Abstract
The Fyn related kinase FRK, originally called RAK, is a member of a small family of intracellular Src-related tyrosine kinases that includes PTK6 and Srms. These kinases share a conserved gene structure that is distinct from that of the Src family. Expression of FRK and PTK6 was originally identified in melanoma, breast cancer cells and normal intestinal epithelium, and both FRK and PTK6 have been implicated in the regulation of epithelial cell differentiation and apoptosis. Recently FRK was reported to phosphorylate the tumor suppressor PTEN (phosphatase and tensin homolog deleted from chromosome 10), a negative regulator of phosphatidylinositol 3 kinase (PI3K) signaling and AKT activation. FRK-mediated tyrosine phosphorylation of PTEN suppressed its association with NEDD4–1, an E3 ubiquitin ligase that may target it for polyubiquitination and proteosomal degradation. As a positive regulator of PTEN, FRK suppresses AKT signaling and inhibits breast cancer cell tumorgenicity in xenograft models. Both FRK and the related tyrosine kinase PTK6 appear to have multiple context-dependent functions, including the ability to regulate AKT. Although PTK6 negatively regulates AKT signaling in normal tissues in vivo, it may enhance AKT signaling in breast cancer cells. In contrast, FRK, which is expressed in the normal mammary gland but lost in some breast tumors, has tumor suppressor functions in mammary gland cells.
Keywords: RAK, FRK, PTK6, BRK, PTEN, AKT, Tyrosine Kinase, Tumor Suppressor
INTRODUCTION
Tyrosine kinases play important roles orchestrating cellular functions including cell migration, differentiation, cell-cell adhesion, exocrine signaling, proliferation, and death 1. Unique extracellular domains and a variety of domains that facilitate intra and inter protein-protein interactions mediate their versatility 2. Tyrosine kinases are regulated by external and internal signals, and play an integral part in signaling within multicellular organisms. Aberrant tyrosine kinase signaling leads to an array of problems and contributes to diseases such as cancer. Src, the first tyrosine kinase and oncogene to be identified, was discovered thirty years ago. Src family members are composed of several distinct functional domains referred to as Src-homology (SH) domains. These include the SH1 tyrosine kinase domain, the phosphotyrosine-binding SH2 and polyproline-binding SH3 domains, which regulate enzymatic activity and confer substrate specificity, and the SH4 domain that promotes membrane targeting.
The FRK/PTK6 family of tyrosine kinases is distantly related to the Src-family, but members of this family share an exon-intron structure distinct from Src-family members 3. A recent study postulated that an ancestral FRK/PTK6 kinase in metazoans duplicated and diverged to give rise to the FRK/PTK6 and Src tyrosine kinase families 4. Members of the vertebrate FRK/PTK6 family include Fyn-related kinase (FRK; also called RAK and in rodents Bsk, Gtk, Iyk), Protein tyrosine kinase 6 (PTK6; also called BRK and in mice Sik), and SRMS (also called SRM). Herein we use the approved HUGO gene symbols FRK and PTK6. Like Src family members, FRK/PTK6 family kinases are composed of a catalytic tyrosine kinase domain, SH2 and SH3 domains, and a regulatory carboxy-terminal tyrosine. However, most FRK/PTK6 family members lack N-terminal myristoylation/palmitoyaltion signals, and are therefore not targeted to the membrane, thus having flexibility in intracellular localization. The exception is rodent FRK that contains a partial myristoylation signal and is composed of 512 amino acids compared with 505 amino acids for the human homolog 5. The FRK SH2 domain contains a bipartite nuclear localization signal, which can direct FRK localization to the nucleus.6 Both FRK and PTK6 appear to have nuclear functions 6–10.
FRK Expression and Function
FRK was first identified in primary human breast cancer and the 600PEI breast cancer cell line 11, in human melanocytes 12, and the human hepatoma cell line Hep3B 13. In human tissues, FRK expression was shown in the epithelium of the kidney and liver, as well as in breast and colon cancer cell lines 7, 11. The rodent homolog of FRK was identified in rat small intestine 14, 15, insulin producing beta-cells 16, and the mouse mammary gland 5. In a survey of FRK expression in rodent tissues, expression was highest in the small intestine 5.
