Abstract
Recent findings suggest that the association between inflammation-related genes and preterm delivery may be stronger in the presence of bacterial vaginosis (BV). Tumor necrosis factor alpha (TNFα) and interleukin 1 beta (IL-1β) are proinflammatory cytokines capable of inducing preterm labor in non-human primates. In this study the authors tested associations among two TNFα promoter polymorphisms (−G308A and −G238A), a single IL-1β polymorphism (+C3954T), vaginal microbial findings, and risk of preterm delivery. Data were from the Pregnancy Outcomes and Community Health (POUCH) Study (n=777 term and n=230 preterm deliveries). Vaginal smears collected at mid-pregnancy (15–27 weeks gestation) were scored according to Nugent’s criteria. A Nugent score of ≥ 4 was modeled as the cut-point for intermediate and positive BV. Logistic regression was used to estimate odds ratios for associations among independent covariates (vaginal flora, genotype) and preterm delivery. Results showed that women with a Nugent Score of ≥ 4 and the TNFα −238 A/G or A/A were at increased risk of delivering preterm (race/ethnicity adjusted OR 2.6, 95% CI 1.2, 5.8). The p-value for the genotype and Nugent score interaction = 0.02. This study points to one more example of a potential gene-environment interaction in a preterm delivery pathway. Future tests of this finding will determine the robustness of these results.
Keywords: Preterm birth, TNFalpha, IL-1beta, Bacterial vaginosis, Gene-environment interaction
1. Introduction
In the United States, preterm delivery (before completing 37 weeks of gestation) accounts for 12.7 % of all births and 18.4% of births among African-American women (Hamilton et al., 2006) The preterm delivery rate has risen more than 30% since 1981. Preterm delivery is a leading cause of infant mortality and a major contributor to child neuro-developmental problems (Callaghan et al., 2006). The Institute of Medicine estimated the 2005 societal economic burden of preterm delivery in the U.S. to be at least $26.2 billion (Behrman et al., 2006).
The role of inflammation in the etiology of preterm delivery is not clearly understood. Some but not all studies report elevated levels of proinflammatory cytokines in mid-pregnancy maternal serum, mid-pregnancy amniotic fluid, and mid-pregnancy cervical samples among women who have a spontaneous preterm delivery (Goepfert et al., 2001, Genc et al., 2004, Wenstrom et al., 1998, Gargano et al., 2008). Higher levels of proinflammatory cytokines were also found in the placental tissues of spontaneous preterm deliveries, and evidence of histologic chorioamnionitis is more common in placentas of spontaneous preterm deliveries (El-Shazly et al., 2004, Holzman et al., 2007).
Genetic variants in the tumor necrosis factor-alpha (TNFα) and interleukin 1-beta (IL-1β) genes have been inconsistently associated with an increased risk for preterm delivery (Edwards et al., 2006, Menon et al., 2006, Genc et al., 2002). TNFα and IL-1β are proinflammatory cytokines involved in the innate immune response to microbial products, and intramniotic infusions of either can stimulate increased synthesis of prostaglandins and subsequent preterm labor in non-human primates (Sadowsky et al., 2006).
A gene-environment interaction was reported in a study of African-American women where the strength of the association between a single nucleotide polymorphism within the promoter of TNFα (−G308A) and spontaneous preterm delivery was increased in the presence of bacterial vaginosis (BV) (Macones et al., 2004). In a separate publication, this interaction was replicated in African Americans delivering spontaneously preterm with the TNFα–G308A polymorphism as well as for women with a polymorphism in the IL-1β (+C3954T) gene and BV(Engel et al., 2005).
BV is a condition that occurs when the flora in the vaginal tract is altered from one typically predominated by Lactobacillus to a more polymicrobial community, often including Gardnerella vaginalis, Mobiluncus, Bacteroides, and Mycoplasma. BV has been associated with an increased risk for preterm delivery and chorioamnionitis, but antibiotic trials to treat BV before and during pregnancy have not universally resulted in a lowering of the risk for preterm delivery (Leitich et al., 2003a, Leitich et al., 2003b, Andrews et al., 2006). Further investigation into the potentially modifying effects of genetic variation in immune response to vaginal flora may help elucidate the relationship between BV and preterm delivery.
The purpose of this project was to determine the associations of three proinflammatory cytokine gene polymorphisms (two TNFα promoter polymorphisms −G308A and −G238A and a single IL-1β polymorphism +C3954T) with preterm delivery, along with their potential interaction with vaginal flora in the Pregnancy Outcomes and Community Health (POUCH) Study. The three candidate polymorphisms were selected based on prior published evidence that they are associated with altered production of TNFα and IL-1β, and to attempt a replication of the previously found gene-environment interactions (Genc et al., 2007, Hajeer and Hutchinson, 2000)
2. Materials and Methods
2.1 Cohort Enrollment
Women were enrolled mid-pregnancy (15–27 weeks gestation, with 86% enrolled by 24 weeks) to participate in a cohort study designed to assess biologic and psychosocial risk factors for preterm delivery. Detailed methods are described elsewhere (Holzman et al., 2001a) The POUCH Study enrolled 3,038 women from 52 participating clinics among five Michigan communities from 1998 to 2004. The eligibility criteria included maternal serum alpha-fetoprotein (MSAFP) screening at 15–22 weeks of pregnancy, maternal age of at least 15 years, English-speaking, no pre-existing diabetes, no known fetal anomalies or chromosomal defects, and singleton pregnancy. Women with unexplained high (two or more multiples of the race-specific median) MSAFP were oversampled (7% of final cohort) because of an interest in the relation between this biomarker and preterm delivery. At enrollment, subjects completed questionnaires and provided biologic samples. Nineteen women were lost to follow up, leaving a cohort sample of 3,019.
