Abstract
Intraoperative blood requirements were analyzed in patients undergoing primary orthotopic liver transplantation and divided into two groups on the basis of panel reactive antibody of pretransplant serum measured by lymphocytotoxicity testing. One group of highly sensitized patients (n = 25) had PRA values of over 70% and the second group of patients (n = 26) had 0% PRA values and were considered nonsensitized. During the transplant procedure, the 70% PRA group received considerably greater quantities of blood products than the 0% PRA group—namely, red blood cells: 21.1±3.7 vs. 9.8±0.8 units (P = 0.002), and platelets: 17.7±3.2 vs. 7.5±1.5 units (P = 0.003). Similar differences were observed for fresh frozen plasma and cryoprecipitate. Despite the larger infusion of platelets, the blood platelet counts in the 70% PRA group were lower postoperatively than preoperatively. Twenty patients in the 70% PRA group received platelet transfusions, and their mean platelet count dropped from 95,050±11,537 preoperatively to 67,750±8,228 postoperatively (P = 0.028). In contrast, nearly identical preoperative (84,058±17,297) and postoperative (85,647±12,445) platelet counts were observed in the 17 0% PRA patients who were transfused intraoperatively with platelets. Prothrombin time, activated partial thromboplastin time, and fibrinogen levels showed no significant differences between both groups. These data demonstrate that lymphocytotoxic antibody screening of liver transplant candidates is useful in identifying patients with increased risk of bleeding problems and who will require large quantities of blood during the transplant operation.
During liver transplantation, major blood loss is of considerable concern and many patients require intraoperative transfusions of rather large quantities of blood components (1–3). In a previous series of 87 liver transplant procedures, the median red blood cell use was 24 units with a mean of 43 units (3). Portal hypertension (4, 5) and deficient coagulation profiles (4, 6–10) are major causes of intraoperative bleeding, with an increased morbidity and mortality in liver transplant patients (2, 3).
Although the introduction of a venovenous bypass during the anhepatic phase of the operation (11), the guidance of intra-operative blood replacement by thrombelastography (7), and the use of epsilon-aminocaproic acid (12, 13) have contributed to continued reduction in intraoperative blood use, many patients still experience severe blood loss problems that are not readily corrected by blood transfusions.
We have investigated the role of HLA alloimmunization in intraoperative blood transfusion requirements during liver transplantation. This alloimmunization can result from previous exposure to HLA antigens through transfusion, transplantation, and pregnancy and is usually detected by serum screening for lymphocytotoxic antibodies. HLA-specific antibodies can mediate hyperacute rejection, and a positive crossmatch between patient serum and donor cells represents a contraindication for successful kidney or heart transplantation (14–18). However, liver transplant outcome appears generally unaffected by a positive donor-specific crossmatch (19, 20). During combined liver-kidney transplantation in patients with preformed donor-specific antibodies, we have observed that liver allograft protects the subsequent kidney allograft from humoral rejection (21, 22). Thus, it appears that the liver is relatively resistant to the potentially deleterious effects of donor-specific HLA antibodies.
On the other hand, HLA alloimmunization plays a significant role in blood transfusions, especially those involving platelets. It is well known that platelets express class I HLA antigens, and specific alloimmunization to these antigens is a major cause of refractoriness of thrombocytopenic patients to platelet transfusions. Alloimmunization-induced platelet refractoriness is generally observed in patients with lymphoproliferative disease or aplastic anemia who receive long-term transfusion support. Such refractory patients will not respond to platelet transfusions from random ABO-matched donors but require HLA-compatible platelets for effective hemostasis (23–25).
In this paper we present evidence that HLA alloimmunization is associated with increased blood transfusion requirements during liver transplantation. This effect is particularly apparent for platelet transfusions, which appear to be less effective in alloimmunized liver transplant recipients. Our findings indicate that pretransplant lymphocytotoxic antibody screening of liver transplant candidates is useful in estimating blood transfusion requirements during the transplant procedure.
MATERIALS AND METHODS
Two groups of adult patients were selected from our liver transplant file on the basis of panel-reactive antibody of pretransplant serum as determined by lymphocytotoxicity testing with a panel of 45–60 HLA-typed donors as previously described (20, 22) One group of 25 highly alloimmunized patients showed serum reactivity of greater than 70% of the cell panel (the 70% PRA group). The second group of 26 patients had PRA values of 0% and were not considered alloimmunized (the 0% PRA group). All serum samples were obtained within one month pretransplant; 20/26 0% PRA and 18/25 70% PRA patients provided serum samples the day before the transplant operation. Although previous reports have indicated no correlation between history of prior operation and blood loss during liver transplantation (2), we excluded patients who had undergone a major operation immediately prior to transplantation. Thus, except for PRA, both groups of patients were selected randomly. All cases were first transplants performed from 1985 to 1987. There were no significant differences between the two groups of patients with regard to age, body weight, or primary liver disease distribution (Table 1). The number of female patients was slightly higher in the alloimmunized group but the difference was statistically insignificant (P > 0.05).
