Abstract
gp130 is a ubiquitously expressed glycoprotein ad signal transducer of interleukin 6 family of cytokines. It has been reported that gp130 has 11 potential N-glycosylation sites in the extracellular domain, and 9 of them are actually N-glycosylated. However, the structure and functional role of the carbohydrate chains carried by gp130 are totally unknown. In this study, we examined the functional role of N-glycans of gp130 in mouse neuroepithelial cells. In neuroepithelial cells treated with tunicamycin, an N-glycosylation inhibitor, unglycosylated form of gp130 was detected. The unglycosylated gp130 was not phosphorylated in response to leukemia inhibitory factor stimulation. Although the unglycosylated gp130 was found to be expressed on the cell surface, it could not form a heterodimer with leukemia inhibitory factor receptor. These results suggest that N-glycans are required for the activation, but not for the localization, of gp130 in neuroepithelial cells.
Keywords: Glycoprotein, IL-6 family of cytokine, N-glycosylation, signal transduction, tunicamycin
Introduction
gp130 (CD130) is a ubiquitously expressed transmembrane protein which has a wide variety of roles in, for instance, the immune system and the nerve system [1]. gp130 was originally found as a receptor component and signal transducer of interleukin 6 (IL-6) [2], but further studies revealed that this molecule mediates signaling activated by all of eight IL-6 family of cytokines, such as IL-11, leukemia inhibitory factor (LIF), ciliary neurotrophic factor, oncostatin M, cardiotrophin-1, cardiotrophin-like cytokine/novel neurotrophin-1/B-cell stimulating factor 3 and neuropoietin. As the downstream signaling pathways activated by gp130, the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, the Ras-mitogen-activated protein kinase (MAPK) pathway and the phosphatidylinositol 3-kinase-Akt pathway are known. The signals to these pathways are mediated via homodimerization of gp130 or heterodimerization of gp130 and another related signal transducer such as LIF receptor (LIFR) or oncostatin M receptor in a cytokine binding-dependent manner.
Neural stem cells (NSCs) and neural precursor cells (NPCs), undifferentiated neural cells endowed with a high potential for proliferation and a capacity for self-renewal with retention of multipotency to differentiate into neuronal and glial cells, have been the focus of attention in the fields of basic biology and clinical use of NSCs because of their biological potentials in neurogenesis and neural repair. In NSCs and NPCs, gp130 has been shown to play critical roles in the following cellular events. First, gp130 is involved in maintenance of the self-renewal ability of NSCs [3, 4]. Second, gp130 is involved in the induction of astrocytic differentiation of NPCs [5]. Furthermore, the gp130 signaling has been reported to support NPC survival via activation of the phosphatidylinositol 3 kinase-Akt pathway [6].
Recently, the importance of “glycosignaling”, signaling mediated or modulated by glycoconjugates and carbohydrate antigens, has been proposed in NSCs and NPCs [7]. Since its first identification, gp130 has been known as a glycoprotein [2]. So far, it has been reported that gp130 has 11 potential N-glycosylation sites in the extracellular domain, and 9 of them are actually N-glycosylated [8]. As compared with the diverse biological activities of gp130, however, the structure and function of the carbohydrate chains carried by gp130 are totally unknown. In this study, we evaluated the functional role of N-glycans of gp130 molecule using mouse primary neuroepithelial cells (NECs) that are known to be rich in NSCs and can differentiate into astrocytes in response to stimulation with IL-6 family of cytokine [9].
Materials and Methods
NEC culture
NECs were isolated from telencephalons of ICR mouse embryos (embryonic day 14.5) and cultured in N2-supplemented Dulbecco’s modified Eagle’s medium/F12 medium containing 10 ng/ml of bFGF (Peprotech) on dishes coated with poly-L-ornithine (Sigma-Aldrich) and fibronectin (Sigma-Aldrich) as previously described [5, 10]. These NECs were treated with tunicamycin (Sigma-Aldrich), an inhibitor of N-linked glycosylation, and stimulated with mouse LIF (ESGRO; Millipore). Mice used for NEC preparation were treated according to the guidelines of the Laboratory Animal Service Committee of the Medical College of Georgia.