Race/Ethnicity-specific characteristics of study participants were compared to birth certificate data from the five Michigan communities for the year 2000. There were no significant differences in parity, education level, proportion with Medicaid insurance, preterm birth rate, rate of previous stillbirth, rate of previous preterm infant, and rate of previous low birth weight infant. The percentage of African Americans over 30 years of age was lower in the POUCH Study than in the birth certificate data (14% versus 21%). The study protocol was approved by the Michigan State University Committee for Research on Human Subjects, institutional review boards at nine participating hospitals, and the Michigan Department of Community Health human subjects committee.
2.2 Study Sample
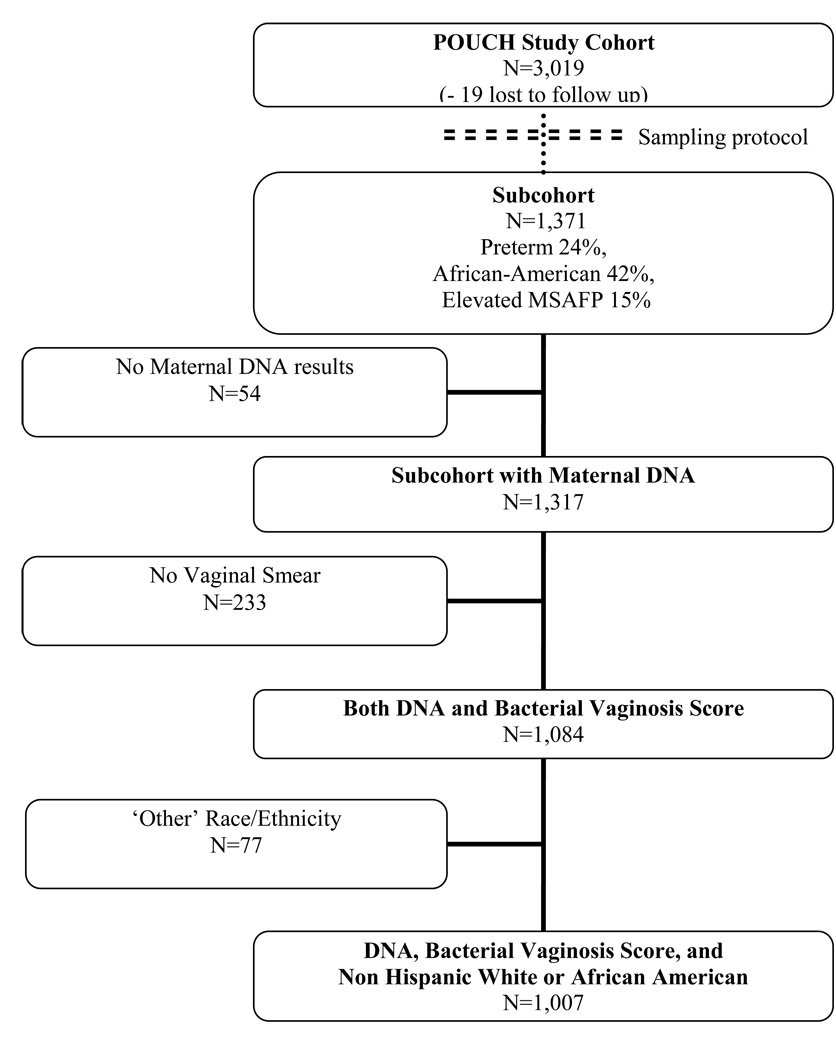
A sub-cohort (N=1,371) was sampled from the cohort for more intensive follow-up (Figure 1). The sub-cohort was designed to maximize statistical power for studying high risk subgroups while conserving resources. The sub-cohort included all cohort women who delivered preterm, all cohort women with unexplained high MSAFP, and a stratified random sample of cohort women who delivered at term and had normal MSAFP, with oversampling of African Americans with these latter criteria. All analyses incorporated weights based on the cohort and sub-cohort sampling scheme. In weighted analyses model estimates reflect the prevalence and odds in the original population, and the effect of oversampling high-risk subgroups into the sub-cohort is statistically accounted for. All models were stratified by race/ethnicity (defined for both groups by self-report).
Figure 1.
Study sample population
Prenatal and labor and delivery records were abstracted for sub-cohort women, and polymorphisms were measured on those with available stored DNA samples (N=1,317). Vaginal fluid smears were collected for 82% of sub-cohort women for whom DNA samples were available (N=1,084). Racial groups that contained small numbers of women (18 Asian women, 44 Hispanic women, and 15 Native American women) were excluded for this analysis. The final study sample of 1,007 women (777 term and 230 preterm deliveries) included African-American and non-Hispanic white sub-cohort women with both DNA results and vaginal smear findings.