TABLE 1.
Distribution of age, weight, sex, and original disease in the 0% PRA and 70% PRA groups
| Patient criteria | 0% PRA group |
70% PRA group |
|---|---|---|
| Age (years) | 44.1±1.7 | 44.3±2.2 |
| Weight (kg) | 67.5±3.2 | 63.1±3.3 |
| Female | 12 | 19 |
| Ma1e | 14 | 6 |
| Primary biliary cirrhosis | 6 | 6 |
| Chronic active hepatitis | 7 | 9 |
| Laennec's disease | 6 | 3 |
| Fulminant hepatitis | 2 | 1 |
| Antitrypsin deficiency | 1 | 3 |
| Others | 4 | 3 |
Orthotopic transplantation of the liver was performed as already described (27) utilizing a venovenous bypass (11). The venous bypass without systemic heparin anticoagulation restores normal hemodynamic physiology during the critical anhepatic phase, lowers the intraoperative blood loss, and increases transplant success (28). In the 70% PRA group, only two patients and none of the 0% PRA patients underwent splenectomy during transplantation.
Intraoperative blood replacement therapy was guided by thrombilastographic monitoring as previously described (7, 29).
In each patient group, the intraoperative infusion of red blood cells, platelets (PLT),* fresh frozen plasma (FFP), and cryoprecipitate (CRYO) was retrospectively analyzed. In most cases, 1 unit (250 ml) of platelets was given together with 1 unit (200 ml) of fresh frozen plasma and 260 ml of crystalloids.
Statistical analysis was done with Student’s t test and the Mann-Whitney U test. Data are shown as mean values ± SE.
RESULTS
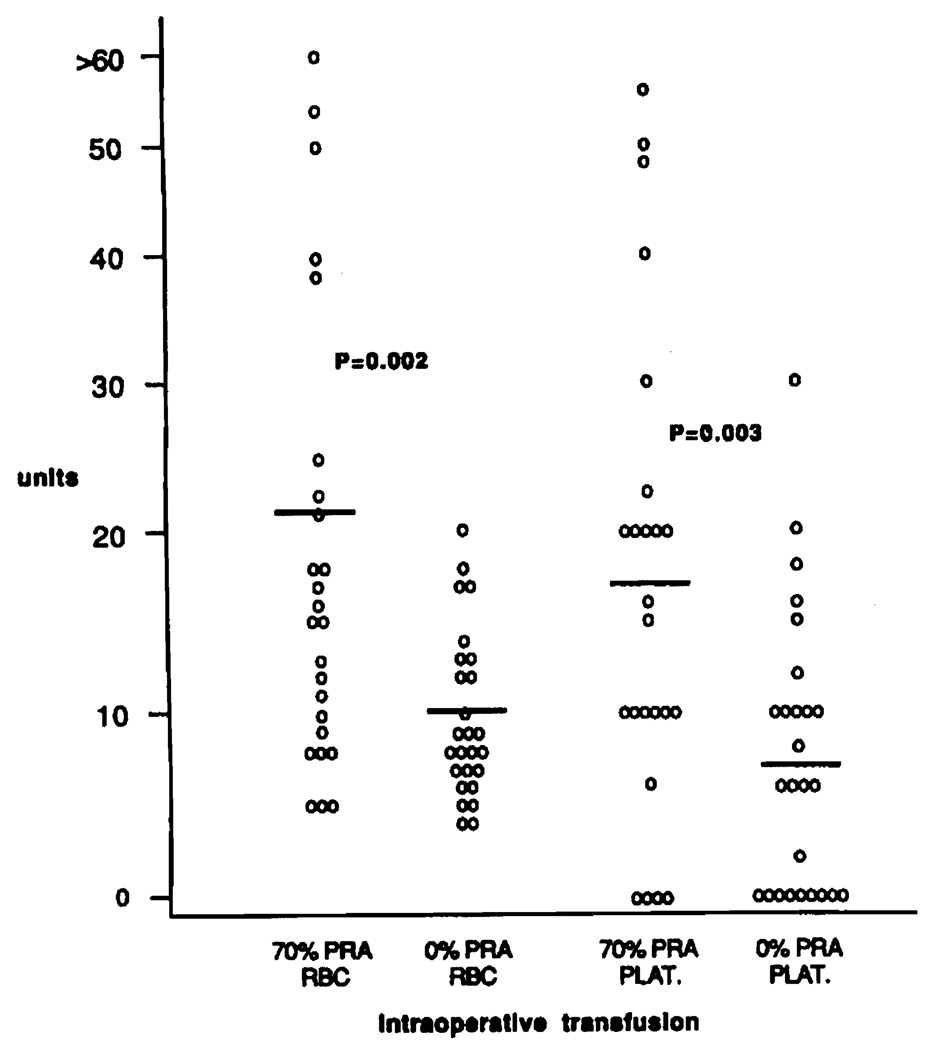
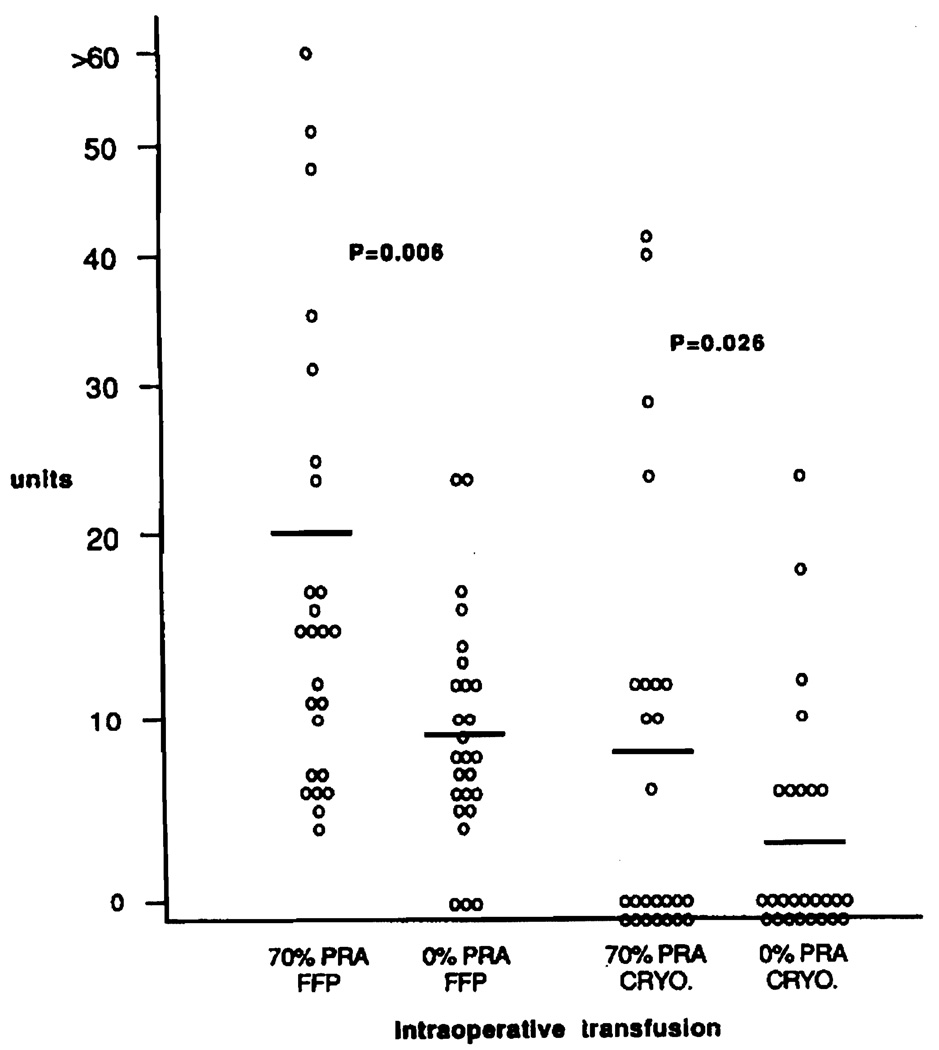