Western-blot analysis and immunoprecipitation
Western-blot analysis and immunoprecipitation were performed as previously described [11]. For immunoprecipitation, anti-gp130 antibody (M-20; Santa Cruz Biotechnology) or anti-LIFR antibody (C-19; Santa Cruz Biotechnology) with protein A Sepharose (GE Healthcare Life Sciences) was used. For Western-blot analysis, antibodies to HNK-1 (BD Biosciences), β-actin (Sigma-Aldrich), gp130, phospho-tyrosine (4G10; Millipore), LIFR, phospho-STAT3 (Cell Signaling Technology), STAT3 (Santa Cruz Biotechnology), phospho-extracellular signal-regulated kinase (phospho-ERK; Cell Signaling Technology) and ERK (Santa Cruz Biotechnology) were used. As the secondary antibody, horseradish peroxidase-conjugated anti-mouse IgM antibody (Jackson ImmunoResearch), anti-mouse IgG antibody (GE Healthcare Life Sciences) or anti-rabbit IgG antibody (GE Healthcare Life Sciences) was used. Protein bands reacted with the antibodies were detected using WesternLightning Western Blot Chemiluminescence Reagent Plus (Perkin Elmer Life And Analytical Sciences).
Cell surface protein biotinylation assay
Cell surface localization of gp130 and LIF was evaluated by a cell surface protein biotinylation assay. After washing NECs three times with ice-cold PBS, proteins expressed on the cell surface were biotinylated by treating the NECs with 0.5 mg/ml of EZ-link sulfo-NHS-LC-biotin (Thermo Fisher Scientific) in PBS at 4°C for 30 min. Excess sulfo-NHS-LC-biotin was quenched with 100 mM glycine in PBS for 20 min at 4°C. Then, NECs were washed once with ice-cold PBS and lyzed with a lysis buffer. Biotinylated cell surface proteins in the lysates were pulled down by gentle agitation in the presence of streptavidin-conjugated agarose (EMD Biosciences) at 4°C overnight, washed three times with the lysis buffer, denatured in Laemmli sample buffer by boiling and subjected to Western-blot analysis using an anti-gp130 antibody or an anti-LIFR antibody.
Cell death assay and caspase-3 activity assay
NEC death induced by tunicamycin was assayed by measuring the activities of lactate dehydrogenase (LDH) in the culture media released from apoptotic or necrotic NECs using a Lactate Dehydrogenase Cytotoxicity Assay Kit (Cayman Chemical Company). In brief, the culture media of NECs treated with or without tunicamycin were collected and incubated with a reaction solution containing NAD+, lactic acid, iodonitrotetrazolium chloride and diaphorase at room temperature for 30 min. The spectrophotometric absorbance of formazan produced after incubation was measured at the wavelength of 490 nm using a Benchmark Plus microplate spectrophotometer (Bio-Rad Laboratories). Caspase-3 activities in the NECs were measured using a Caspase-3/CPP32 Colorimetric Kit (BioVision) [11]. In brief, NECs treated with or without tunicamycin were lyzed in a Cell Lysis buffer, and then the NEC lysates containing the same amount of protein were incubated with 200 μM of chromophore p-nitroanilide-conjugated caspase-3 substrate tetrapeptide (Asp-Glu-Val-Asp-pNA) in the Reaction Buffer at 37°C for 4 h. The spectrophotometric absorbance of p-nitroanilide cleaved from the Asp-Glu-Val-Asp-pNA by active caspase-3 was measured at the wavelength of 405 nm using a Benchmark Plus microplate spectrophotometer.