2.3 Definition of Preterm Delivery
Gestational age at delivery was calculated from the date of the last menstrual period (LMP). Gestational age estimates from early ultrasound (before 20 weeks) were given preference if the ultrasound gestational age differed from the LMP gestational age by at least two weeks or if the LMP was missing (18% of the cohort). Preterm deliveries were those delivered before 37 weeks’ gestation.
2.4 Genotyping
Maternal blood was obtained through venipuncture at enrollment. Genomic DNA was prepared from venous samples using the Gentra Systems (Minneapolis, MN) Puregene™ kit. Single nucleotide polymorphism (SNP) genotyping was performed using TaqMan® Assays-on-Demand™, which consist of PCR primers and a fluorescently-labeled probe (Applied Biosystems, Foster City, CA). Three primer/probe sets were used that were specific to SNPs in IL-β (rs1143634) or TNFα (−308 G>A rs1800629 and −238 G>A rs361525).
Assays were performed in 384-well plates in 5 µL reactions containing 25 ng of template DNA or no-template control and 0.25 µL of primer/probe in 1X TaqMan® PCR Universal Master Mix. Thermal cycling conditions were according to manufacturer guidelines, as follows: 2 minutes at 50°C, 10 minutes at 95°C, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. End-point fluorescent detection was performed on an ABI Prism® 7900HT Sequence Detection System. Genotypes were evaluated using SDS software (v.2.1). Duplicate genotypes were generated on a random sampling representing ≥ 15% of the individuals with a concordance rate of 99.9%.
2.5 Vaginal Flora
Dacron swabs were used to obtain vaginal fluid samples at enrollment. Heat-fixed gram-stained vaginal smears were prepared and bacterial morphotypes were scored according to Nugent’s criteria(Nugent et al., 1991) A score of 0–3 is considered normal vaginal flora, 4–6 is an intermediate score, and a score of 7 or greater is defined as BV positive.
2.6 Analytic Methods
The goals of this analysis were to examine associations among genotype, vaginal flora, and preterm delivery. The heterozygote and homozygote rare allele genotypes were grouped together and compared to the homozygote non-rare allele genotype. The three polymorphisms were analyzed separately. Women with intermediate and positive BV were grouped together (Nugent scores ≥ 4) and compared to women with a Nugent score < 4. Grouping strategies were motivated by a consideration of the biology and a goal of obtaining more precise confidence intervals for effect size estimates. Analyses were repeated after removing women with intermediate BV (Nugent score 4–6) to assess the impact of our grouping strategy on the overall results.
Regression analyses modeled covariates (genotype, vaginal flora) in relation to preterm delivery. All regression models incorporated inverse probability weights to account for the POUCH Study sampling scheme (for both the cohort and sub-cohort). This statistical method removes any potential bias from oversampling of subgroups. Because race/ethnicity has been consistently linked to variations in vaginal flora (Nugent scores) (Holzman et al., 2001b) (Ness et al., 2003, Allsworth and Peipert, 2007) and risk of preterm delivery (Zhang and Savitz, 1992, Savitz et al., 2005, Hitti et al., 2007), we conducted both race/ethnicity-adjusted and race/ethnicity-stratified analyses.
Two-way interactions were examined, first between genotype and race/ethnicity in relation to vaginal flora, and then among three covariates (genotype, vaginal flora, race/ethnicity) in relation to preterm delivery. The Wald test was used to evaluate the statistical significance of interactions.
3. Results
Maternal characteristics of the study sample are presented in Table 1. The weighted estimates reflect the prevalence in the cohort. After incorporating sampling weights, the prevalence of BV was 7% in Non-Hispanic white women and 20% in African-American women. There were 149 preterm births in non-Hispanic white women and 81 preterm births in African-American women. The prevalence of preterm delivery was 9% in non-Hispanic white women and 14% in African-American women. Unweighted race/ethnicity-specific allele frequencies for women with normal MSAFP who delivered at term were calculated and did not differ significantly from Hardy-Weinberg equilibrium (data not shown in table: TNFα −238A non-Hispanic white = 0.05 and African American = 0.04; TNFα −308A non-Hispanic white = 0.19 and African American = 0.14; IL-1β +3954T non-Hispanic white = 0.23 and African American = 0.16). The allele frequencies in POUCH Study African-American and non-Hispanic white women were similar to previous reports (Roberts et al., 1999, Moore et al., 2004, Engel et al., 2005).
Table 1.