Serum antibody analysis was performed on 932 liver transplants, and 227 patients (24%) had evidence of alloimmunization (PRA≥10%) prior to operation, 67 patients (7%) were highly sensitized (PRA≥70%). Highly sensitized patients (70% PRA group) required considerably larger quantities of blood products intraoperatively than patients with no indication of alloimmunization (0% PRA group). Patients in the 70% PRA group received 21.1±3.7 units of RBC and 17.7±3.2 units of PLT, significantly more than 9.8±0.8 units of RBC and 7.5±1.5 units of PLT in the 0% PRA group (Fig. 1) (RBC: P = 0.002, PLT: P = 0.003). Similarly, significantly larger quantities of fresh frozen plasma and cryoprecipitate were given intraoperatively to the highly alloimmunized group of liver transplant recipients (Fig. 2).
FIGURE 1.
Intraoperative transfusion of red blood cells (RBC) and platelet (PLT) in 25 highly sensitized (70% PRA group) and 26 non-sensitized (0% PRA group) patients. Mean values are depicted with thick bars.
FIGURE 2.
Intraoperative transfusion of fresh frozen plasma (FFP) and cryoprecipitate (CRYO) in 25 highly sensitized (70% PRA group) and 26 nonsensitized (0% PRA group) patients. Mean values are depicted by thick bars.
The preoperative hematocrit values were below the normal range of 35–46 in both groups (Tables 2 and 3). Hematocrit values were significantly lower in the 70% PRA group (24.6±0.7) than in the 0% PRA group (28.8±1.0) (P = 0.01). However, there was no correlation between decreased preoperative hematocrit values and the intraoperative requirement for infusions of blood products in both groups of patients (data not shown).
TABLE 2.