In the rat, FRK has been shown to localize to the brush border membrane of the columnar gut epithelium and it can be activated by MET 15, 17. In normal human breast tissue, FRK is localized to the cytoplasm and nucleus during the follicular phase of the menstrual cycle, and it becomes more cytoplasmic during the luteal phase, suggesting that cytoplasmic FRK localization correlates with cell proliferation 18. Phosphorylation of the C-terminal tyrosine Tyr497 (rodent Tyr504) of FRK regulates activity and localization, and activating mutations of Tyr497 to phenylalanine decrease cell proliferation 19, suggesting that nuclear FRK may have a tumor suppressor function.
FRK can regulate production of endocrine cells during pancreatic development. At embryonic day 15.5, reduced numbers of insulin producing beta-cells and increased numbers of glucagon producing alpha-cells were observed, although no differences were detected in adult mice 20. Expression of FRK in the PC12 pheochromocytoma cell line resulted in neurite outgrowth, supporting a role in differentiation, and increased SHB phosphorylation and association with FAK1 were observed 21, 22. Transgenic mice overexpressing kinase active FRK under the insulin promoter showed increased beta-cells mass 23, 24. Partial pancreatectomy of transgenic mice also showed increased cell growth 24, albeit increased sensitivity to cytokine-induced cell death was also observed 23, 25. Cells isolated from Frk−/− mice showed resistance to cell death 26.
Overexpression of human 6, 27 and murine FRK 19, 28 induces growth arrest. Association of FRK with the tumor suppressor protein pRB during G1 and S phase was observed 6. FRK protein levels are lowest during mitosis and highest during G1 arrest, and over-expression of FRK was shown to cause G1 arrest and decrease growth and colony formation 6, 27. Although FRK was shown to bind the A/B binding pocket of RB 6, G1 arrest occurred independent of pRB 27. Interestingly, FRK kinase activity, but not the SH2 or SH3 domains, was required for cell cycle arrest 27.
FRK positively regulates the tumor suppressor PTEN
PTEN (phosphatase and tensin homolog deleted from chromosome ten) is a tumor suppressor that is often mutated or deleted in cancers 29, 30. PTEN antagonizes phosphatidylinositol 3 kinase (PI3K) by dephosphorylating PIP3 to PI(4,5)P2, thereby preventing PIP3-mediated activation of AKT. Through its regulation of AKT, PTEN can negatively regulate proliferation, survival, and cell motility (reviewed in 31). PTEN also plays a role in the nucleus, maintaining chromosome integrity by binding to Cenp-c in the centromere 32. A role of PTEN in promoting DNA repair has also been established because it can coordinate with E2F to upregulate expression of the repair protein Rad51, as well as block AKT-dependent inactivation of the checkpoint protein Chk1 (reviewed in 33, 34).
Recently FRK was identified as a PTEN associated protein, and a direct correlation between FRK levels and PTEN levels was observed in breast cancer tissue samples 35. PTEN is degraded via the proteosomal degradation pathway, and NEDD4–1 is an E3 ubiquitin ligase that is involved in the ubiquitination of PTEN 36. To determine if ubiquitination of PTEN was influenced by FRK, Yim and colleges determined that PTEN ubiquitination was increased when FRK was knocked down. Furthermore, knock down of FRK resulted in increased association of PTEN with NEDD4–1. FRK was found to phosphorylate PTEN at tyrosine residue 336, and mutation of this residue negated stabilization of PTEN protein by FRK, and resulted in increased PTEN association with NEDD4–1. Phosphorylation of PTEN tyrosine residue 336 by FRK appeared to be sufficient and necessary to protect PTEN from NEDD4–1 mediated degradation 35.
Ectopic expression of FRK in the MCF7 breast cancer cell line increased PTEN protein levels, and knockdown of FRK in the nontransformed immortalized MCF10A cell line reduced PTEN protein expression 35. Alteration of FRK in either cell line did not affect PTEN mRNA levels, supporting a role for FRK in post-transcriptional regulation of PTEN. In fact, knockdown of FRK reduced the half-life of PTEN, while FRK over-expression increased PTEN stability. Increased PTEN activity in the presence of FRK was verified by lipid phosphatase assays, detection of reduced levels of activated phospho-AKT, a shift of nuclear to cytoplasmic β-catenin, and reduced β-catenin/Tcf4 signaling. Knockdown of FRK was sufficient to transform the MCF10A cell line, showing increased cell growth, colony formation and tumor growth in xenografts. Overexpression of FRK in MCF7 breast cancer cells, on the other hand, suppressed tumor growth 35.