Race/Ethnic specific maternal characteristics of POUCH subcohort women with maternal DNA and vaginal smears collected
| Maternal Characteristics | Non-Hispanic White N=560 |
African American N=447 |
||||
|---|---|---|---|---|---|---|
| N | (%) Unweighted |
(%) weighted |
N | (%) Unweighted |
(%) weighted |
|
| Maternal Age (years) | ||||||
| <20 | 53 | (10) | (9) | 123 | (28) | (28) |
| 20–30 | 309 | (55) | (57) | 265 | (59) | (60) |
| >30 | 198 | (35) | (34) | 59 | (13) | (13) |
| Education Level (years) | ||||||
| ≤ 12 | 223 | (40) | (38) | 306 | (68) | (69) |
| > 12 | 337 | (60) | (62) | 141 | (32) | (31) |
| Medicaid Insureda | ||||||
| No | 351 | (63) | (64) | 72 | (16) | (16) |
| Yes | 208 | (37) | (36) | 375 | (84) | (84) |
| Primiparousa | ||||||
| No | 333 | (60) | (61) | 261 | (58) | (58) |
| Yes | 226 | (40) | (39) | 186 | (42) | (42) |
| Gram Stain Results | ||||||
| BV Negative (Score 0–3) | 474 | (85) | (85) | 305 | (68) | (68) |
| BV Intermediate (Score 4–6) | 43 | (8) | (8) | 53 | (12) | (12) |
| BV Positive (Score ≥ 7) | 43 | (8) | (7) | 89 | (20) | (20) |
| Pregnancy Outcome | ||||||
| Term Delivery | 411 | (73) | (91) | 366 | (82) | (86) |
| Preterm Delivery | 149 | (27) | (9) | 81 | (18) | (14) |
| TNF-α -238 | ||||||
| GG | 506 | (90) | (90) | 414 | (93) | (93) |
| GA | 53 | (10) | (10) | 31 | (7) | (7) |
| AA | 1 | (<1) | (<1) | 2 | (<1) | (<1) |
| TNF-α -308 | ||||||
| GG | 382 | (68) | (66) | 338 | (76) | (75) |
| GA | 167 | (30) | (32) | 101 | (22) | (23) |
| AA | 11 | (2) | (2) | 8 | (2) | (2) |
| IL-1β +3954b | ||||||
| CC | 337 | (60) | (60) | 334 | (75) | (74) |
| CT | 189 | (34) | (34) | 98 | (22) | (23) |
| TT | 32 | (6) | (6) | 15 | (3) | (3) |
Percentages are presented both unweighted and weighted for the cohort and subcohort sampling scheme.
Missing data for 1 non-Hispanic white women.
Two non-Hispanic white woman are missing IL-1β genotype
3.1 Genotype and Preterm Delivery
The risk of preterm delivery was not significantly associated with any of the three genotypes (TNFα −238, IL-1β +3954, or TNFα −308) in race/ethnicity stratified models (Table 2). There were no statistically significant race/ethnicity by genotype interactions (all p > 0.20) for preterm delivery.
Table 2.
Preterm delivery by genotype among non-Hispanic white and African-American women in the POUCH subcohort
| Non-Hispanic White N=560 |
African American N=447 |
|||||
|---|---|---|---|---|---|---|
| Genotype | Term Deliveries N (weighted %) |
Preterm Deliveries N (weighted %) |
OR (95% CI) a | Term Deliveries N (weighted %) |
Preterm Deliveries N (weighted %) |
OR (95% CI) a |
| TNF-α −G238A | ||||||
| Common allele | 372 (90.6%) | 134 (9.4%) | 341 (86.1%) | 73 (13.9%) | ||
| Rare allele | 39 (90.3%) | 15 (9.7%) | 1.0 (0.5, 1.9) | 25 (79.5%) | 8 (20.5%) | 1.6 (0.7, 3.4) |
| TNF-α −G308A | ||||||
| Common allele | 274 (89.8%) | 108 (10.2%) | 274 (85.1 %) | 64 (14.9%) | ||
| Rare allele | 137 (92.2%) | 41 (7.8%) | 0.7 (0.5, 1.0) | 92 (87.3%) | 17 (12.7%) | 0.8 (0.5, 1.4) |
| IL-1β +C3954Tb | ||||||
| Common allele | 248 (90.5%) | 89 (9.5%) | 268 (84.0 %) | 66 (16.0%) | ||
| Rare allele | 162 (90.8%) | 59 (9.2%) | 1.0 (0.8, 1.5) | 98 (90.3%) | 15 (9.7%) | 0.8 (0.4, 1.4) |
OR= Odds Ratio and 95 % confidence intervals for preterm birth was calculated by weighted logistic regression (with inverse probability weights to account for the POUCH Study sampling scheme). The heterozygote and homozygote rare allele genotypes were grouped together (rare allele) and compared to the homozygote non-rare allele genotype (common allele). The term common allele group was the reference category.
Two non-Hispanic white woman are missing IL-1β genotype.
3.2 Genotype and Vaginal Flora
In both racial/ethnic groups women with the TNFα −238 minor allele were more likely to have Nugent scores ≥ 4 (OR 1.6), but the confidence intervals for the odds ratios included one (Table 3). The other two genotypes (IL-1β +3954 or TNFα −308) were unrelated to vaginal flora. There were no statistically significant race/ethnicity by genotype interactions (all p > 0.70) for vaginal flora.
Table 3.