Pre- and posttransplant coagulation profiles in the 0% PRA group
| 0% PRA group | Pretransplant | Posttransplant | Significance (paired t test) |
|---|---|---|---|
| Hematocrit (%) | 27.8±1.0 | 31.5±1.1 | P=0.034 |
| Platelet count (×103/mm3) | 124.0±18.4 | 114.0±14.6 | NS |
| Fibrinogen (mg/dl) | 190.0±23.9 | 162.6±19.1 | NS |
| PT (sec) | 14.2±0.5 | 14.7±0.4 | NS |
| APTT (sec) | 41.7±1.8 | 40.1±1.4 | NS |
TABLE 3.
Pre- and posttransplant coagulation profiles in the 70% PRA group
| 70% PRA group | Pretransplant | Posttransplant | Significance (paired t test) |
|---|---|---|---|
| Hematocrit (%) | 24.6±0.7 | 28.6±0.8 | P=0.001 |
| Platelet count (×103/mm3) | 106.8±13.7 | 82.0±13.7 | P=0.006 |
| Fibrinogen (mg/dl) | 169.5±16.8 | 150±11.2 | NS |
| PT (sec) | 14.7±0.5 | 15.5±0.3 | NS |
| APTT (sec) | 42.6±1.9 | 44.8±1.6 | NS |
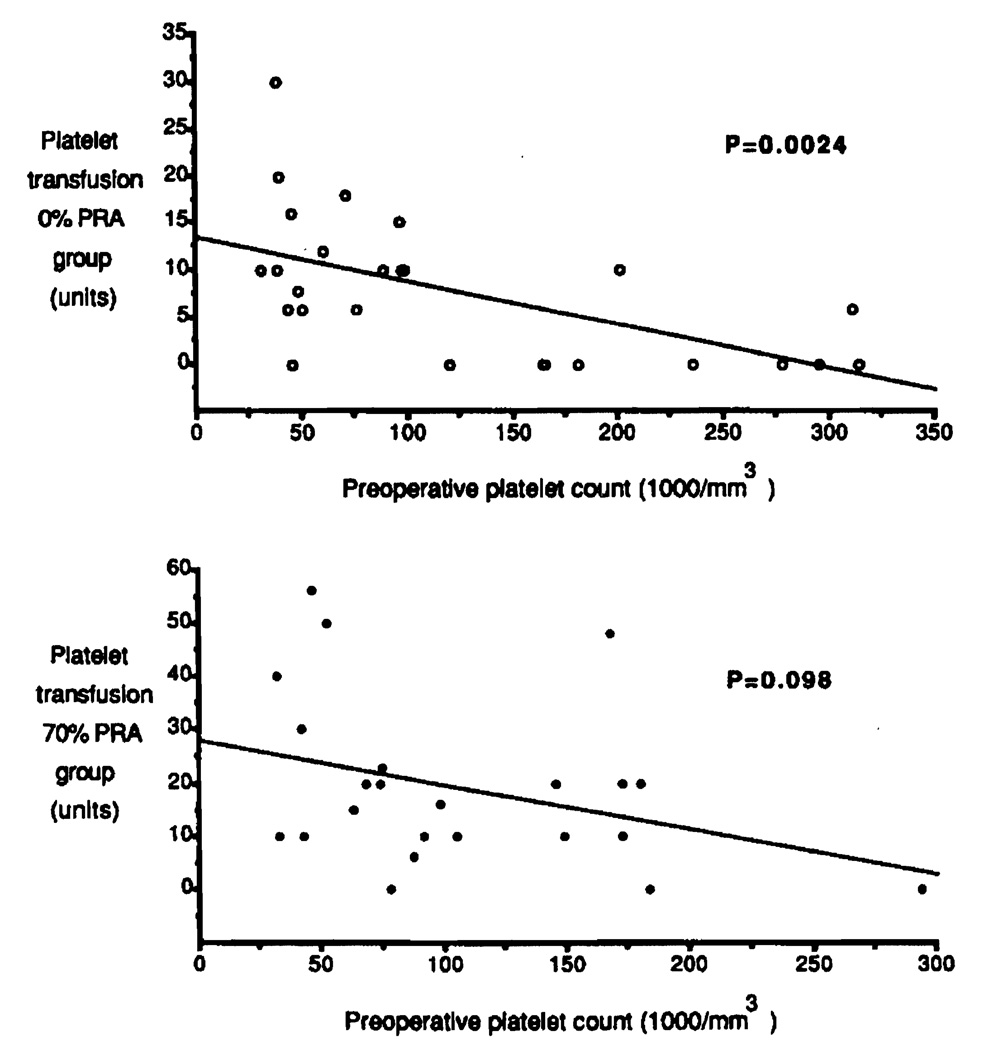
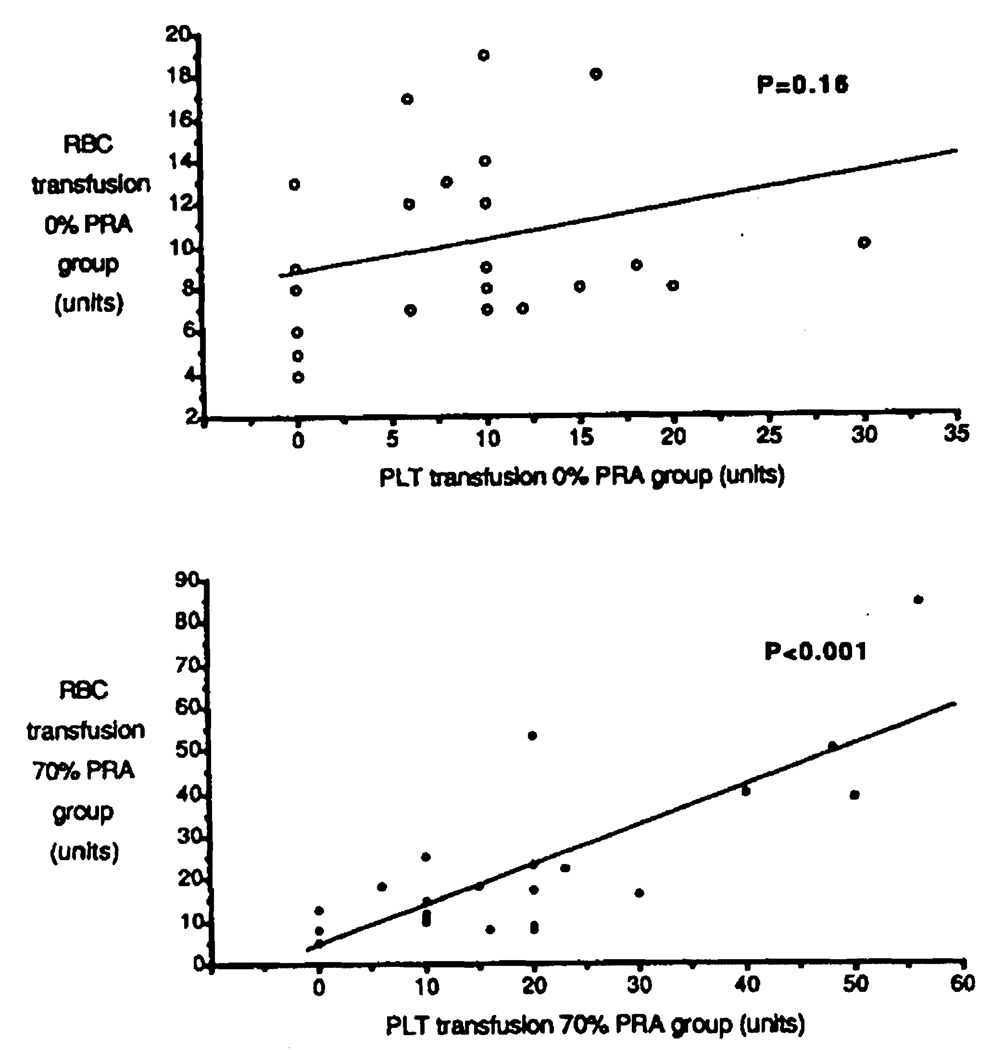
The preoperative platelet counts had similar profiles in both groups, with 124,038±18,495 in the 0% PRA group and 106,826±13,714 in the 70% PRA group, (P = 0.52). Intraoperative substitution with platelets can be expected to depend in part on the preoperative platelet count, since patients with intraoperative bleeding for technical reasons were excluded from this study. The lower the basic platelet count before operation, the higher the quantity of platelets that should be required for transfusion. As shown in Figure 3 (top), this expected correlation was observed in the 0% PRA group (P = 0.0024). In contrast, platelet transfusion requirements showed no significant