Results
Unglycosylated gp130 in NECs treated with tunicamycin
Tunicamycin is a nucleoside antibiotic that inhibits N-glycosylation of cell surface and secreted glycoproteins in the endoplasmic reticulum and Golgi by preventing the transfer of N-acetylglucosamine-1-phosphate from UDP-N-acetylglucosamine to dolichol phosphate catalyzed by N-acetylglucosamine phosphotransferase [12]. To evaluate the functional role of N-glycans of gp130 molecules, we treated NECs with tunicamycin. The inhibitory effect of tunicamycin on N-glycosylation was confirmed by a severe decrease of proteins positive for HNK-1, a sulfated carbohydrate antigen that is carried by glycoproteins expressed in NECs [13] (Fig. 1; top panel). In these tunicamycin-treated NECs, as reported by Wang and Fuller [14], two bands of gp130 molecules were detected (Fig. 1; second panel from the bottom). The molecular masses indicate that the upper band (approximately 130 kDa) and the lower band (approximately 100 kDa) are mature glycosylated gp130 and unglycosylated gp130, respectively [2]. Existence of the glycosylated gp130 in tunicamycin-treated NECs is probably due to the stability of this molecule; the half life of gp130 is approximately 14 hours in hepatocytes [14]. Interestingly, while the mature glycosylated gp130 was phosphorylated in response to LIF stimulation (Fig. 1; bottom panel, second to fifth lanes), the unglycosylated gp130 was not phosphorylated (Fig. 1; bottom panel, third to fifth lanes). This result suggests that N-glycans are required for the activation of gp130.
Fig. 1.
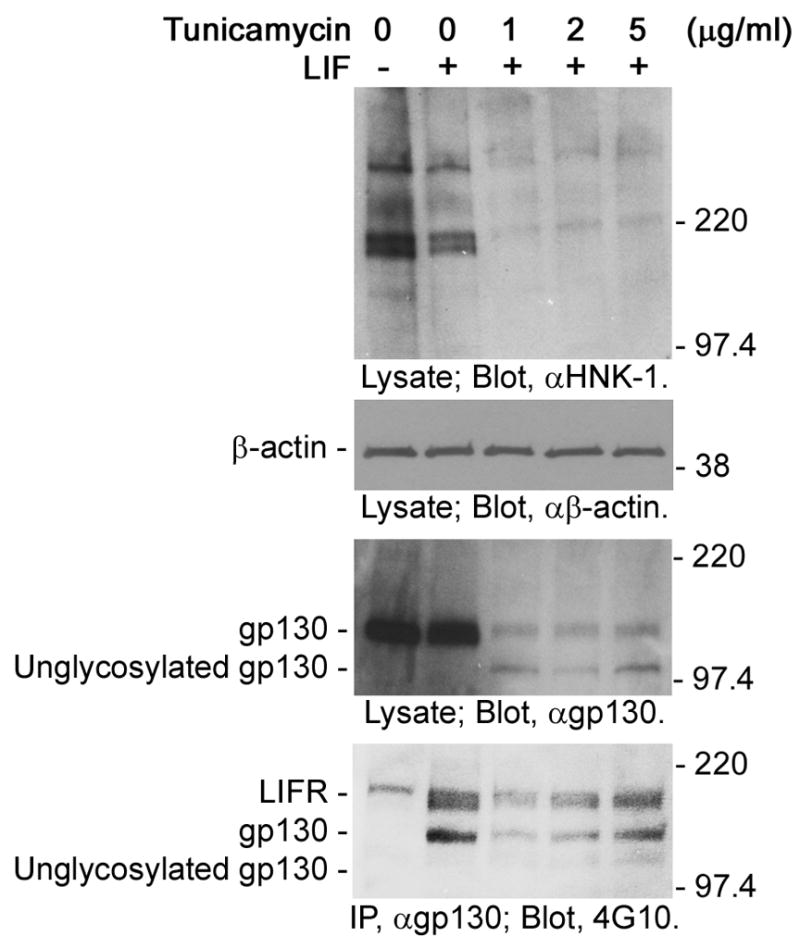
Unglycosylated gp130 in NECs treated with tunicamycin. NECs were treated with tunicamycin for 8 h, stimulated with LIF (0 or 40 ng/ml) for 10 min and then lyzed. Lysates and immunoprecipitated gp130 were subjected to Western-blot analysis. NEC lysates were analyzed with anti-HNK-1 antibody, anti-β-actin antibody and anti-gp130 antibody. Anti-HNK-1 antibody was used to detect glycoproteins as a control. Anti-β-actin antibody was used to confirm that the same amount of proteins was applied to each lane. Immunoprecipitated gp130 was analyzed with anti-phospho-tyrosine antibody (4G10) to evaluate the activation. The molecular masses are indicated on the right of each panel.