Vaginal smear results by genotype among non-Hispanic white and African-American women in the POUCH Study subcohort
| Non-Hispanic White N=560 |
African American N=447 |
|||||
|---|---|---|---|---|---|---|
| Genotype | Nugent Score < 4 N (weighted %) |
Nugent Score ≥ 4 N (weighted %) |
OR (95%CI) a | Nugent Score < 4 N (weighted %) |
Nugent Score ≥ 4 N (weighted %) |
OR (95%CI) a |
| TNF-α −G238A | ||||||
| Common allele | 436 (85.8%) | 70 (14.2%) | 286 (69.0%) | 128 (31.0%) | ||
| Rare allele | 38 (78.0%) | 16 (22.0%) | 1.6 (0.8, 3.3) | 19 (54.9%) | 14 (45.1%) | 1.6 (0.8, 3.3) |
| TNF-α −G308A | ||||||
| Common allele | 323 (85.4%) | 59 (14.6%) | 231 (67.8%) | 107 (32.2%) | ||
| Rare allele | 151 (84.2%) | 27 (15.7%) | 1.0 (0.6, 1.8) | 74 (68.7%) | 35 (31.3%) | 0.9 (0.6, 1.3) |
| IL-1β +C3954Tb | ||||||
| Common allele | 283 (84.8%) | 54 (15.2%) | 229 (68.1%) | 105 (31.9%) | ||
| Rare allele | 189 (85.2%) | 32 (14.8%) | 0.9 (0.6, 1.4) | 76 (67.9%) | 37 (32.1%) | 1.0 (0.7, 1.5) |
OR= Odds Ratio and 95 % confidence intervals (CI) for preterm birth was calculated by weighted logistic regression (with inverse probability weights to account for the POUCH Study sampling scheme). The heterozygote and homozygote rare allele genotypes were grouped together (rare allele) and compared to the homozygote non-rare allele genotype (common allele). The term common allele group was the reference category.
Two non-Hispanic white woman are missing IL-1β genotype.
3.4 Vaginal Flora and Preterm Delivery
In race/ethnicity-adjusted models and race/ethnicity-stratified models, women who had Nugent Scores ≥ 4 were not at significantly increased risk for delivering preterm and there was no significant interaction between race/ethnicity and vaginal flora in relation to preterm birth risk. The association between Nugent Scores and preterm delivery was not significant in the race/ethnicity-adjusted model (adjusted OR 1.2, 95% CI 0.8, 1.7), nor the race/ethnicity stratified model for non-Hispanic Whites (OR 1.3, 95% CI 0.8, 2.2), nor for African Americans (OR 1.0, 95 % CI 0.6, 1.8; interaction p-value=0.57). After removing women with intermediate Nugent Scores (4–6) and repeating the analysis, there was no statistically significant relation between Nugent Score and preterm delivery; although, the association between BV and preterm delivery in the non-Hispanic white women approached significance (OR 1.8, 95 % CI 0.9, 3.2; p-value for race/ethnicity interaction = 0.19).
3.5 Genotype, Vaginal Flora, and Preterm Delivery
Compared to women who had a Nugent score < 4 and TNFα −238 G/G, women who had Nugent Scores ≥ 4 and were TNFα −238 A/G or −238 A/A were at a significantly increased risk for preterm delivery (adjusted OR 2.6, 95% CI 1.2, 5.8; p-value for interaction=0.02) (Table 4). When stratified by race/ethnicity, the effect size was slightly higher among non-Hispanic White women (TNFα −238 A/G or −238 A/A and Nugent Score ≥ 4: OR 3.1, 95% CI 1.1, 9.0) than among African Americans (TNFα −238 A/G or −238 A/A and Nugent Score ≥ 4 OR 2.0, 95% CI 0.6, 6.7), but small samples sizes preclude inferences from these stratified analyses.
Table 4.
TNF-alpha −G238A, vaginal flora, and preterm delivery
| Nugent Score and TNF-α genotype | Term | Preterm | AOR (95% CI) |
|---|---|---|---|
| Nugent Score < 4 and -238 G/G | 557 | 165 | Reference |
| Nugent Score ≥ 4 and -238 G/G | 156 | 42 | 1.0 (0.6, 1.5) |
| Nugent Score < 4 and -238 A/G or A/A | 46 | 11 | 0.7 (0.3, 1.4) |
| Nugent Score ≥ 4 and -238 A/G or A/A | 18 | 12 | 2.6 (1.2, 5.8) |
AOR= race/ethnicity adjusted odds ratio; CI = confidence interval
There were no other significant polymorphism - vaginal flora interactions (interaction p values ranged from 0.41 to 0.75) in relation to preterm delivery. When the analysis was repeated with the intermediate vaginal flora group removed (Nugent scores 4–6), the association between TNFα −238 genotype and preterm delivery did not change (adjusted OR 2.8, 95% CI 1.0, 7.2). However, the estimate was based on a small number of women. Removal of the women with intermediate BV did not change the null findings for the other two polymorphisms (i.e., no significant interactions).
4. Discussion
We found a significant interaction between the TNFα −238 polymorphism and vaginal flora in relation to preterm delivery. The risk of preterm delivery was elevated in women who had both the minor allele and a Nugent Score ≥ 4, but not in women who had only one or the other. This association was evident in race/ethnicity adjusted and race/ethnicity stratified models. We did not detect previously reported interactions between Nugent scores and the TNFα −308 and IL-1β+3954 polymorphisms (Macones et al., 2004, Engel et al., 2005). Possible explanations for varied results among studies include: 1) the polymorphisms are not truly functional but are closely linked to other nearby functional gene changes; 2) there are gene-gene interactions that complicate reproducibility; 3) the typical approach of characterizing vaginal flora by using Nugent criteria at one point in time has limitations and 4) other important environmental exposures have not been fully measured and are inconsistent across study populations
In the history of examining preterm delivery risk, studies have frequently considered BV (Leitich et al., 2003a) or candidate genes, (Anum et al., 2009) but there are fewer reports on interactions among these factors. Overall, investigations into associations among TNFα −308, IL-1β +3954 and preterm delivery have produced inconsistent findings (Menon et al., 2006, Genc et al., 2002, Moore et al., 2004, Edwards et al., 2006). Recently, a large case-control study tested over 1400 SNPs in 130 candidate genes including TNFα −308 and IL-1β +3954 in relation to preterm delivery (Menon et al., 2009). This study did not find significant associations among these genes and preterm delivery after using a variety of analytic techniques. While this and other case-control studies have accrued a large number of preterm delivery cases and have power to test multiple gene associations, they often lack information on potential effect modifiers during pregnancy such as BV. One value of smaller cohort studies is that they can more fully explore specific gene-environment interactions.