correlation with pretransplant platelet counts in the 70% PRA group (P = 0.098) (Fig. 3, bottom). This group of highly sensitized patients experienced more bleeding problems that were not readily corrected by platelet transfusions. These bleeding problems were also evident from the increased RBC transfusion requirements, which strongly correlated with an increase in PLT transfusions in the 70% PRA group (Fig. 4, bottom) (P < 0.001). In contrast, no correlation was found between RBC and PLT transfusion requirements in the 0% PRA group (Fig. 4, top) (P = 0.16).
FIGURE 3.
Correlation between preoperative blood platelet counts and intraoperative platelet transfusions in the 0% PRA group (top) and 70% PRA group (bottom).
FIGURE 4.
Correlation between intraoperative transfusion of platelets (PLT) and red blood cells (RBC) in the 0% PRA group (top) and 70% PRA group (bottom).
Despite the larger platelet substitution in the group of highly sensitized patients, as shown in Figure 1, their postoperative platelet counts were significantly reduced as compared with preoperative values (Table 3). At the same time, the 0% PRA group had similar pre- and postoperative platelet counts (Table 2). Since not all patients of each group were treated with platelet transfusions—and thus the overall decreased postoperative platelet counts in the 70% PRA group might be due to some few patients with no platelet infusions but with deteriorated coagulation profile as a result of operation—we compared only patients who received intraoperative platelet transfusions during the transplant operation.