DISCUSSION
Yim and colleagues have provided evidence that FRK is a tumor suppressor that associates with and phosphorylates PTEN, leading to its subsequent stabilization by preventing its ubiquitination by NEDD4–1 in breast cancer cells 35. While NEDD4–1 appears critical for targeting PTEN for degradation in breast cancer cells, Fouladkou et al. showed that disruption of NEDD4–1 expression had no impact on PTEN stability and localization in MEFs and hearts from two strains of knockout mice 37. However, it is possible that another mechanism compensates for NEDD4–1 loss in mice, or that tissue specific factors play an important role in determining how PTEN is regulated by NEDD4–1. In addition, it has been proposed that NEDD4–1 may target PTEN only in response to specific signals 38.
Previous studies of FRK function using Frk −/− mice did not yield evidence for a tumor suppressive role in vivo 20, 39. Only a mild phenotype in circulating T3 hormone levels was observed in adult mice 39, and FRK seems to be involved in determining glucagon versus insulin producing cell differentiation in the pancreas of day 15 embryos and newborns, but not in adults 20. Frk −/− mice may be able to compensate for loss of FRK through a redundant mechanism, such as overlapping functions with another FRK or SRC family member, such as PTK6 (Table 1). It is possible that a conditional loss of FRK in the adult mouse is less likely to be compensated, and the effects would be more apparent.
Table 1.
Similar functions for the FRK and PTK6 tyrosine kinases.
| FUNCTION | OBSERVATION | REFERENCE | |
|---|---|---|---|
| Differentiation | FRK | Neurite outgrowth; generation of insulin producing cells | 20, 21 |
| PTK6 | Differentiation of keratinocytes and enterocytes | 42, 43, 45 | |
| AKT Regulation | FRK | Decreased PI3K signaling; Upregulation of PTEN | 35, 64 |
| PTK6 | Inhibition of AKT activation in vivo; Direct binding, phosphorylation, and inhibition of AKT in unstimulated cells | 43, 47, 48 | |
| Nuclear Functions | FRK | Nuclear localization; Binding to RB | 6, 7, 18 |
| PTK6 | Nuclear localization; Binding and regulation of Sam68, SLM-1 and SLM-2 | 8–10, 49, 65 | |
| Growth Arrest | FRK | G1 arrest | 6, 19, 27, 28 |
| PTK6 | Negative regulator of proliferation in normal intestine | 43 | |
| Apoptosis | FRK | Sensitization of islet cells to cytokine-induced death | 23, 25, 26 |
| PTK6 | Sensitization of non-transformed cells to UV and serum starvation; DNA-damage-induced apoptosis in small intestine | 46, 47 |
Like FRK, PTK6 was first identified in melanocytes 12, breast cancer cells 40 and the normal intestine 41, 42. PTK6 positively regulates enterocyte 43 and keratinocyte differentiation 44, 45. Both FRK and PTK6 promote cell death; FRK promotes pancreatic islet cell death in response to cytotoxic cytokines 26, while PTK6 sensitizes nontransformed cells to apoptotic stimuli in culture 46, and promotes DNA damage induced apoptosis in the intestine 47. Both kinases can negatively regulate AKT; PTK6 has been shown to associate with and phosphorylate AKT directly 48. In addition, increased AKT activation has been reported in the intestines of Ptk6 null mice 43, 47 (see Figure 1). It will be important to examine the phenotypes of FRK/PTK6 double null mice to determine if they are more prone to developing tumors, particularly in tissues where they are coexpressed, such as in the intestine. Untreated Ptk6 −/− mice have a mild growth and differentiation phenotype 43.
Figure 1.