The decision to investigate interactions among Nugent score and the three selected polymorphisms was guided by previous reports on preterm delivery as well as biological plausibility. While the relation between BV and preterm delivery is not fully understood, the presence of BV is associated with elevated levels of TNFα and IL-1β in vaginal fluid. (Sturm-Ramirez et al., 2000, Hedges et al., 2006). Both TNFα and IL-1β are proinflammatory cytokines capable of inducing the release of prostaglandins and matrix metalloproteinases in in vitro experiments with decidual and amniotic cells (So et al., 1992, Rauk and Chiao, 2000, Arechavaleta-Velasco et al., 2002, Romero et al., 1989, Mitchell et al., 1990, Oner et al., 2008). Elevated levels of either TNFα or IL-1β in connection with the development of BV could stimulate degradation of membranes or myometrial contractions as part of initiation of the process of early labor. The three polymorphisms selected for this study are hypothesized to alter gene production and maternal response to infection and therefore impact risk of preterm delivery in the presence of BV (Genc et al., 2007, Hernandez-Guerrero et al., 2003, Kazzi et al., 2004).
There are several strengths in this study. The sample of women is socioeconomically diverse and from multiple communities. The assessment of vaginal flora was part of the study protocol and did not rely on diagnosis from medical records. Our study takes place in a population with different races and therefore tests the importance of shared genetic background in relation to shared environment exposures.
There are also some important limitations. We did not measure gene product throughout pregnancy to determine the functionality of the polymorphisms in this population. Although we have a large cohort, the frequency of the rare alleles was low, so there was insufficient power to consider gene-gene interactions or to consider subgroups of early preterm delivery where infection is more strongly implicated as an underlying mechanism. We combined both heterozygotes and homozygyotes into a single group and therefore we could not test for a gene-dose effect. The POUCH Study has adequate power (>0.80) to detect odds ratios greater than 2.0 for preterm delivery and genotypes with a minor allele prevalence of 15%. It has much more limited power to detect gene-environment interactions and our non-significant findings could be the result of type II error. Our measurement of vaginal flora was limited to a single assessment in mid-pregnancy. In addition we did not assess multiple polymorphisms within the candidate genes, and this may be necessary to fully describe gene function. For this study we focused on two candidate genes and therefore are missing information on the role of other genes or potential gene-gene interactions in the possible relationship between BV and preterm birth.
Our findings highlight the importance of considering other relevant cofactors when testing for associations among genes and preterm delivery. In addition to large, genome wide studies, smaller prospective studies with detailed information on conditions in pregnancy will help test targeted hypotheses of interactions. Results from these smaller studies can then be compared to determine the strength and consistency of results across diverse populations. This process can help elucidate causal mechanisms underlying preterm delivery and potential interventions.
Acknowledgements
We thank Isabel Leader and Judith Leventhal for microbiology assistance and Bertha Bullen for invaluable oversight of data collection in the POUCH Study. This work was supported by funding from the National Institute of Child Health and Human Development and the National Institute for Nursing Research grant numbers R01 HD34543-01 and R01 HD034543-07, the March of Dimes Perinatal Epidemiological Research Initiative Program grants 20-FY98-0697 through 20-FY04-37, the Thrasher Research Foundation grant number 02816-7, and cooperative agreement number U01 DP000143-02 from the Centers for Disease Control and Prevention. N.M. Jones was supported by an Institutional T32 grant (T32 HD046377) in Perinatal Epidemiology awarded to Michigan State University. Study sponsors did not have a role in the collection, analysis and interpretation of data or in the writing of the manuscript.
