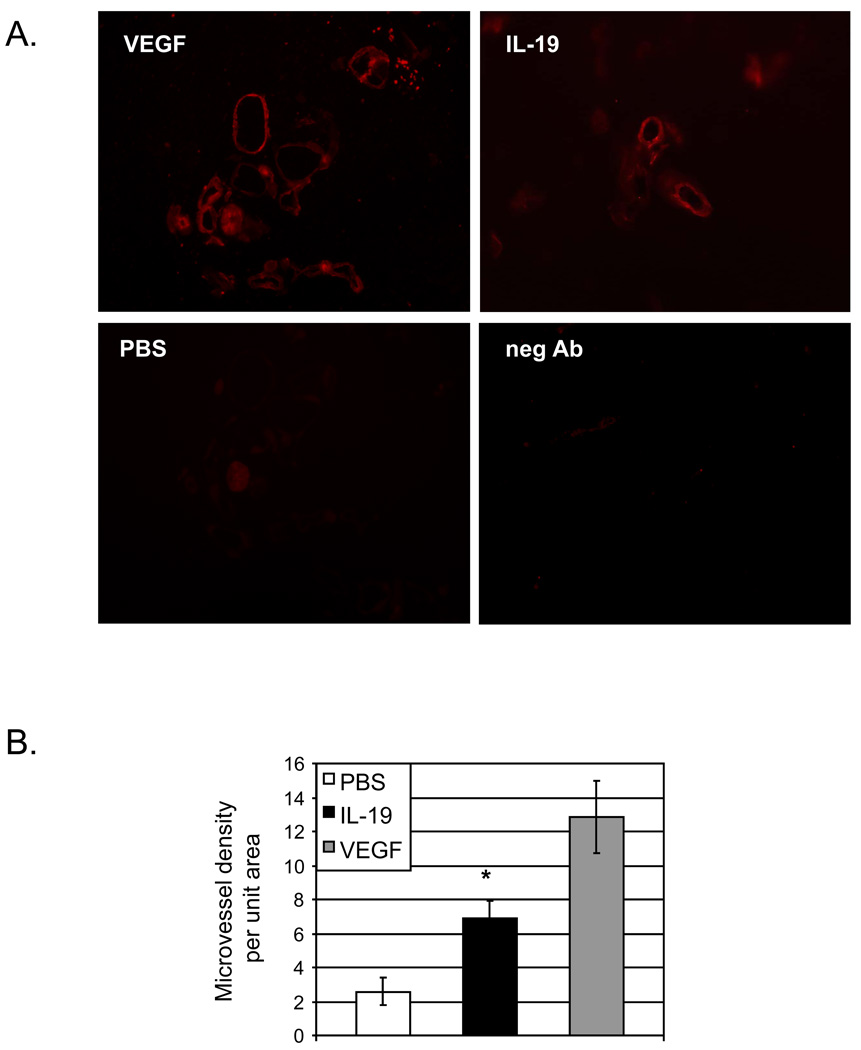
To determine if IL-19 could stimulate microvessel formation in vivo, 0.5 ml growth-factor free matrigel was mixed with 200ng IL-19, 200ng VEGF or PBS control, and delivered as a single plug subcutaneously. After 10 days, the plug was recovered, and microvessels containing lumen determined by immunohistochemistry using PECAM1 antibody. Figure 6 shows that Matrigel containing IL-19 contained significantly more PECAM1-positive microvessels per unit area than did PBS control plugs (2.5+/−.08, 6.9+/−1.0, and 12.8+/−2.1 microvessels/unit area for PBS, IL-19, and VEGF, respectively, P<0.05,). These PECAM1 positive structures surrounded a lumen, indicating that IL-19 can induce microvessel formation in vivo.
Figure 6.
IL-19 stimulates Matrigel plug angiogenesis in vivo. A. C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with PBS control, VEGF, or IL-19 and injected subcutaneously. Ten days later, Matrigel plugs were harvested processed for immunofluorescence PECAM1 staining. Image is representative of 4 plugs, from four different mice. B. PECAM1 positive cells surrounding lumens normalized to unit area were quantitated (*= P<0.05 Vs PBS control).