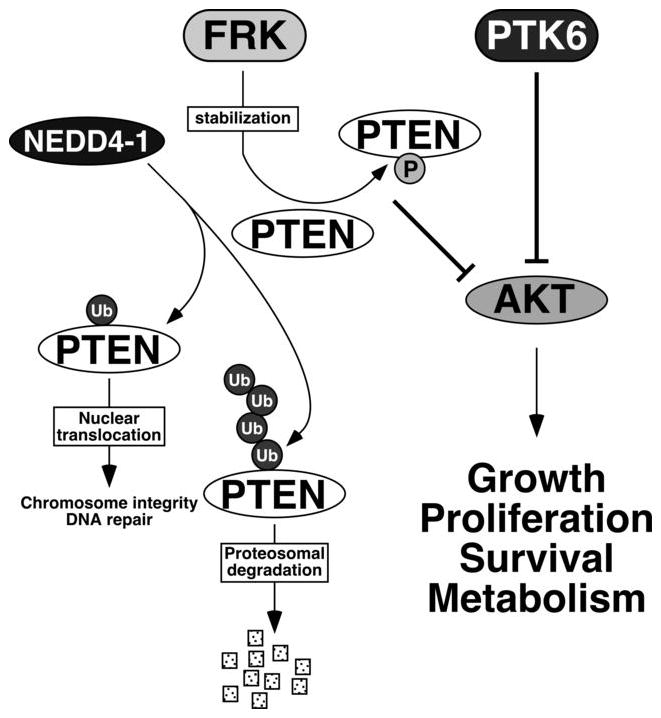
FRK and PTK6 negatively regulate AKT activity. The ubiquitin ligase NEDD4–1 can monoubiquinate or polyubiquitinate PTEN, leading to its nuclear translocation or proteosomal degradation respectively 32, 36. FRK directly phosphorylates PTEN and prevents its association with NEDD4–1, leading to stabilization and accumulation of PTEN 35. PTEN antagonizes the phosphoinositol-3-kinase/Akt pathway. PTK6 may directly associate with and phosphorylate AKT, and has been shown to inhibit AKT in unstimulated cells 48. Increased AKT activation has been detected in the intestines of Ptk6 null mice 43, 47.
Context specific functions of FRK and PTK6 may be dictated by the intracellular localization of these kinases. It was first proposed that intracellular localization of PTK6 may play a significant role in regulating signaling outcomes 10. Indeed, targeting PTK6 to the membrane or nucleus influences whether PTK6 has oncogenic or growth suppressive functions 49, (Palka-Hamblin and Tyner, unpublished). FRK contains a bipartite nuclear localization signal, and it will be interesting to determine if it plays a distinct role in regulating nuclear localization and/or nuclear functions of PTEN 34, 50. NEDD4–1 has been shown to regulate both monoubiquination and polyubiquitination of PTEN, leading to its nuclear translocation or proteosomal degradation respectively 32, 36.
While FRK and PTK6 signaling have many parallels, each kinase appears to have its own tissue specific functions. Human FRK and PTK6 share only 44% amino acid identity. Unlike FRK, PTK6 is not expressed in the normal mammary gland 10, 51, 52, but it is expressed in a high proportion of breast tumors 51, 53. The PTK6 gene maps to human chromosome 20q13.3 54, a region often amplified in breast cancer, and PTK6 is coamplified with HER2 in some human breast tumors 55, 56. Several studies suggest that PTK6, referred to as the breast tumor kinase BRK, promotes breast cancer cell proliferation and migration (for examples see 48, 53, 56–60).
In contrast to PTK6, FRK maps to human chromosome 6q21-q22.3, a region lost in some breast cancers 61, and loss of FRK expression has been reported in human mammary gland tumors 18. However, a few reports have also suggested that FRK may have oncogenic potential. FRK appears to be overexpressed in some primary human colon tumors 7 and a third of mammary tumors examined 7, 62. In a patient with acute myelogenous leukemia, the Frk gene was found fused with the ETS transcription factor ETV6 and the ETV6/FRK protein had oncogenic properties 63. It will be important to determine if promoting expression/activity of FRK during mammary gland tumorigenesis in vivo leads to repression of tumor growth through stabilization of PTEN, and if this is sufficient to counteract tumor promoting functions of the related kinase PTK6.
Acknowledgments
A.L.T. is supported by National Institutes of Health Grants DK44525 and DK068503.