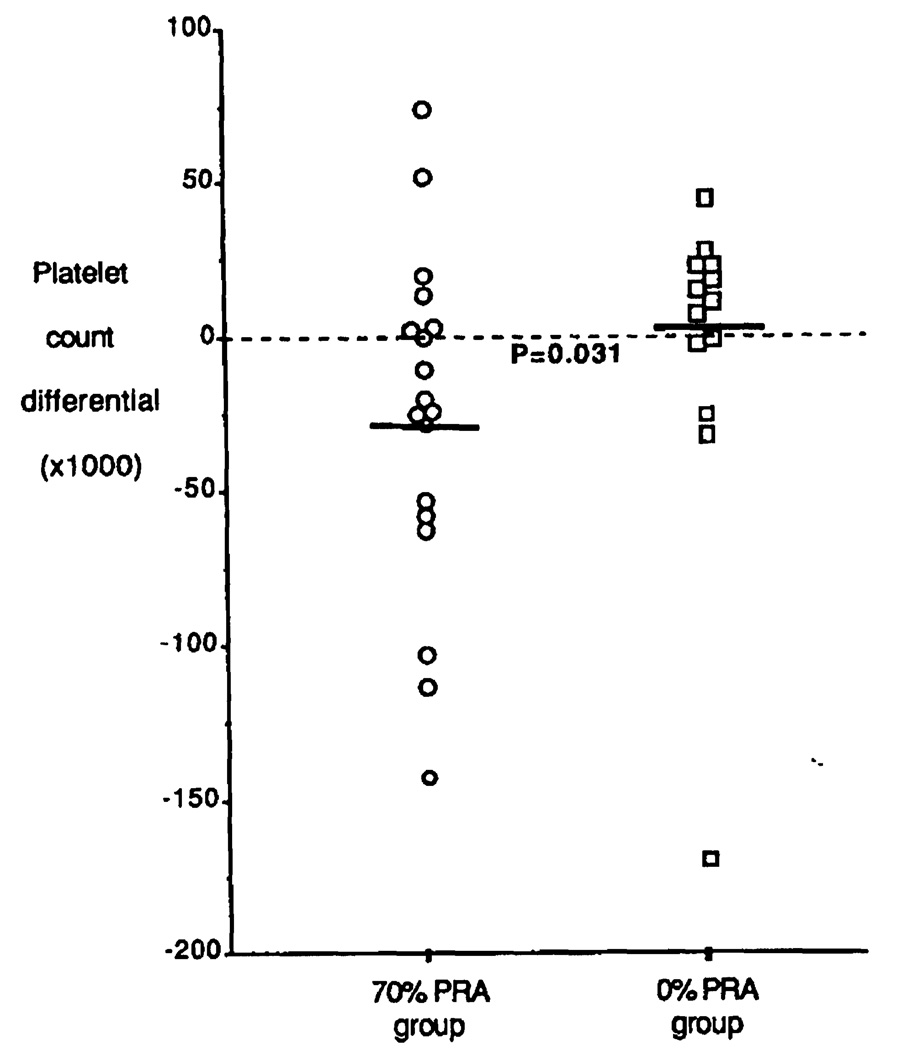
In the 70% PRA group, 20 of 25 were intraoperatively given platelet infusions. The platelet count showed a significant drop from 95,050±11,537 preoperatively to 67,750±8,223 postoperatively (P = 0.028). In contrast, 17 of 26 nonsensitized patients received platelets during the transplant operation. Their pre- (84,058±17,297) and postoperative (85,647±12,445) platelet counts were almost identical. Figure 5 shows the differential in pre- and postoperative platelet count. The 70% PRA group had an average platelet drop of 27,300±11,591, whereas platelet 0% counts in the 0% PRA group slightly increased by 1588±7756 (P = 0.018.
FIGURE 5.
Differential in blood platelet counts before and after operation in transfused patients in the 70% (○) and 0% PRA group (□). Mean values are depicted with thick bars.
Prothrombin time (normal range 10.8–13.0 sec) partial thromboplastin time (normal range 26–34 sec) showed similar prolongation in both groups before and after operation (Tables 2 and 3). Fibrinogen levels varied widely in both groups, but without any statistical significance. The postoperative hematocrit values were about an average of 3–4% higher than the corresponding preoperative values (0% PRA group: P = 0.034, 70% PRA group P = 0.001), and borderline statistically significant differences were observed between the postoperative hematocrit in the 0% and 70% PRA groups (P = 0.053).
DISCUSSION
In this study we present evidence that lymphocytotoxic antibody activity in liver transplant recipients is associated with intraoperative blood transfusion requirements. Highly sensitized patients with PRA values of greater than 70% received during transplant operation much larger quantities of red blood cells, platelets, cryoprecipitate, and fresh frozen plasma than nonsensitized patients with 0% PRA. This analysis was done on patients receiving a first transplant and selected solely on the basis of their PRA. Both groups had similar distributions of liver diseases, and none of these patients had a previous surgical history or intraoperative complications that could have been responsible for greater bleeding problems during the transplant procedure.