Cell surface localization of unglycosylated gp130 in NECs treated with tunicamycin
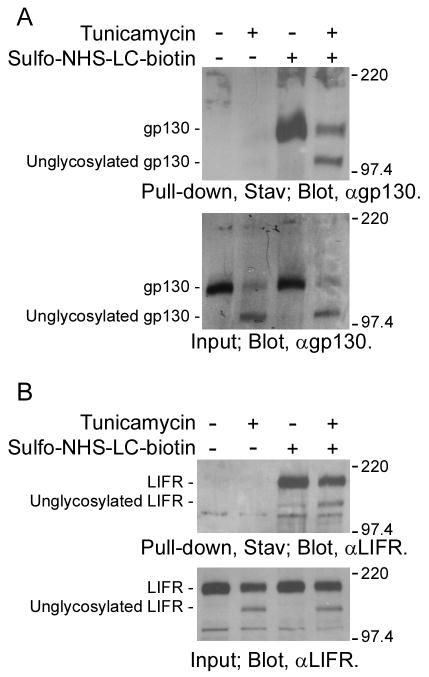
N-glycosylation is important for the folding of proteins. Improperly folded proteins that fail to mature are eventually degraded via the ubiquitin-proteasome system. The possibility that unglycosylated gp130 was degraded and not transported to cell surface could not be eliminated. So, we examined the cell surface localization of unglycosylated gp130 in tunicamycin-treated NECs. For this purpose, we biotinylated cell surface proteins by treating NECs with sulfo-NHS-LC-biotin, and then pulled down the biotinylated cell surface proteins with streptavidin-conjugated agarose. As shown in Fig. 2A (upper panel, third and forth lanes), in biotinylated cell surface proteins, not only mature gp130 but also unglycosylated gp130 was detected. In addition, LIFR which forms a heterodimer with gp130 in response to LIF stimulation was also found in the biotinylated cell surface proteins regardless of their N-glycosylation status (Fig. 2B; upper panel, third and forth lanes). These results indicate that unglycosylated gp130 and LIFR were able to be transport to the NEC surface. Therefore, it also suggests that activation of unglycosylated gp130 is inhibited on the cell surface.
Fig. 2.
Cell surface localization of unglycosylated gp130 in NECs treated with tunicamycin. To confirm the cell surface localization of unglycosylated gp130, cell surface proteins expressed in NECs treated with or without tunicamycin (2 μg/ml) for 8 h were biotinylated by treating the cells with sulfo-NHS-LC-biotin. After lysis, biotinylated cell surface proteins were pulled down with streptavidin-conjugated agarose and then analyzed by Western-blot using anti-gp130 antibody (A) or anti-LIFR antibody (B).
Heterodimerization of unglycosylated gp130 and LIFR in NECs with tunicamycin
It is well known that gp130 mediates the signaling of LIF via heterodimerization with LIFR [1]. LIF binds to LIFR and induces formation of a gp130/LIF heterodimer by recruitment of gp130. This heterodimerization then triggers the phosphorylation of the cytoplasmic regions of gp130 and LIFR via induction of the activation of a protein tyrosine kinase, JAK, which is constitutively associated with the cytoplasmic region of LIFR and gp130. To clarify the molecular mechanism underlying the inactivation of unglycosylated gp130, we examined the heterodimerization ability of unglycosylated gp130 and LIFR in tunicamycin-treated NECs. As shown in Fig. 3, the mature gp130 was found in immunoprecipitates with anti-LIFR antibody in a LIF stimulation-dependent manner (top panel; second and forth lanes); it was confirmed that gp130 forms a heterodimer with LIFR in response to LIF stimulation. In contract, unglycosylated gp130 was not co-immunoprecipitated with LIFR (top panel; fourth lane). This result clearly indicates that unglycosylated gp130 cannot form a heterodimer with LIFR even in the presence of LIF. Therefore, it is suggested that the inability of unglycosylated gp130 to mediate signaling is attributed to the defective dimerization ability.