References
- 1.Hunter T. Tyrosine phosphorylation: thirty years and counting. Curr Opin Cell Biol. 2009;21:140–6. doi: 10.1016/j.ceb.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pawson T, Kofler M. Kinome signaling through regulated protein-protein interactions in normal and cancer cells. Curr Opin Cell Biol. 2009;21:147–53. doi: 10.1016/j.ceb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Serfas MS, Tyner AL. Brk, Srm, Frk, and Src42A form a distinct family of intracellular Src-like tyrosine kinases. Oncol Res. 2003;13:409–19. doi: 10.3727/096504003108748438. [DOI] [PubMed] [Google Scholar]
- 4.D’Aniello S, Irimia M, Maeso I, Pascual-Anaya J, Jimenez-Delgado S, Bertrand S, Garcia-Fernandez J. Gene expansion and retention leads to a diverse tyrosine kinase superfamily in amphioxus. Molecular biology and evolution. 2008;25:1841–54. doi: 10.1093/molbev/msn132. [DOI] [PubMed] [Google Scholar]
- 5.Thuveson M, Albrecht D, Zurcher G, Andres AC, Ziemiecki A. iyk, a novel intracellular protein tyrosine kinase differentially expressed in the mouse mammary gland and intestine [published erratum appears in Biochem Biophys Res Commun 1995 Jun 26;211(3):1100] Biochem Biophys Res Commun. 1995;209:582–9. doi: 10.1006/bbrc.1995.1540. [DOI] [PubMed] [Google Scholar]
- 6.Craven RJ, Cance WG, Liu ET. The nuclear tyrosine kinase Rak associates with the retinoblastoma protein pRb. Cancer Res. 1995;55:3969–72. [PubMed] [Google Scholar]
- 7.Cance WG, Craven RJ, Bergman M, Xu L, Aitalo K, Liu ET. Rak, a novel nuclear tyrosine kinase expressed in epithelial cells. Cell Growth Diff. 1994;5:1347–55. [PubMed] [Google Scholar]
- 8.Derry JJ, Richard S, Valderrama Carvajal H, Ye X, Vasioukhin V, Cochrane AW, Chen T, Tyner AL. Sik (BRK) Phosphorylates Sam68 in the Nucleus and Negatively Regulates Its RNA Binding Ability. Mol Cell Biol. 2000;20:6114–26. doi: 10.1128/mcb.20.16.6114-6126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derry JJ, Prins GS, Ray V, Tyner AL. Altered localization and activity of the intracellular tyrosine kinase BRK/Sik in prostate tumor cells. Oncogene. 2003;22:4212–20. doi: 10.1038/sj.onc.1206465. [DOI] [PubMed] [Google Scholar]
- 10.Haegebarth A, Heap D, Bie W, Derry JJ, Richard S, Tyner AL. The nuclear tyrosine kinase BRK/Sik phosphorylates and inhibits the RNA-binding activities of the Sam68-like mammalian proteins SLM-1 and SLM-2. J Biol Chem. 2004;279:54398–404. doi: 10.1074/jbc.M409579200. [DOI] [PubMed] [Google Scholar]
- 11.Cance WG, Craven RJ, Weiner TM, Liu ET. Novel protein kinases expresed in human breast cancer. Int J Cancer. 1993;54:571–7. doi: 10.1002/ijc.2910540409. [DOI] [PubMed] [Google Scholar]
- 12.Lee ST, Strunk KM, Spritz RA. A survey of protein tyrosine kinase mRNAs expressed in normal human melanocytes. Oncogene. 1993;8:3403–10. [PubMed] [Google Scholar]
- 13.Lee J, Wang Z, Luoh S-M, Wood WL, Scadden DT. Cloning of FRK, a novel human intracellular SRC-like tyrosine kinase-encoding gene. Gene. 1994;138:247–51. doi: 10.1016/0378-1119(94)90817-6. [DOI] [PubMed] [Google Scholar]
- 14.Sunitha I, Avigan MI. A newly identified tyrosine kinase is preferentially expressed in the gastrointestinal tract. Biochim Biophys Acta. 1994;1221:348–52. doi: 10.1016/0167-4889(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 15.Sunitha I, Avigan MI. The apical membranes of maturing gut columnar epithelial cells contain the enzymatically active form of a newly identified fyn-related tyrosine kinase. Oncogene. 1996;13:547–59. [PubMed] [Google Scholar]
- 16.Oberg-Welsh C, Welsh M. Cloning of BSK, a murine FRK homologue with a specific pattern of tissue distribution. Gene. 1995;152:239–42. doi: 10.1016/0378-1119(94)00718-8. [DOI] [PubMed] [Google Scholar]