Lymphocytotoxic antibodies are generally specific for HLA antigens. In particular, antibodies specific for class I HLA antigens controlled by the HLA-A and HLA-B loci are important in platelet transfusions (23–25). HLA alloimmunization is a major cause of platelet refractoriness of thrombocytopenic patients requiring transfusions (23). Such refractory patients respond poorly to random ABO-compatible platelets even when given in larger quantities. Our data suggest that platelet transfusions are also less effective in correcting the bleeding problems of highly sensitized patients undergoing liver transplantation. This may even be more apparent from our finding that, although the group of patients with high PRA values received larger quantities of platelets, they ended up having significantly lower platelet counts after the transplant procedure. This lower platelet count might be due to the “innocent bystander” effect whereby the patients own platelets become involved in the immune clearance of transfused incompatible platelets (26). Thus, in certain alloimmunized patients, random platelet transfusions would not only be ineffective, but also might worsen the bleeding problems during the transplant procedure.
Our data also show a close correlation between the quantities of transfused platelets and erythrocytes in the 70% PRA group but not in the 0% PRA group. This may be because platelet transfusions in the 70% PRA group are not hemostatistically effective in correcting intraoperative bleeding problems, which would lead to increased requirements for red blood cell substitution in the group of sensitized patients. Nevertheless, recent studies have indicated a low expression of HLA class I antigens on red blood cells (30), and an involvement of red blood cells in the alloimmune response—either direct or indirect—cannot be completely ruled out. The lack of correlation between preoperative platelet counts and subsequent intraopertive platelet transfusion in the 70% PRA group further indicates that hemostasis was not merely a function of absolute platelet counts but of platelet activity. In contrast, the platelet substitution in the 0% PRA group was, as expected, inversely proportional to the preoperative platelet counts.
The decreased coagulation status in highly alloimmunized patients was also documented by differences in plasma replacement requirements and substitution with concentrated fractions of coagulation factors. The 70% PRA group needed significantly higher substitution with fresh frozen plasma and cryoprecipitates than the 0% PRA group. Further studies are necessary to evaluate intraoperative changes in these coagulation factors in alloimmunized patients as a possible cause of bleeding.
Our preliminary data presented here show that prothrombin time, activated thromboplastin time, and fibrinogen levels had identical pre- and postoperative values in both groups. Thus, on the basis of a considerably larger substitution in the 70% PRA group, we conclude from these findings that major blood loss in highly alloimmunized liver transplant patients is caused by direct or indirect interference of preformed alloreactive antibodies that cause refractoriness to platelet transfusions from random donors. The administration of histocompatible platelets in these patients might lead to a more effective hemostasis and thus lower the quantities of other blood components required for substitution. Preoperative screening of patient sera for panel-reactive antibodies, indicating the degree of alloimmunization, would identify liver transplant candidates at increased risk of bleeding problems that would require larger quantities of blood during transplantation. Highly sensitized patients might also benefit from HLA-matched rather than random platelet transfusions.
Footnotes
This work was supported in part by Grants HL-35069 and AM-29961 from the National Institutes of Health.
Recipient of a Fellowship grant from the Deutsche Forschungsgemeinschaft.
Abbreviations: CRYO, cryoprecipitate; FFP, fresh frozen plasma; PLT, platelets.