Abbreviations
- TNFα
tumor necrosis factor-alpha
- IL-1β
interleukin 1-beta
- BV
bacterial vaginosis
- POUCH Study
Pregnancy Outcomes and Community Health
- MSAFP
maternal serum alphafetoprotein
- LMP
last menstrual period
- SNP
Single nucleotide polymorphism
- CI
Confidence interval
- OR
Odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet. Gynecol. 2007;109:114–120. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Copper R, Conner M. Interconceptional antibiotics to prevent spontaneous preterm birth: a randomized clinical trial. Am. J. Obstet. Gynecol. 2006;194:617–623. doi: 10.1016/j.ajog.2005.11.049. [DOI] [PubMed] [Google Scholar]
- Anum EA, Springel EH, Shriver MD, Strauss JF., 3rd Genetic contributions to disparities in preterm birth. Pediatr. Res. 2009;65:1–9. doi: 10.1203/PDR.0b013e31818912e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arechavaleta-Velasco F, Ogando D, Parry S, Vadillo-Ortega F. Production of matrix metalloproteinase-9 in lipopolysaccharide-stimulated human amnion occurs through an autocrine and paracrine proinflammatory cytokine-dependent system. Biol. Reprod. 2002;67:1952–1958. doi: 10.1095/biolreprod.102.004721. [DOI] [PubMed] [Google Scholar]
- Behrman RE, Butler AS Institute of Medicine (U.S.). Committee on Understanding Premature Birth and Assuring Health Outcomes. Institute of Medicine (U.S.). Board on Health Sciences Policy. Preterm birth : causes, consequences, and prevention. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- Callaghan WM, Macdorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118:1566–1573. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- Edwards RK, Ferguson RJ, Duff P. The interleukin-1 beta +3953 single nucleotide polymorphism: cervical protein concentration and preterm delivery risk. Am. J. Reprod. Immunol. 2006;55:259–264. doi: 10.1111/j.1600-0897.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- El-Shazly S, Makhseed M, Azizieh F, Raghupathy R. Increased expression of pro-inflammatory cytokines in placentas of women undergoing spontaneous preterm delivery or premature rupture of membranes. Am. J. Reprod. Immunol. 2004;52:45–52. doi: 10.1111/j.1600-0897.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- Engel SA, Erichsen HC, Savitz DA, Thorp J, Chanock SJ, Olshan AF. Risk of spontaneous preterm birth is associated with common proinflammatory cytokine polymorphisms. Epidemiology. 2005;16:469–477. doi: 10.1097/01.ede.0000164539.09250.31. [DOI] [PubMed] [Google Scholar]
- Gargano JW, Holzman C, Senagore P, Thorsen P, Skogstrand K, Hougaard DM, et al. Mid-pregnancy circulating cytokine levels, histologic chorioamnionitis and spontaneous preterm birth. J. Reprod. Immunol. 2008;79:100–110. doi: 10.1016/j.jri.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc MR, Gerber S, Nesin M, Witkin SS. Polymorphism in the interleukin- 1 gene complex and spontaneous preterm delivery. Am. J. Obstet. Gynecol. 2002;187:157–163. doi: 10.1067/mob.2002.122407. [DOI] [PubMed] [Google Scholar]
- Genc MR, Vardhana S, Delaney ML, Witkin SS, Onderdonk AB. TNFA-308G>A polymorphism influences the TNFalpha response to altered vaginal flora. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007;134:188–191. doi: 10.1016/j.ejogrb.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Genc MR, Witkin SS, Delaney ML, Paraskevas LR, Tuomala RE, Norwitz ER, et al. A disproportionate increase in IL-1beta over IL-1ra in the cervicovaginal secretions of pregnant women with altered vaginal microflora correlates with preterm birth. Am. J. Obstet. Gynecol. 2004;190:1191–1197. doi: 10.1016/j.ajog.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Goepfert AR, Goldenberg RL, Andrews WW, Hauth JC, Mercer B, Iams J, et al. The Preterm Prediction Study: association between cervical interleukin 6 concentration and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am. J. Obstet. Gynecol. 2001;184:483–488. doi: 10.1067/mob.2001.109653. [DOI] [PubMed] [Google Scholar]
- Hajeer AH, Hutchinson IV. TNFalpha gene polymorphism: clinical and biological implications. Microsc. Res. Tech. 2000;50:216–228. doi: 10.1002/1097-0029(20000801)50:3<216::AID-JEMT5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Hamilton BE, Martin J, Ventura S. National Vital Statistics Reports. CDC; 2006. Births: Preliminary Data for 2005. [PubMed] [Google Scholar]
- Hedges SR, Barrientes F, Desmond RA, Schwebke JR. Local and systemic cytokine levels in relation to changes in vaginal flora. J. Infect. Dis. 2006;193:556–562. doi: 10.1086/499824. [DOI] [PubMed] [Google Scholar]
- Hernandez-Guerrero C, Monzon-Bordonaba F, Jimenez-Zamudio L, Ahued-Ahued R, Arechavaleta-Velasco F, Strauss JF, 3rd, et al. In-vitro secretion of proinflammatory cytokines by human amniochorion carrying hyper-responsive gene polymorphisms of tumour necrosis factor-alpha and interleukin-1beta. Mol. Hum. Reprod. 2003;9:625–629. doi: 10.1093/molehr/gag076. [DOI] [PubMed] [Google Scholar]