- 17.Sunitha I, Shen R, McKillop IH, Lee JH, Resau J, Avigan M. A src-related kinase in the brush border membranes of gastrointestinal cells is regulated by c-met. Exp Cell Res. 1999;250:86–98. doi: 10.1006/excr.1999.4550. [DOI] [PubMed] [Google Scholar]
- 18.Berclaz G, Altermatt HJ, Rohrbach V, Dreher E, Ziemiecki A, Andres AC. Hormone-dependent nuclear localization of the tyrosine kinase iyk in the normal human breast epithelium and loss of expression during carcinogenesis. Int J Cancer. 2000;85:889–94. doi: 10.1002/(sici)1097-0215(20000315)85:6<889::aid-ijc25>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Oberg-Welsh C, Anneren C, Welsh M. Mutation of C-terminal tyrosine residues Y497/Y504 of the Src-family member Bsk/Iyk decreases NIH3T3 cell proliferation. Growth Factors. 1998;16:111–24. doi: 10.3109/08977199809002122. [DOI] [PubMed] [Google Scholar]
- 20.Akerblom B, Anneren C, Welsh M. A role of FRK in regulation of embryonal pancreatic beta cell formation. Mol Cell Endocrinol. 2007;270:73–8. doi: 10.1016/j.mce.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Anneren C, Reedquist KA, Bos JL, Welsh M. GTK, a Src-related tyrosine kinase, induces nerve growth factor-independent neurite outgrowth in PC12 cells through activation of the Rap1 pathway. Relationship to Shb tyrosine phosphorylation and elevated levels of focal adhesion kinase. J Biol Chem. 2000;275:29153–61. doi: 10.1074/jbc.M003926200. [DOI] [PubMed] [Google Scholar]
- 22.Anneren C, Lindholm CK, Kriz V, Welsh M. The FRK/RAK-SHB signaling cascade: a versatile signal-transduction pathway that regulates cell survival, differentiation and proliferation. Curr Mol Med. 2003;3:313–24. doi: 10.2174/1566524033479744. [DOI] [PubMed] [Google Scholar]
- 23.Anneren C, Welsh M. Increased cytokine-induced cytotoxicity of pancreatic islet cells from transgenic mice expressing the Src-like tyrosine kinase GTK. Mol Med. 2001;7:301–10. [PMC free article] [PubMed] [Google Scholar]
- 24.Anneren C, Welsh M, Jansson L. Glucose intolerance and reduced islet blood flow in transgenic mice expressing the FRK tyrosine kinase under the control of the rat insulin promoter. Am J Physiol Endocrinol Metab. 2007;292:E1183–90. doi: 10.1152/ajpendo.00168.2006. [DOI] [PubMed] [Google Scholar]
- 25.Anneren C. Dual role of the tyrosine kinase GTK and the adaptor protein SHB in beta-cell growth: enhanced beta-cell replication after 60% pancreatectomy and increased sensitivity to streptozotocin. J Endocrinol. 2002;172:145–53. doi: 10.1677/joe.0.1720145. [DOI] [PubMed] [Google Scholar]
- 26.Welsh M, Welsh C, Ekman M, Dixelius J, Hagerkvist R, Anneren C, Akerblom B, Mahboobi S, Chandrasekharan S, Liu ET. The tyrosine kinase FRK/RAK participates in cytokine-induced islet cell cytotoxicity. Biochem J. 2004;382:261–8. doi: 10.1042/BJ20040285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer T, Xu L, Chang J, Liu ET, Craven RJ, Cance WG. Breast cancer cell line proliferation blocked by the Src-related Rak tyrosine kinase. Int J Cancer. 2003;104:139–46. doi: 10.1002/ijc.10925. [DOI] [PubMed] [Google Scholar]
- 28.Anneren C, Welsh M. Role of the Bsk/Iyk non-receptor tyrosine kinase for the control of growth and hormone production in RINm5F cells. Growth Factors. 2000;17:233–47. doi: 10.3109/08977190009028969. [DOI] [PubMed] [Google Scholar]
- 29.Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci U S A. 1997;94:9052–7. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 31.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and crosstalks. Oncogene. 2008;27:5527–41. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 32.Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, Tempst P, Chi SG, Kim HJ, Misteli T, Jiang X, Pandolfi PP. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–56. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin Y, Shen WH. PTEN: a new guardian of the genome. Oncogene. 2008;27:5443–53. doi: 10.1038/onc.2008.241. [DOI] [PubMed] [Google Scholar]