Fig. 3.
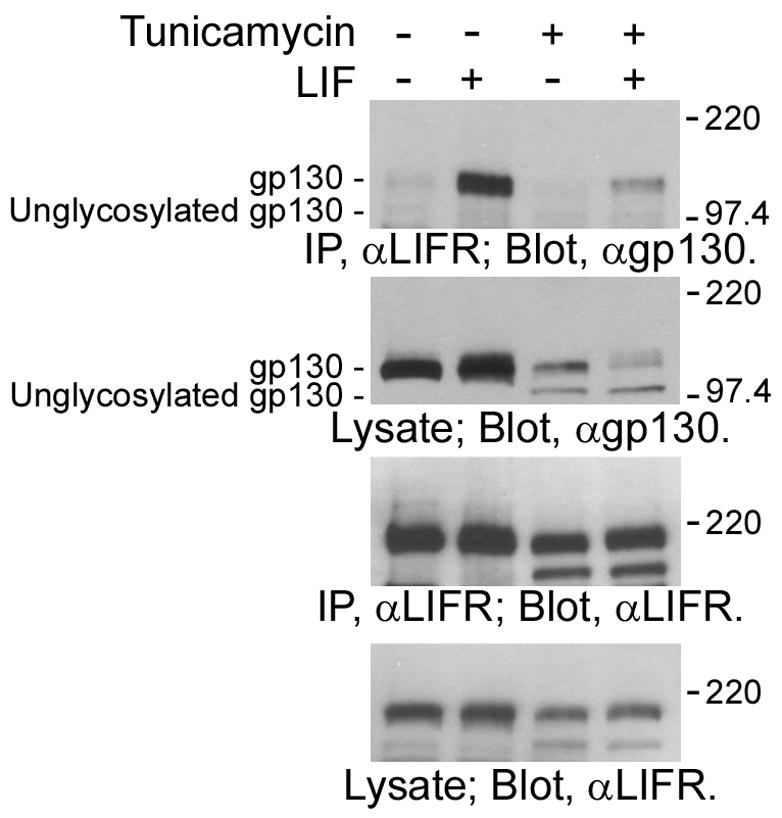
Heterodimerization of unglycosylated gp130 and LIFR in NECs. Lysates of NECs treated with tunicamycin (0 or 2μg/ml) for 8 h and then stimulated with LIF (0 or 40 ng/ml) for 10 min were immunoprecipitated with anti-LIFR antibody. gp130 co-immunoprecipitated with LIFR was analyzed by Western-blot analysis using anti-gp130 antibody.
Stress-mediated cell death signaling in NECs with tunicamycin
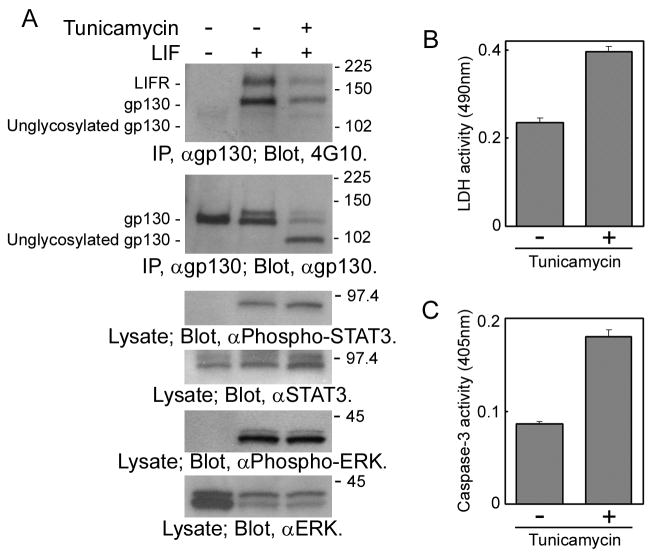
LIF can induce astrocytic differentiation of NECs [5]. Therefore, it was expected that tunicamycin-treated NECs cannot differentiate into astrocytes in the presence of LIF, if gp130 is activated in an N-glycan-dependent manner. However, the cellular effect of tunicamycin such as inhibition of astrocytic differentiation could not be evaluated due to two technical difficulties. First, the downstream signaling pathways of gp130, the JAK-STAT pathway and the Ras-MAPK pathway, were not inhibited even in the presence of tunicamycin; STAT3 and ERK (MAPK) were phosphorylated in NECs treated with tunicamycin and then stimulated with LIF (Fig. 4A; third and fifth panels from the top, third lane). It should be due to signaling via glycosylated gp130 that is still expressed in the tunicamycin-treated NECs (Fig. 1 & 4A). Second, tunicamycin activated the cell stress signaling by causing accumulation of unglycosylated glycoproteins. In NSCs, tunicamycin has been reported to induce stress-mediated cell death [15]. As shown in Fig. 4B, in culture media of NECs treated with tunicamycin, the activity of LDH released from apoptotic or necrotic cells increased. In addition, in NECs treated with tunicamycin, the activity of caspase-3, a cysteine protease and a critical executioner of apoptosis or programmed cell death signaling, also increased (Fig. 4C). To evaluate defects caused by unglycosylated gp130 at the cellular level, these technical difficulties should be solved.