DISCUSSION
The major finding of this study is that IL-19 is a novel, pro-angiogenic factor expressed in EC. IL-19 is a Th2 anti-inflammatory interleukin, and nothing has been reported on the expression or mechanism(s) of IL-19 effects on EC. Basal levels of IL-19 can be detected in human monocytes, B, and T lymphocytes, and IL-19 expression can be upregulated in these cells by stimulation. This is the first report regarding IL-19 expression in endothelial cells, and IL-19 expression in inflamed EC suggests a role distinct from Th1/Th2 phenotype modulation in lymphocytes. The observation that low basal levels of IL-19 expression can be increased by fetal calf serum is consistent with the finding that IL-19 is undetectable in uninjured human arteries, but is increased in arteries from patients diagnosed with CAV, a chronic inflammatory disease in allografted hearts. Expression in injured arteries is also consistent with an in vivo murine tissue survey indicating that IL-19 is not present in unstimulated tissue, but can be induced in immune and local tissue cells in inflamed tissue, and cultured keratinocytes when stimulated with IL-1β (24,25). Expression of certain anti-inflammatory cytokines; IL-9, IL-10 or IL-11, in EC have not been reported. However, IL-4 is detected in EC from atherosclerotic plaques, and can increase VCAM-1, MCP-1, and IL-6, consistent with a pro-inflammatory effect on in endothelium (11,26).
IL-19 shares 20% amino acid identity with IL-10, but does not engage the IL-10 receptor, but rather the IL-20 receptor, which is known to activate STAT proteins in several cell types (17,27). In our study in HVEC, IL-19 did activate both STAT3 and p44/42 MAPK activity, but not STAT1. There is a diversity in utilization of various members of the STAT family by interleukins, much of which is regulated by tissue specific expression of receptor subunits (27,28). The IL-20 receptor is composed of α and β subunits; this study shows that each EC type examined expressed both IL-20 receptor chains. IL-19 can only engage and signal through a heterodimer of alpha and beta chains (16,17,24). In another study in EC, IL-20 also activated p44/42, but activated STAT1 rather than STAT3 (12). Interestingly, IL-20 did stimulate STAT3 in keratinocytes (27). Some of these differences in signaling and gene expression may be explained by IL-20 receptor complexity, and tissue distribution (27,29).
The present study demonstrated that IL-19 was capable of meditating EC spreading and migration and proliferation. STAT3, p44/42, and the small GTPase Rac1 are involved in EC proliferation, migration and angiogenesis. STAT3 in particular participates in angiogenesis by multiple mechanisms, including potentiation of microtubule polymerization leading to increased migration and induction of VEGF expression (30,31). p44/42 regulates membrane protrusions and focal adhesion dynamics, and specific p44/42 inhibitors decrease migration of multiple cell types, including EC (32,33). Rac1 also has a key role in cell migration by influencing actin cytoskeleton and membrane ruffling, and IL-19 activation of any one of these three enzymes may be responsible for EC cell spreading and migration (34,35). Interestingly, EC pre-treated with IL-19 for 24 hours migrated more rapidly than those singly incubated with either IL-19 or VEGF, and migrated to the same degree as those co-incubated with both VEGF and IL-19 as chemotactic agents in the lower chamber, suggesting that IL-19 could induce expression of a factor or factors which played a part in migration.
Migration and proliferation of EC in response to peptide factors is a key event in angiogenesis. IL-19 promoted cord-like structure formation on Matrigel by HUVECs, and also enhanced microvessel formation in isolated mouse aortic rings, and microvessel formation in matrigel plugs in vivo. PECAM1-positive structures in matrigel plugs surrounded a lumen. These assays assess EC migration, proliferation, differentiation, and polarization of primary, quiescent EC into organized sprouts. Microvessels sprouting from aortic rings were immunoreactive with PECAM1 antibody, confirming the effects of IL-19 on EC in these structures.