- 34.Planchon SM, Waite KA, Eng C. The nuclear affairs of PTEN. J Cell Sci. 2008;121:249–53. doi: 10.1242/jcs.022459. [DOI] [PubMed] [Google Scholar]
- 35.Yim EK, Peng G, Dai H, Hu R, Li K, Lu Y, Mills GB, Meric-Bernstam F, Hennessy BT, Craven RJ, Lin SY. Rak functions as a tumor suppressor by regulating PTEN protein stability and function. Cancer Cell. 2009;15:304–14. doi: 10.1016/j.ccr.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, Jiang X. NEDD4–1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–39. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fouladkou F, Landry T, Kawabe H, Neeb A, Lu C, Brose N, Stambolic V, Rotin D. The ubiquitin ligase Nedd4–1 is dispensable for the regulation of PTEN stability and localization. Proc Natl Acad Sci U S A. 2008;105:8585–90. doi: 10.1073/pnas.0803233105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Jiang X. Post-translational regulation of PTEN. Oncogene. 2008;27:5454–63. doi: 10.1038/onc.2008.242. [DOI] [PubMed] [Google Scholar]
- 39.Chandrasekharan S, Qiu TH, Alkharouf N, Brantley K, Mitchell JB, Liu ET. Characterization of mice deficient in the Src family nonreceptor tyrosine kinase Frk/rak. Mol Cell Biol. 2002;22:5235–47. doi: 10.1128/MCB.22.14.5235-5247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell PJ, Barker KT, Martindale JE, Kamalati T, Lowe PN, Page MJ, Gusterson BA, Crompton MR. Cloning and characterisation of cDNAs encoding a novel non-receptor tyrosine kinase, brk, expressed in human breast tumours. Oncogene. 1994;9:2383–90. [PubMed] [Google Scholar]
- 41.Siyanova EY, Serfas MS, Mazo IA, Tyner AL. Tyrosine kinase gene expression in the mouse small intestine. Oncogene. 1994;9:2053–7. [PubMed] [Google Scholar]
- 42.Vasioukhin V, Serfas MS, Siyanova EY, Polonskaia M, Costigan VJ, Liu B, Thomason A, Tyner AL. A novel intracellular epithelial cell tyrosine kinase is expressed in the skin and gastrointestinal tract. Oncogene. 1995;10:349–57. [PubMed] [Google Scholar]
- 43.Haegebarth A, Bie W, Yang R, Crawford SE, Vasioukhin V, Fuchs E, Tyner AL. Protein tyrosine kinase 6 negatively regulates growth and promotes enterocyte differentiation in the small intestine. Mol Cell Biol. 2006;26:4949–57. doi: 10.1128/MCB.01901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasioukhin V, Tyner AL. A role for the epithelial-cell-specific tyrosine kinase Sik during keratinocyte differentiation. Proc Natl Acad Sci U S A. 1997;94:14477–82. doi: 10.1073/pnas.94.26.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang TC, Jee SH, Tsai TF, Huang YL, Tsai WL, Chen RH. Role of breast tumour kinase in the in vitro differentiation of HaCaT cells. Br J Dermatol. 2005;153:282–9. doi: 10.1111/j.1365-2133.2005.06604.x. [DOI] [PubMed] [Google Scholar]
- 46.Haegebarth A, Nunez R, Tyner AL. The Intracellular Tyrosine Kinase Brk Sensitizes Non-Transformed Cells to Inducers of Apoptosis. Cell Cycle. 2005;4:1239–46. doi: 10.4161/cc.4.9.1965. [DOI] [PubMed] [Google Scholar]
- 47.Haegebarth A, Perekatt AO, Bie W, Gierut JJ, Tyner AL. Induction of Protein Tyrosine Kinase 6 in Mouse Intestinal Crypt Epithelial Cells Promotes DNA-Damage Induced Apoptosis. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang P, Ostrander JH, Faivre EJ, Olsen A, Fitzsimmons D, Lange CA. Regulated association of protein kinase B/Akt with breast tumor kinase. J Biol Chem. 2005;280:1982–91. doi: 10.1074/jbc.M412038200. [DOI] [PubMed] [Google Scholar]
- 49.Kim HI, Lee ST. Oncogenic Functions of PTK6 Are Enhanced by Its Targeting to Plasma Membrane But Abolished by Its Targeting to Nucleus. Journal of biochemistry. 2009 doi: 10.1093/jb/mvp050. [DOI] [PubMed] [Google Scholar]