REFERENCES
- 1.Lewis JH, Bontempo FA, Cornell F, et al. Blood use in liver transplantation. Transfusion. 1987;27:222. doi: 10.1046/j.1537-2995.1987.27387235624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw BW, Jr, Wood PR, Gordon RD, Iwatsuki S, Gillquist WP, Starzl TE. Influence of selected variables and operative blood loss on six-months survival following liver transplantation. Semin Liv. 1987;5:385. doi: 10.1055/s-2008-1040637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bontempo FA, Lewis JH, Van Thiel DH, et al. The relations of preoperative coagulation findings to diagnosis, blood usage, and survival in adult liver transplantation. Transplantation. 1985;39:532. doi: 10.1097/00007890-198505000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calne RY. Management of bleeding. In: Sir Roy Calne, editor. Liver transplantation. 2nd ed. London: Grune & Stratton; 1987. p. 247. [Google Scholar]
- 5.Starzl TE, Iwatsuki S, Esquivel CO, et al. Requirements in the surgical technique of liver transplantation. Semin Liv Dis. 1985;5:349. doi: 10.1055/s-2008-1040632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groth CG, Pechet L, Stanl TE. Coagulation during and after orthotopic transplantation of the human liver. Arch Surg. 1969;98:31. doi: 10.1001/archsurg.1969.01340070049006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888. [PMC free article] [PubMed] [Google Scholar]
- 8.Groth CG. Changes in coagulation. In: Starzl TE, editor. Experience in hepatic transplantation. Philadelphia: Saunders; 1969. p. 159. [Google Scholar]
- 9.Pechet L, Groth CG, Daloze PM. Changes in coagulation and fibrinolysis after orthotopic canine liver transplantation. J Lab Clin Med. 1969;73:91. [PubMed] [Google Scholar]
- 10.Lewis JH, Bontempo FA, Kang YG, Spero JA, Ragni MV, Starzl TE. Intraoperative coagulation changes in liver transplantation. In: Winter PM, Kang YG, editors. Hepatic transplantation: anesthetic and perioperative management. New York: Praeger; 1986. p. 142. [Google Scholar]
- 11.Shaw BW, Jr, Martin DJ, Marquez JM, et al. Venous by-pass in clinical liver transplantation. Ann Surg. 1984;200:524. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flute PT, Rake MO, Williams R, Seaman MJ, Calne RY. Liver transplantation in man: IV. Haemorrhage and thrombosis. Br Med J. 1969;3:20. doi: 10.1136/bmj.3.5661.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang YG, Lewis JH, Navalgund A, et al. Epsilon-aminocapzoic acid for treatment of fibrinolysis during liver transplantation. Anesthesiology. 1987;66:766. [PMC free article] [PubMed] [Google Scholar]
- 14.Terasaki PI, Marchioro TL, Starzl TE. Sero-typing of human lymphocyte antigens: preliminary trials on long-term kidney homograft survivors. In: Russell PS, Winn JH, Amos DR, editors. Histocompatibility testing 1964. Washington, DC: National Academy of Science; 1965. p. 83. [Google Scholar]
- 15.Kissmeyer-Nielsen F, Olson S, Peterson VP, Fieldborg O. Hyperacute rejection of kidney allografts associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2:662. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 16.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1965;280:735. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner WA, Reitz BA, Oyer PE, Stinson EB, Shumway NE. Cardiac hemotransplantation. Curr Prob Surg. 1979;16:1. doi: 10.1016/s0011-3840(79)80010-6. [DOI] [PubMed] [Google Scholar]
- 18.Singh G, Rabin BS, Thompson ME, et al. Positive warm T-cell crossmatch in cardiac transplantation: with transient vasculitis and without hyperacute rejection. Transplantation. 1982;33:564. doi: 10.1097/00007890-198205000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwatsuki S, Iwaki Y, Kano T, et al. Successful liver transplantation from crossmatch positive donors. Transplant Proc. 1981;13:286. [PMC free article] [PubMed] [Google Scholar]
- 20.Iwatsuki S, Rabin BS, Shaw BW, Starzl TE. Liver transplantation against T-cell positive warm crossmatches. Transplant Proc. 1984;16:1427. [PMC free article] [PubMed] [Google Scholar]
- 21.Fung JJ, Makowka L, Tzakis A, et al. Combined liver-kidney transplantation: analysis of patients with preformed lymphocytotoxic antibodies. Transplant Proc. 1988;20:88. [PMC free article] [PubMed] [Google Scholar]
- 22.Fung JJ, Makowka L, Griffin M, Duquesnoy RJ, Tzakis A, Starzl TE. Successful sequential liver-kidney transplantation in patients with preformed lymphocytotoxic antibodies. Clin Transplant. 1987;1:187. [PMC free article] [PubMed] [Google Scholar]
- 23.Yankee RA, Graft KS, Dawling R, Henderson ES. Selection of unrelated compatible HLA select donors by lymphocyte HLA matching. N Engl J Med. 1973;280:760. doi: 10.1056/NEJM197304122881504. [DOI] [PubMed] [Google Scholar]
- 24.Duquesnoy RJ. Donor selection in platelet transfusion therapy of alloimmunized thrombocytopenic patients. In: Greenwalt TJ, Jamieson GA, editors. The blood platelet in transfusion therapy. New York: Liss; 1978. p. 229. [PubMed] [Google Scholar]
- 25.Schiffer CA, Slichter JJ. Platelet transfusions from single donors. N Engl J Med. 1982;307:245. doi: 10.1056/NEJM198207223070410. [DOI] [PubMed] [Google Scholar]
- 26.Heal JM, Blumberg N, Masel D. An evaluation of crossmatching, HLA and ABO matching for platelet transfusions to refractory patients. Blood. 1987;70:23. [PubMed] [Google Scholar]
- 27.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marquez JM, Jr, Martin D. Anesthesia for liver transplantation. In: Winter PM, Kang YG, editors. Hepatic transplantation: anesthetic and perioperative management. New York: Praeger; 1986. p. 44. [Google Scholar]
- 29.Di Nicola P. Thrombelastography. Springfield, IL: Charles C. Thomas; 1957. [Google Scholar]
- 30.Everett ET, Kao KJ, Scornik JC. Class I HLA molecules on human erythrocytes: quantitation and transfusion effects. Transplantation. 1987;44:123. doi: 10.1097/00007890-198707000-00025. [DOI] [PubMed] [Google Scholar]