Various pro-inflammatory interleukins such has IL-1, IL-8, IL-17 and IL-20 have been identified to be angiogenic, and increase EC migration, cord-like structure organization, and MMP production (4–6,36). However, the effects of anti-inflammatory interleukins on angiogenesis is not well characterized. The archetypal anti-inflammatory cytokine, IL-10, is anti-angiogenic, as it reduces EC migration, and down regulates VEGF production (7,8). While IL-4 is anti-angiogenic in that it inhibits VEGF production and reduces vascularization (10,38), it can also induce migration and cord like structure formation in EC, consistent with angiogenesis (9,11). Similarly, IL-13 attenuates EC tube formation by induction of the JAK2-STAT6 pathway (37). IL-20 is expressed by endothelial cells, and has both pro and anti-angiogenic effects on EC (12,14). IL-20 angiogenic effects are indirect, as it also induces FGF2 and VEGF expression (12). In contrast to IL-20, IL-19 does not induce VEGF, or its own expression, and VEGF does not induce IL-19 expression, implying that IL-19 effects on EC proliferation are direct. It also explains why IL-19 neutralizing antibody had no effect on VEGF induced migration. It is also noteworthy that neither VEGF or bFGF could significantly increase the proliferative effects of IL-19 on EC, implying independent pathways of activity. IL-19 effects on cellular proliferation also appear to be cell type-specific, as IL-19 has anti-proliferative effects in both a breast cancer cell line and human vascular smooth muscle cells (VSMC) (17,21). This may also be a function of tissue specific expression of receptor subunits, and distribution of receptor chain expression on EC and VSMC requires further investigation.
The notion that a Th2 anti-inflammatory interleukin can directly regulate EC pathophysiology is novel, and has important implications for the role of anti-inflammatory mediators in vasculogenesis. This is an inaugural study describing IL-19 effects in EC, and there are several unique findings in this report. Expression of IL-19, a Th2 interleukin, can be induced in cultured EC and in vivo by local inflammation. Treatment of cultured human EC with IL-19 results in activation of STAT3, p44/42 MAPK, and Rac1. IL-19 treatment of EC results in increased cell spreading and enhanced migration. IL-19 is mitogenic for EC, and enhances cord-like structure formation and microvessel sprouting from aortic rings. IL-19 can induce microvessel formation in matrigel plugs in vivo. Angiogenesis is a multi-step process requiring EC activation, basement membrane degradation, proliferation, migration, and cord formation. This work suggests that IL-19 can participate in each of these processes, and has important implications for wound healing, vasculogenesis, and vascular biology.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Victor Rizzo (Temple University School of Medicine) for critical reading of the manuscript.
Sources of Funding. This work was supported by grants HL063810 and HL090885 from the National Heart Lung, and Blood Institute, and grant 0455562U from the American Heart Association, to M.V.A..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure. There are no relationships that could be percieved as apparent conflict of interest.