- 50.Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–97. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barker KT, Jackson LE, Crompton MR. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene. 1997;15:799–805. doi: 10.1038/sj.onc.1201241. [DOI] [PubMed] [Google Scholar]
- 52.Llor X, Serfas MS, Bie W, Vasioukhin V, Polonskaia M, Derry J, Abbott CM, Tyner AL. BRK/Sik expression in the gastrointestinal tract and in colon tumors. Clin Cancer Res. 1999;5:1767–77. [PubMed] [Google Scholar]
- 53.Ostrander JH, Daniel AR, Lofgren K, Kleer CG, Lange CA. Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Res. 2007;67:4199–209. doi: 10.1158/0008-5472.CAN-06-3409. [DOI] [PubMed] [Google Scholar]
- 54.Park SH, Lee KH, Kim H, Lee ST. Assignment of the human PTK6 gene encoding a non-receptor protein tyrosine kinase to 20q13.3 by fluorescence in situ hybridization. Cytogenet Cell Genet. 1997;77:271–2. doi: 10.1159/000134595. [DOI] [PubMed] [Google Scholar]
- 55.Born M, Quintanilla-Fend L, Braselmann H, Reich U, Richter M, Hutzler P, Aubele M. Simultaneous over-expression of the Her2/neu and PTK6 tyrosine kinases in archival invasive ductal breast carcinomas. J Pathol. 2005;205:592–6. doi: 10.1002/path.1720. [DOI] [PubMed] [Google Scholar]
- 56.Xiang B, Chatti K, Qiu H, Lakshmi B, Krasnitz A, Hicks J, Yu M, Miller WT, Muthuswamy SK. Brk is coamplified with ErbB2 to promote proliferation in breast cancer. Proc Natl Acad Sci U S A. 2008;105:12463–8. doi: 10.1073/pnas.0805009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamalati T, Jolin HE, Fry MJ, Crompton MR. Expression of the BRK tyrosine kinase in mammary epithelial cells enhances the coupling of EGF signalling to PI 3-kinase and Akt, via erbB3 phosphorylation. Oncogene. 2000;19:5471–6. doi: 10.1038/sj.onc.1203931. [DOI] [PubMed] [Google Scholar]
- 58.Harvey AJ, Crompton MR. Use of RNA interference to validate Brk as a novel therapeutic target in breast cancer: Brk promotes breast carcinoma cell proliferation. Oncogene. 2003;22:5006–10. doi: 10.1038/sj.onc.1206577. [DOI] [PubMed] [Google Scholar]
- 59.Chen HY, Shen CH, Tsai YT, Lin FC, Huang YP, Chen RH. Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol Cell Biol. 2004;24:10558–72. doi: 10.1128/MCB.24.24.10558-10572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weaver AM, Silva CM. Signal transducer and activator of transcription 5b: a new target of breast tumor kinase/protein tyrosine kinase 6. Breast Cancer Res. 2007;9:R79. doi: 10.1186/bcr1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheng ZM, Marchetti A, Buttitta F, Champeme MH, Campani D, Bistocchi M, Lidereau R, Callahan R. Multiple regions of chromosome 6q affected by loss of heterozygosity in primary human breast carcinomas. Br J Cancer. 1996;73:144–7. doi: 10.1038/bjc.1996.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armistead PM, Thorp HH. Electrochemical detection of gene expression in tumor samples: overexpression of Rak nuclear tyrosine kinase. Bioconjugate chemistry. 2002;13:172–6. doi: 10.1021/bc000129y. [DOI] [PubMed] [Google Scholar]
- 63.Hosoya N, Qiao Y, Hangaishi A, Wang L, Nannya Y, Sanada M, Kurokawa M, Chiba S, Hirai H, Ogawa S. Identification of a SRC-like tyrosine kinase gene, FRK, fused with ETV6 in a patient with acute myelogenous leukemia carrying a t(6;12)(q21; p13) translocation. Genes Chromosomes Cancer. 2005;42:269–79. doi: 10.1002/gcc.20147. [DOI] [PubMed] [Google Scholar]
- 64.Anneren C, Welsh M. GTK tyrosine kinase-induced alteration of IRS-protein signalling in insulin producing cells. Mol Med. 2002;8:705–13. [PMC free article] [PubMed] [Google Scholar]
- 65.Coyle JH, Guzik BW, Bor YC, Jin L, Eisner-Smerage L, Taylor SJ, Rekosh D, Hammarskjold ML. Sam68 enhances the cytoplasmic utilization of intron-containing RNA and is functionally regulated by the nuclear kinase Sik/BRK. Mol Cell Biol. 2003;23:92–103. doi: 10.1128/MCB.23.1.92-103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