Fig. 4.
(A) Activation of the JAK-STAT pathway and the Ras-MAPK pathway in NECs treated with tunicamycin. Lysates prepared from NECs treated with tunicamycin (0 or 5 μg/ml) for 8 h and then stimulated with LIF (0 or 40 ng/ml) for 10 min were analyzed by Western-blot analysis. Stress-mediated cell death signaling was evaluated by measuring (B) LDH activities in culture media of tunicamycin-treated NECs and (C) caspase 3 activities in tunicamycin-treated NECs.
Discussion
In this study, we evaluated the functional role of N-glycans of gp130 using mouse NECs treated with tunicamycin, and found that unglycosylated gp130 can be transported to cell surface but not be activated to mediate signaling in response to LIF stimulation. These results suggest that N-glycans are required for activation, but not translocation, of gp130 in NECs. The molecular mechanism underlying the inactivation of unglycosylated gp130 is not yet fully understood. Generally, glycosylation is considered to be involved in the proper folding of newly synthesized polypeptides and in the subsequent maintenance of protein solubility and conformation. Therefore, there is a strong possibility that the conformational change of unglycosylated gp130 leads to inactivation. In fact, it was found in this study that unglycosylated gp130 cannot form a heterodimer with LIFR in response to LIF stimulation. This loss of heterodimerization ability of N-glycosylated gp130 may be due to the conformational change. So far, it has been reported that N-glycosylation is required for ligand binding in certain receptor molecules [16–18]. LIFR unglycosylated in tunicamycin-treated NECs (Fig. 2B; bottom panel, second and forth lanes) may also lack the ligand binding ability.
In the clinical use of NSCs and NPCs such as cell therapy by transplantation, carbohydrate antigens are considered useful for (i) defining NSCs/NPCs and (ii) isolating NSCs/NPCs by cell staining and sorting with anti-carbohydrate probes, and (iii) enriching NSCs/NPCs and (iv) modulating the fate to differentiate into beneficial neuronal or oligodendroglial lineage by modifying the signaling pathways [19]. As the functional roles of gp130 in NSCs and NPCs, maintenance of self-renewal and induction of astrocytic differentiation have been known [3–5]. By modulating N-glycans of gp130 and the signaling, it was expected to be possible to modify the fate of NECs regulated by the gp130 signaling. Unfortunately, because of the existence of glycosylated gp130 molecules and the activation of the cell stress signaling in NECs treated with tunicamycin, involvement of N-glycans of gp130 in the cell fate regulation could not be evaluated in this study. Future studies should be directed toward a full understanding of functional role of N-glycans of gp130 at the cellular level and evaluate whether it is possible to enhance the self-renewal or inhibit the astrocytic differentiation for cell therapy.