REFERENCES
- 1.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in Physiology and pathophysiology of Vascular Disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 2.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 3.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 4.Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J. Immunol. 2001;167:1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 5.Salven P, Hattori K, Heissig B, Rafii S. Interleukin-1 alpha promotes angiogenesis in vivo via VEGFR-2 pathway by inducing inflammatory cell VEGF synthesis and secretion. FASEB J. 2002;16:1471–1483. doi: 10.1096/fj.02-0134fje. [DOI] [PubMed] [Google Scholar]
- 6.Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, Gudas JM, Bar-Eli M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am. J. Pathol. 2002;161:125–134. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvestre JS, Mallat Z, Duriez M, Tamarat R, Bureau MF, Scherman D, Duverger N, Branellec D, Tedgui A, Levy BI. Antiangiogenic effect of interleukin-10 in ischemia-induced angiogenesis in mice hindlimb. Circ Res. 2000;87:448–452. doi: 10.1161/01.res.87.6.448. [DOI] [PubMed] [Google Scholar]
- 8.Mtairag M, Chollet-Martin S, Oudghiri M, Laquay N, Jacob M, Michel J, Feldman L. Effects of interleukin-10 on monocyte/endothelial cell adhesion and MMP-9/TIMP-1 secretion. Cardiovasc Res. 2001;49:882–890. doi: 10.1016/s0008-6363(00)00287-x. [DOI] [PubMed] [Google Scholar]
- 9.Walch L, Massade L, Dufilho M, Brunet A, Rendu F. Pro-atherogenic effect of interleukin-4 in endothelial cells: modulation of oxidative stress, nitric oxide and monocyte chemoattractant protein-1 expression. Atherosclerosis. 2006;187:285–291. doi: 10.1016/j.atherosclerosis.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Haas CS, Amin MA, Allen BB, Ruth JH, Haines GK, 3rd, Woods JM, Koch AE. Inhibition of angiogenesis by interleukin-4 gene therapy in rat adjuvant-induced arthritis. Arthritis Rheum. 2006;54:2402–2414. doi: 10.1002/art.22034. [DOI] [PubMed] [Google Scholar]
- 11.Lee YW, Eum SY, Chen KC, Hennig B, Toborek M. Gene expression profile in interleukin-4-stimulated human vascular endothelial cells. Mol Med. 2004;10:19–27. doi: 10.2119/2004-00024.lee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh MY, Chen WY, Jiang MJ, Cheng BC, Huang TY, Chang MS. Interleukin-20 promotes angiogenesis in a direct and indirect manner. Genes Immun. 2006;7:234–242. doi: 10.1038/sj.gene.6364291. [DOI] [PubMed] [Google Scholar]
- 13.Tritsaris K, Myren M, Ditlev SB, Hübschmann MV, van der Blom I, Hansen AJ, Olsen UB, Cao R, Zhang J, Jia T, Wahlberg E, Dissing S, Cao Y. IL-20 is an arteriogenic cytokine that remodels collateral networks and improves functions of ischemic hind limbs. Proc Natl Acad Sci U S A. 2007;104:15364–15369. doi: 10.1073/pnas.0707302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heuzé-Vourc'h N, Liu M, Dalwadi H, Baratelli FE, Zhu L, Goodglick L, Põld M, Sharma S, Ramirez RD, Shay JW, Minna JD, Strieter RM, Dubinett SM. IL-20, an anti-angiogenic cytokine that inhibits COX-2 expression. Biochem Biophys Res Commun. 2005;333:470–475. doi: 10.1016/j.bbrc.2005.05.122. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, Vazquez N, Pestka S, Donnelly RP, Kotenko SV. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10) Genes Immun. 2000;1:442–450. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- 16.Oral HB, Kotenko SV, Yilmaz M, Mani O, Zumkehr J, Blaser K, Akdis CA, Akdis M. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol. 2006;36:380–388. doi: 10.1002/eji.200425523. [DOI] [PubMed] [Google Scholar]
- 17.Parrish-Novak J, Xu W, Brender T, Yao L, Jones C, West J, Brandt C, Jelinek L, Madden K, McKernan PA, Foster DC, Jaspers S, Chandrasekher YA. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological function. J Biol Chem. 2002;277:47517–47523. doi: 10.1074/jbc.M205114200. [DOI] [PubMed] [Google Scholar]
- 18.Jordan W, Eskdale J, Boniotto M, Lennon GP, Peat J, Campbell JDM, Gallagher G. Human IL-19 regulates immunity through auto-induction of IL-19 and production of IL-10. Eur J Immunol. 2005;35:1576–1582. doi: 10.1002/eji.200425317. [DOI] [PubMed] [Google Scholar]