- Hitti J, Nugent R, Boutain D, Gardella C, Hillier SL, Eschenbach DA. Racial disparity in risk of preterm birth associated with lower genital tract infection. Paediatr. Perinat. Epidemiol. 2007;21:330–337. doi: 10.1111/j.1365-3016.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- Holzman C, Bullen B, Fisher R, Paneth N, Reuss L, Group PS. Pregnancy outcomes and community health: the POUCH study of preterm delivery. Paediatr. Perinat. Epidemiol. 2001a;15 Suppl 2:136–158. doi: 10.1046/j.1365-3016.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- Holzman C, Leventhal JM, Qiu H, Jones NM, Wang J. Factors linked to bacterial vaginosis in nonpregnant women. Am. J. Public Health. 2001b;91:1664–1670. doi: 10.2105/ajph.91.10.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman C, Lin X, Senagore P, Chung H. Histologic chorioamnionitis and preterm delivery. Am. J. Epidemiol. 2007;166:786–794. doi: 10.1093/aje/kwm168. [DOI] [PubMed] [Google Scholar]
- Kazzi SN, Jacques SM, Qureshi F, Quasney MW, Kim UO, Buhimschi IA. Tumor necrosis factor-alpha allele lymphotoxin-alpha+250 is associated with the presence and severity of placental inflammation among preterm births. Pediatr. Res. 2004;56:94–98. doi: 10.1203/01.PDR.0000130474.12948.A4. [DOI] [PubMed] [Google Scholar]
- Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am. J. Obstet. Gynecol. 2003a;189:139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- Leitich H, Brunbauer M, Bodner-Adler B, Kaider A, Egarter C, Husslein P. Antibiotic treatment of bacterial vaginosis in pregnancy: a meta-analysis. Am. J. Obstet. Gynecol. 2003b;188:752–758. doi: 10.1067/mob.2003.167. [DOI] [PubMed] [Google Scholar]
- Macones GA, Parry S, Elkousy M, Clothier B, Ural SH, Strauss JF., 3rd A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am. J. Obstet. Gynecol. 2004;190:1504–1508. doi: 10.1016/j.ajog.2004.01.001. discussion 1503A. [DOI] [PubMed] [Google Scholar]
- Menon R, Merialdi M, Betran AP, Dolan S, Jiang L, Fortunato SJ, et al. Analysis of association between maternal tumor necrosis factor-alpha promoter polymorphism (-308), tumor necrosis factor concentration, and preterm birth. Am. J. Obstet. Gynecol. 2006;195:1240–1248. doi: 10.1016/j.ajog.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Menon R, Pearce B, Velez DR, Merialdi M, Williams SM, Fortunato SJ, et al. Racial disparity in pathophysiologic pathways of preterm birth based on genetic variants. Reprod. Biol. Endocrinol. 2009;7:62. doi: 10.1186/1477-7827-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MD, Edwin S, Romero RJ. Prostaglandin biosynthesis by human decidual cells: effects of inflammatory mediators. Prostaglandins Leukot. Essent. Fatty Acids. 1990;41:35–38. doi: 10.1016/0952-3278(90)90128-8. [DOI] [PubMed] [Google Scholar]
- Moore S, Ide M, Randhawa M, Walker JJ, Reid JG, Simpson NA. An investigation into the association among preterm birth, cytokine gene polymorphisms and periodontal disease. BJOG. 2004;111:125–132. doi: 10.1046/j.1471-0528.2003.00024.x-i1. [DOI] [PubMed] [Google Scholar]
- Ness RB, Hillier S, Richter HE, Soper DE, Stamm C, Bass DC, et al. Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J. Natl. Med. Assoc. 2003;95:201–212. [PMC free article] [PubMed] [Google Scholar]
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oner C, Schatz F, Kizilay G, Murk W, Buchwalder LF, Kayisli UA, et al. Progestin-inflammatory cytokine interactions affect matrix metalloproteinase-1 and -3 expression in term decidual cells: implications for treatment of chorioamnionitis-induced preterm delivery. J. Clin. Endocrinol. Metab. 2008;93:252–259. doi: 10.1210/jc.2007-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauk PN, Chiao JP. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am. J. Reprod. Immunol. 2000;43:152–159. doi: 10.1111/j.8755-8920.2000.430304.x. [DOI] [PubMed] [Google Scholar]
- Roberts AK, Monzon-Bordonaba F, Van Deerlin PG, Holder J, Macones GA, Morgan MA, et al. Association of polymorphism within the promoter of the tumor necrosis factor alpha gene with increased risk of preterm premature rupture of the fetal membranes. Am. J. Obstet. Gynecol. 1999;180:1297–1302. doi: 10.1016/s0002-9378(99)70632-0. [DOI] [PubMed] [Google Scholar]
- Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22. doi: 10.1016/0090-6980(89)90028-2. [DOI] [PubMed] [Google Scholar]
- Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am. J. Obstet. Gynecol. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Savitz DA, Dole N, Herring AH, Kaczor D, Murphy J, Siega-Riz AM, et al. Should spontaneous and medically indicated preterm births be separated for studying aetiology? Paediatr. Perinat. Epidemiol. 2005;19:97–105. doi: 10.1111/j.1365-3016.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- So T, Ito A, Sato T, Mori Y, Hirakawa S. Tumor necrosis factor-alpha stimulates the biosynthesis of matrix metalloproteinases and plasminogen activator in cultured human chorionic cells. Biol. Reprod. 1992;46:772–778. doi: 10.1095/biolreprod46.5.772. [DOI] [PubMed] [Google Scholar]
- Sturm-Ramirez K, Gaye-Diallo A, Eisen G, Mboup S, Kanki PJ. High levels of tumor necrosis factor-alpha and interleukin-1beta in bacterial vaginosis may increase susceptibility to human immunodeficiency virus. J. Infect. Dis. 2000;182:467–473. doi: 10.1086/315713. [DOI] [PubMed] [Google Scholar]
- Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, Dubard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am. J. Obstet. Gynecol. 1998;178:546–550. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- Zhang J, Savitz DA. Preterm birth subtypes among blacks and whites. Epidemiology. 1992;3:428–433. doi: 10.1097/00001648-199209000-00008. [DOI] [PubMed] [Google Scholar]