Acknowledgments
This work was supported by USPHS grants NS11853, NS26994, AG027199 and a grant from the Childrens’ Medical Research Foundation, Chicago, IL to RKY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 2.Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 3.Shimazaki T, Shingo T, Weiss S. The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J Neurosci. 2001;21:7642–7653. doi: 10.1523/JNEUROSCI.21-19-07642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chojnacki A, Shimazaki T, Gregg C, Weinmaster G, Weiss S. Glycoprotein 130 signaling regulates Notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. J Neurosci. 2003;23:1730–1741. doi: 10.1523/JNEUROSCI.23-05-01730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Yoshida K, Kishimoto T, Sendtner M, Taga T. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci. 1999;19:5429–5434. doi: 10.1523/JNEUROSCI.19-13-05429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang MY, Park CH, Son H, Lee YS, Lee SH. Developmental stage-dependent self-regulation of embryonic cortical precursor cell survival and differentiation by leukemia inhibitory factor. Cell Death Differ. 2004;11:985–996. doi: 10.1038/sj.cdd.4401426. [DOI] [PubMed] [Google Scholar]
- 7.Yu RK, Yanagisawa M. Glycosignaling in neural stem cells: involvement of glycoconjugates in signal transduction modulating the neural stem cell fate. J Neurochem. 2007;103(Suppl 1):39–46. doi: 10.1111/j.1471-4159.2007.04710.x. [DOI] [PubMed] [Google Scholar]
- 8.Moritz RL, Hall NE, Connolly LM, Simpson RJ. Determination of the disulfide structure and N-glycosylation sites of the extracellular domain of the human signal transducer gp130. J Biol Chem. 2001;276:8244–8253. doi: 10.1074/jbc.M009979200. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda S, Taga T. Cell fate determination regulated by a transcriptional signal network in the developing mouse brain. Anat Sci Int. 2005;80:12–18. doi: 10.1111/j.1447-073x.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 11.Yanagisawa M, Yu RK. O-Linked β-N-acetylglucosaminylation in mouse embryonic neural precursor cells. J Neurosci Res. 2009 doi: 10.1002/jnr.22170. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esko JD, Bertozzi CR. Chemical tools for inhibiting glycosylation. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor Press; Cold Spring Harbor, New York: 2008. pp. 705–718. [Google Scholar]
- 13.Yanagisawa M, Taga T, Nakamura K, Ariga T, Yu RK. Characterization of glycoconjugate antigens in mouse embryonic neural precursor cells. J Neurochem. 2005;95:1311–1320. doi: 10.1111/j.1471-4159.2005.03452.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Fuller GM. Biosynthetic and glycosylation events of the IL-6 receptor beta-subunit, gp130. J Cell Biochem. 1995;57:610–618. doi: 10.1002/jcb.240570405. [DOI] [PubMed] [Google Scholar]
- 15.Ohashi H, Nishikawa K, Ayukawa K, Hara Y, Nishimoto M, Kudo Y, Abe T, Aoki S, Wada K. Alpha 1-adrenoceptor agonists protect against stress-induced death of neural progenitor cells. Eur J Pharmacol. 2007;573:20–28. doi: 10.1016/j.ejphar.2007.06.060. [DOI] [PubMed] [Google Scholar]
- 16.Slieker LJ, Lane MD. Post-translational processing of the epidermal growth factor receptor. Glycosylation-dependent acquisition of ligand-binding capacity. J Biol Chem. 1985;260:687–690. [PubMed] [Google Scholar]
- 17.Ding DX, Vera JC, Heaney ML, Golde DW. N-glycosylation of the human granulocyte-macrophage colony-stimulating factor receptor alpha subunit is essential for ligand binding and signal transduction. J Biol Chem. 1995;270:24580–24584. doi: 10.1074/jbc.270.41.24580. [DOI] [PubMed] [Google Scholar]
- 18.Zhou H, Tai HH. Characterization of recombinant human CXCR4 in insect cells: role of extracellular domains and N-glycosylation in ligand binding. Arch Biochem Biophys. 1999;369:267–276. doi: 10.1006/abbi.1999.1368. [DOI] [PubMed] [Google Scholar]
- 19.Yanagisawa M, Yu RK. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 2007;17:57R–74R. doi: 10.1093/glycob/cwm018. [DOI] [PubMed] [Google Scholar]