- 19.Tian Y, Jain S, Kelemen SE, Autieri MV. AIF-1 expression regulates endothelial cell activation, signal transduction, and vasculogenesis. Am J Physiol Cell Physiol. 2009;296:C256–C266. doi: 10.1152/ajpcell.00325.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Rizzo V, Liu Y, Sainz IM, Schmuckler NG, Colman RW. Kininostatin Associates With Membrane Rafts and Inhibits vβ3 Integrin Activation in Human Umbilical Vein Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2007;27:1968–1975. doi: 10.1161/ATVBAHA.107.148759. [DOI] [PubMed] [Google Scholar]
- 21.Tian Y, Sommerville LJ, Cuneo A, Kelemen SE, Autieri MV. Expression and suppressive effects of interleukin-19 on vascular smooth muscle cell pathophysiology and development of intimal hyperplasia. Am J Pathol. 2008;173:901–909. doi: 10.2353/ajpath.2008.080163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu WH, Iurlaro M, MacIntyre A, Fogel E, Nicosia RF. The mouse aorta model: influence of genetic background and aging on bFGF- and VEGF-induced angiogenic sprouting. Angiogenesis. 2003;6:193–199. doi: 10.1023/B:AGEN.0000021397.18713.9c. [DOI] [PubMed] [Google Scholar]
- 23.Kundumani-Sridharan V, Niu J, Wang D, Van Quyen D, Zhang Q, Singh NK, Subramani J, Karri S, Rao GN. 15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis requires Src-mediated Egr-1-dependent rapid induction of FGF-2 expression. Blood. 2010;115:2105–2116. doi: 10.1182/blood-2009-09-241802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsing CH, Li HH, Hsu YH, Ho CL, Chuang SS, Lan KM, Chang MS. The distribution of interleukin-19 in healthy and neoplastic tissue. Cytokine. 2008;44:221–228. doi: 10.1016/j.cyto.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, Sterry W, Asadullah K, Sabat R. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol. 2006;15:991–1004. doi: 10.1111/j.1600-0625.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 26.Barks JL, McQuillan JJ, Iademarco MF. TNF-alpha and IL-4 synergistically increase vascular cell adhesion molecule-1 expression in cultured vascular smooth muscle cells. J Immunol. 1997;159:4532–45388. [PubMed] [Google Scholar]
- 27.Dumoutier L, Leemans Caroline, Lejeune Diane, Kotenko SergeiV, Renauld Jean-Christophe. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J. Immunol. 2001;167:3545–3549. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- 28.Murray PJ. STAT3-mediated anti-inflammatory signalling. Biochem Society Trans. 2006;34:1028–1031. doi: 10.1042/BST0341028. [DOI] [PubMed] [Google Scholar]
- 29.Blumberg H, Conklin D, Xu W, Grossmann A, Brender T, Carollo S, Eagan M, Chandrasekher YA. Interleukin 20: Discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9–19. doi: 10.1016/s0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 30.Ng DC, Lin BH, Lim CP, Huang G, Zhang T, Poli V, Cao X. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J. Cell Biol. 2006;172:245–257. doi: 10.1083/jcb.200503021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2000;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 32.Klemke RL, Cai S, Giannini AL, Gallagher PJ, deLanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anand-Apte B, Zetter BR, Viswanathan A, Qiu RG, Chen J, Ruggieri R, Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J Biol Chem. 1997;272:30688–30692. doi: 10.1074/jbc.272.49.30688. [DOI] [PubMed] [Google Scholar]
- 34.Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res. 2006;98:176–185. doi: 10.1161/01.RES.0000200162.94463.d7. [DOI] [PubMed] [Google Scholar]
- 35.Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J. Cell Sci. 2002;115:1123–1136. doi: 10.1242/jcs.115.6.1123. [DOI] [PubMed] [Google Scholar]
- 36.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. 1. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura Y, Nitto T, Inoue T, Node K. IL-13 Attenuates Vascular Tube Formation Via JAK2-STAT6 Pathway. Circ. J. 2008;72:469–475. doi: 10.1253/circj.72.469. [DOI] [PubMed] [Google Scholar]
- 38.Hong KH, Cho ML, Min SY, Shin YJ, Yoo SA, Choi JJ, Kim WU, Song SW, Cho CS. Effect of interleukin-4 on vascular endothelial growth factor production in rheumatoid synovial fibroblasts. Clin Exp Immunol. 2007;47:573–579. doi: 10.1111/j.1365-2249.2006.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.